Effects of Cranberry Juice Supplementation on Cardiovascular Disease Risk Factors in Adults with Elevated Blood Pressure: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
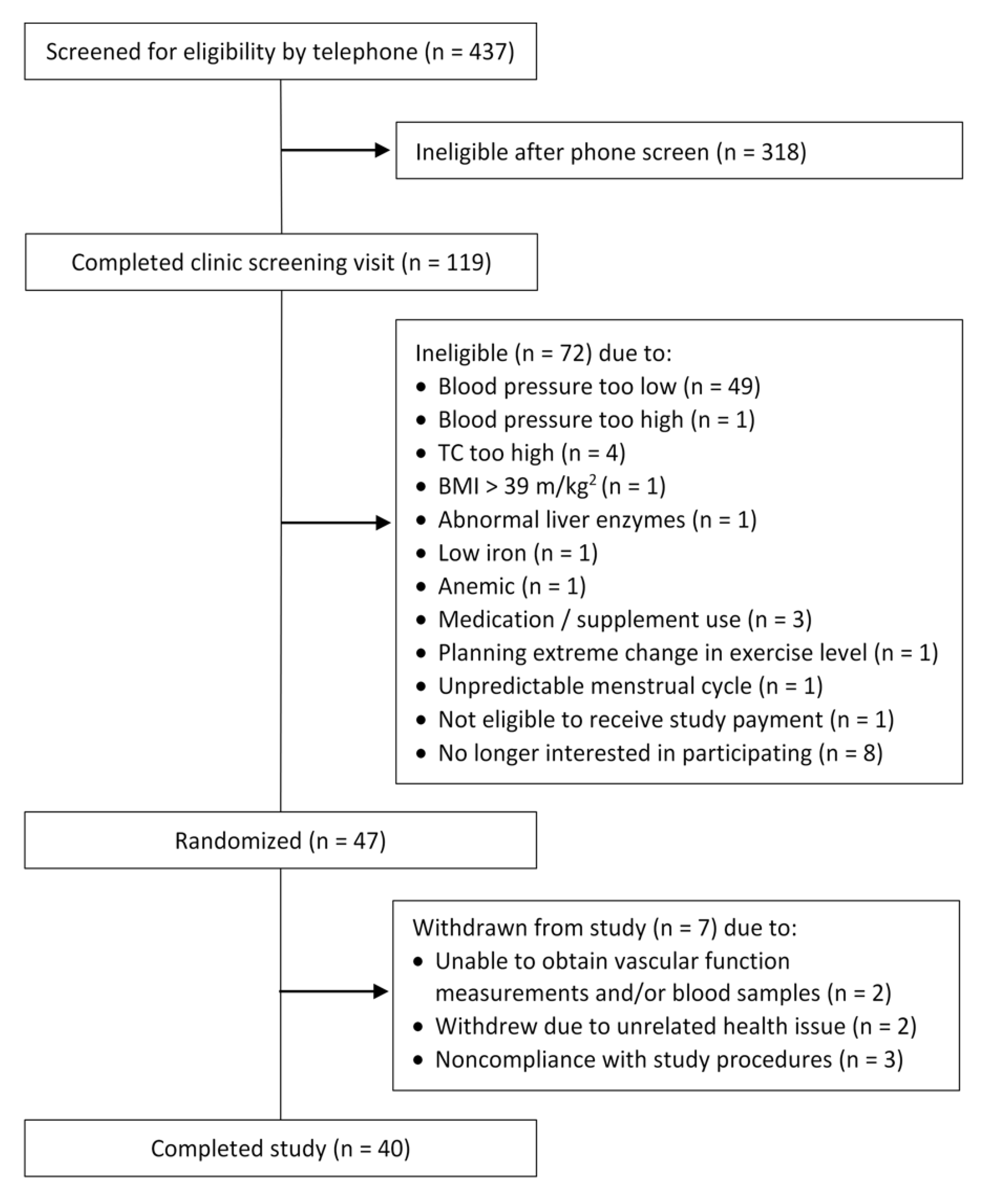
2.2. Participant Recruitment
2.3. Study Design and Intervention
2.4. Vascular Function Measures
2.5. Pulse Wave Analysis (PWA): Central (Aortic) Blood Pressure and Augmentation Index (AI)
2.6. Pulse Wave Velocity (PWV)
2.7. Ambulatory Blood Pressure Monitoring
2.8. Blood Sample Collection and Assay Methods
2.9. Cholesterol Efflux
2.10. Statistical Analyses
3. Results
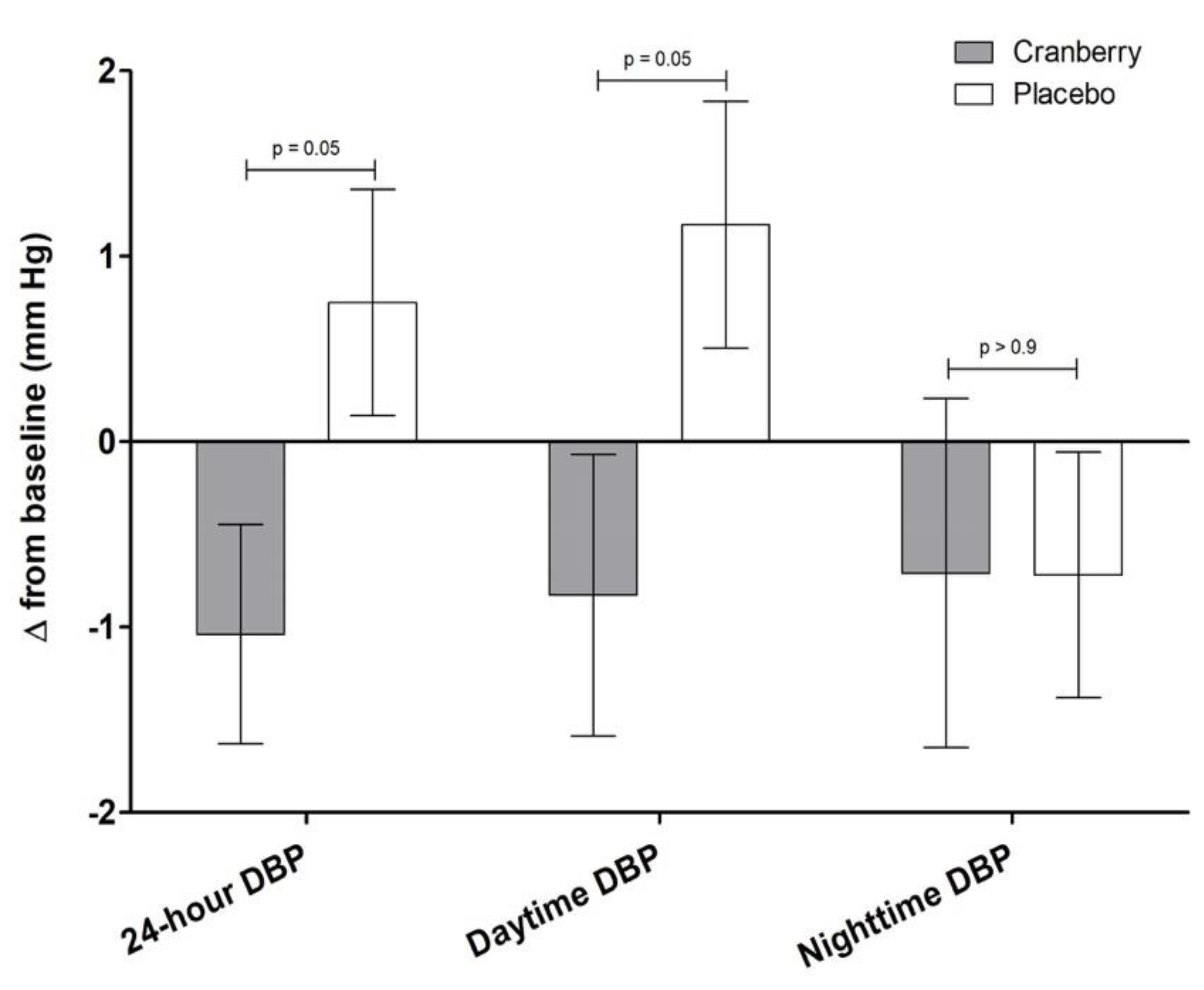
3.1. Effect of Cranberry Juice on Measures of Vascular Function
3.2. Effects of Cranberry Juice on Atherogenic Lipoproteins and Other Blood Markers of CVD Risk
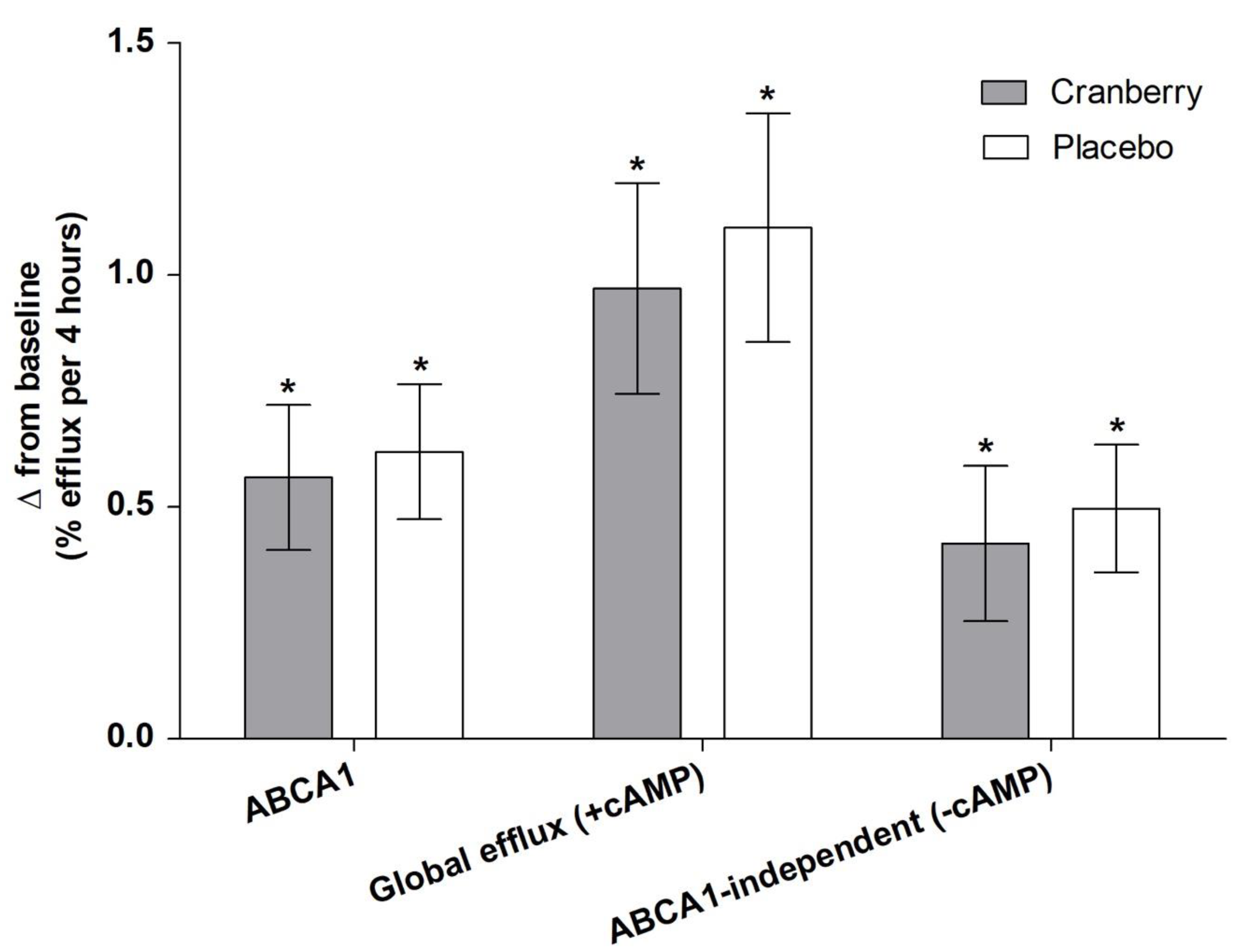
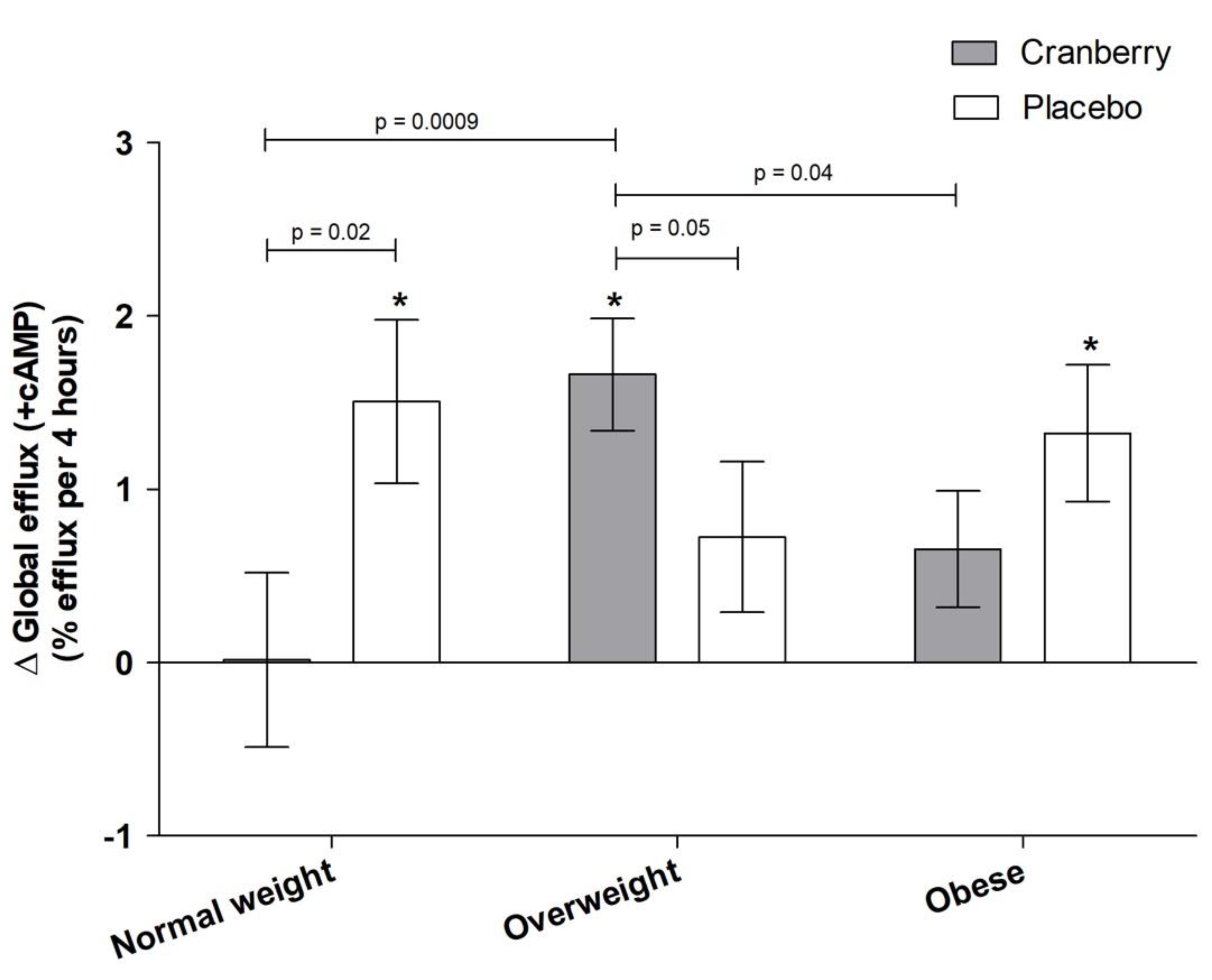
3.3. Effects of Cranberry Juice on HDL Efflux and Role of BMI
3.4. Baseline CRP as a Moderator of Treatment Effects
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control. National Vital Statistics Report—Deaths: Leading causes for 2017; U.S. Department of Health & Human Services: Hyattsville, MD, USA, 2019. [Google Scholar]
- World Health Organization. The Top 10 Causes of Death. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 29 July 2021).
- 2015 Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee; U.S. Department of Agriculture: Washington, DC, USA, 2015. [Google Scholar]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Cote, J.; Caillet, S.; Doyon, G.; Sylvain, J.F.; Lacroix, M. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, M.; Hadi, A.; Najafgholizadeh, A.; Joukar, F.; Mansour-Ghanaei, F. The effects of cranberry on cardiovascular metabolic risk factors: A systematic review and meta-analysis. Clin. Nutr. 2019, 39, 774–788. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B.; Kizer, J.R.; Lee, E.T.; Galloway, J.M.; Ali, T.; Umans, J.G.; Howard, B.V. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The Strong Heart Study. Hypertension 2007, 50, 197–203. [Google Scholar] [CrossRef]
- Waddell, T.K.; Dart, A.M.; Medley, T.L.; Cameron, J.D.; Kingwell, B.A. Carotid pressure is a better predictor of coronary artery disease severity than brachial pressure. Hypertension 2001, 38, 927–931. [Google Scholar] [CrossRef]
- Ruel, G.; Lapointe, A.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Evidence that cranberry juice may improve augmentation index in overweight men. Nutr. Res. 2013, 33, 41–49. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.O.; Blumberg, J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 1223–1235. [Google Scholar] [CrossRef]
- Novotny, J.A.; Baer, D.J.; Khoo, C.; Gebauer, S.K.; Charron, C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015, 145, 1185–1193. [Google Scholar] [CrossRef]
- Shidfar, F.; Heydari, I.; Hajimiresmaiel, S.J.; Hosseini, S.; Shidfar, S.; Amiri, F. The effects of cranberry juice on serum glucose, apoB, apoA-I, Lp(a), and Paraoxonase-1 activity in type 2 diabetic male patients. J. Res. Med. Sci. 2012, 17, 355–360. [Google Scholar]
- Basu, A.; Betts, N.M.; Ortiz, J.; Simmons, B.; Wu, M.; Lyons, T.J. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr. Res. 2011, 31, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Jenkinson, A.M.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chambers, B.K.; Camire, M.E. Can cranberry supplementation benefit adults with type 2 diabetes? Diabetes Care 2003, 26, 2695–2696. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larque, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonne, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.K.; Skulas-Ray, A.C.; Fleming, J.A.; Link, C.J.; Mukherjea, R.; Krul, E.S.; Kris-Etherton, P.M. Effects of isoflavone-containing soya protein on ex vivo cholesterol efflux, vascular function and blood markers of CVD risk in adults with moderately elevated blood pressure: A dose-response randomised controlled trial. Br. J. Nutr. 2017, 117, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Nevo, E.; Fetics, B.; Pak, P.H.; Yin, F.C.; Maughan, W.L.; Kass, D.A. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 1997, 95, 1827–1836. [Google Scholar] [CrossRef]
- Pauca, A.L.; O’Rourke, M.F.; Kon, N.D. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001, 38, 932–937. [Google Scholar] [CrossRef]
- Sharman, J.E.; Lim, R.; Qasem, A.M.; Coombes, J.S.; Burgess, M.I.; Franco, J.; Garrahy, P.; Wilkinson, I.B.; Marwick, T.H. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 2006, 47, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.B.; MacCallum, H.; Flint, L.; Cockcroft, J.R.; Newby, D.E.; Webb, D.J. The influence of heart rate on augmentation index and central arterial pressure in humans. J. Physiol. 2000, 525, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Milne, G.L.; Sanchez, S.C.; Musiek, E.S.; Morrow, J.D. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc. 2007, 2, 221–226. [Google Scholar] [CrossRef]
- Heffron, S.P.; Lin, B.X.; Parikh, M.; Scolaro, B.; Adelman, S.J.; Collins, H.L.; Berger, J.S.; Fisher, E.A. Changes in High-Density Lipoprotein Cholesterol Efflux Capacity After Bariatric Surgery Are Procedure Dependent. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gille, A.; D’Andrea, D.; Tortorici, M.A.; Hartel, G.; Wright, S.D. CSL112 (Apolipoprotein A-I [Human]) Enhances Cholesterol Efflux Similarly in Healthy Individuals and Stable Atherosclerotic Disease Patients. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 953–963. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C. Omega-3 fatty acids and inflammation: A perspective on the challenges of evaluating efficacy in clinical research. Prostaglandins Other Lipid Mediat. 2015, 116, 6390–6404. [Google Scholar] [CrossRef] [PubMed]
- Harada, P.H.N.; Demler, O.V.; Dugani, S.B.; Akinkuolie, A.O.; Moorthy, M.V.; Ridker, P.M.; Cook, N.R.; Pradhan, A.D.; Mora, S. Lipoprotein insulin resistance score and risk of incident diabetes during extended follow-up of 20 years: The Women’s Health Study. J. Clin. Lipidol. 2017, 11, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Shalaurova, I.; Connelly, M.A.; Garvey, W.T.; Otvos, J.D. Lipoprotein insulin resistance index: A lipoprotein particle-derived measure of insulin resistance. Metab. Syndr. Relat. Disord. 2014, 12, 422–429. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef]
- Verdecchia, P. Prognostic value of ambulatory blood pressure: Current evidence and clinical implications. Hypertension 2000, 35, 844–851. [Google Scholar] [CrossRef]
- Verdecchia, P.; Angeli, F.; Cavallini, C. Ambulatory blood pressure for cardiovascular risk stratification. Circulation 2007, 115, 2091–2093. [Google Scholar] [CrossRef][Green Version]
- Staessen, J.A.; Thijs, L.; Fagard, R.; O’Brien, E.T.; Clement, D.; de Leeuw, P.W.; Mancia, G.; Nachev, C.; Palatini, P.; Parati, G.; et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 1999, 282, 539–546. [Google Scholar] [CrossRef]
- Draijer, R.; de Graaf, Y.; Slettenaar, M.; de Groot, E.; Wright, C.I. Consumption of a polyphenol-rich grape-wine extract lowers ambulatory blood pressure in mildly hypertensive subjects. Nutrients 2015, 7, 3138–3153. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Hamburg, N.M.; Holbrook, M.; Kim, B.H.; Duess, M.A.; Levit, A.; Titas, M.; Chung, W.B.; Vincent, F.B.; Caiano, T.L.; et al. Effects of Concord grape juice on ambulatory blood pressure in prehypertension and stage 1 hypertension. Am. J. Clin. Nutr. 2010, 92, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Zock, P.L.; Zebregs, Y.E.; Johnston, N.R.; Webb, D.J.; Draijer, R. Effect of polyphenol-rich grape seed extract on ambulatory blood pressure in subjects with pre- and stage I hypertension. Br. J. Nutr. 2013, 110, 2234–2241. [Google Scholar] [CrossRef]
- de Bock, M.; Derraik, J.G.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Verny, M.A.; Milenkovic, D.; Barber-Chamoux, N.; Mazur, A.; Dubray, C.; Morand, C. Flavanones protect from arterial stiffness in postmenopausal women consuming grapefruit juice for 6 mo: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2015, 102, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef]
- Lynn, A.; Hamadeh, H.; Leung, W.C.; Russell, J.M.; Barker, M.E. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum. Nutr. 2012, 67, 309–314. [Google Scholar] [CrossRef]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; McDougall, G.J.M.; Al-Dujaili, E.A.S. Antioxidant Rich Potato Improves Arterial Stiffness in Healthy Adults. Plant Foods Hum. Nutr. 2018, 73, 203–208. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.Y.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; Dos Santos, C.N.; Ventura, M.R.; Heiss, C. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Marino, M.; Angelino, D.; Del Bo, C.; Del Rio, D.; Riso, P.; Porrini, M. Role of berries in vascular function: A systematic review of human intervention studies. Nutr. Rev. 2019, 78, 189–206. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of Berries Consumption on Cardiovascular Risk Factors: A Meta-analysis with Trial Sequential Analysis of Randomized Controlled Trials. Sci. Rep. 2016, 6, 23625. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.; Vors, C.; Couture, P.; Lamarche, B. LDL particle number and size and cardiovascular risk: Anything new under the sun? Curr. Opin. Lipidol. 2017, 28, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.M. Lipoprotein subfractions and cardiovascular disease risk. Curr. Opin. Lipidol. 2010, 21, 305–311. [Google Scholar] [CrossRef]
- Sacks, F.M.; Campos, H. Clinical review 163: Cardiovascular endocrinology: Low-density lipoprotein size and cardiovascular disease: A reappraisal. J. Clin. Endocrinol. Metab. 2003, 88, 4525–4532. [Google Scholar] [CrossRef]
- Shiffman, D.; Louie, J.Z.; Caulfield, M.P.; Nilsson, P.M.; Devlin, J.J.; Melander, O. LDL subfractions are associated with incident cardiovascular disease in the Malmo Prevention Project Study. Atherosclerosis 2017, 263, 287–292. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, X.; Zhou, Q.; Zhang, Z. High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: A systematic review and meta-analysis. Lipids Health Disord. 2017, 16, 212. [Google Scholar] [CrossRef]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; and Mora, S. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef]
- Ruel, G.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Br. J. Nutr. 2006, 96, 357–364. [Google Scholar] [CrossRef]
- Eftekhari, M.H.; Allaei, M.; Khosropanah, S.; Rajaeifard, A.; Sohrabi, Z. Cranberry Supplement and Metabolic Risk Factors in Obese and Overweight Females. Jentashapir J. Health Res. 2016, 7, e37255. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Fleming, J.A.; Kris-Etherton, P.M. Inclusion of Almonds in a Cholesterol-Lowering Diet Improves Plasma HDL Subspecies and Cholesterol Efflux to Serum in Normal-Weight Individuals with Elevated LDL Cholesterol. J. Nutr. 2017, 147, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar]
- Vinson, J.A.; Bose, P.; Proch, J.; Al Kharrat, H.; Samman, N. Cranberries and cranberry products: Powerful in vitro, ex vivo, and in vivo sources of antioxidants. J. Agric. Food Chem. 2008, 56, 5884–5891. [Google Scholar] [CrossRef] [PubMed]


| Screening Visit | Baseline Visit | ||
|---|---|---|---|
| Pooled | Cranberry-Placebo Sequence | Placebo-Cranberry Sequence | |
| Age (y) | 47 ± 12 (30–64) | 43 ± 12 | 50 ± 10 |
| BMI (kg/m2) | 28.8 ± 4.7 (22.2–39.1) | 28.6 ± 4.6 | 28.9 ± 4.9 |
| Normal weight (18.5–24.9 kg/m2) | n = 9 | n = 4 | n = 5 |
| Overweight (25–29.9 kg/m2) | n = 16 | n = 8 | n = 8 |
| Obese (≥30 kg/m2) | n = 15 | n = 6 | n = 9 |
| Systolic blood pressure (mm Hg) | 124 ± 9 (108–139) | 126 ± 6 | 123 ± 13 |
| Diastolic blood pressure (mm Hg) | 85 ± 6 (72–97) | 82 ± 6 | 79 ± 8 |
| Glucose (mg/dL) | 94 ± 8 (79–113) | 91 ± 7 | 93 ± 7 |
| Total cholesterol (mg/dL) | 195 ± 31 (121–268) | 184 ± 32 | 200 ± 42 |
| HDL-C (mg/dL) | 49 ± 13 (30–84) | 45 ± 11 | 51 ± 12 |
| TC:HDL-C | 4.2 ± 1.2 (1.8–7.8) | 4.3 ± 1.0 | 4.1 ± 1.3 |
| LDL-C (mg/dL) | 124 ± 28 (54–176) | 115 ± 27 | 128 ± 39 |
| Triglycerides (mg/dL) | 112 ± 46 (51–230) | 123 ± 47 | 99 ± 34 |
| Baseline | Cranberry | Placebo | Treatment p-Value | Period p-Value | |
|---|---|---|---|---|---|
| Brachial SBP (mm Hg) | 124 ± 1 | 123 ± 1 | 125 ± 1 | 0.1 | 0.2 |
| Brachial DBP (mm Hg) | 81 ± 1 | 79 ± 1 | 81 ± 1 | 0.3 | 0.3 |
| Central SBP (mm Hg) | 115 ± 1 | 113 ± 1 | 115 ± 1 | 0.2 | 0.2 |
| Central DBP (mm Hg) | 81 ± 1 | 80 ± 1 | 81 ± 1 | 0.3 | 0.3 |
| Central PP (mm Hg) | 33 ± 1 | 33 ± 1 | 34 ± 1 | 0.5 | 0.5 |
| Augmentation Pressure (mm Hg) | 8.8 ± 0.6 | 8.5 ± 0.5 | 8.4 ± 0.4 | 0.8 | 0.3 |
| Augmentation Index | 21.9 ± 1.5 | 20.7 ± 1.0 | 20.7 ± 1.0 | >0.9 | 0.3 |
| Pulse Wave Velocity (m/s) | 7.0 ± 0.1 | 7.1 ± 0.1 | 7.1 ± 0.1 | 0.6 | 0.4 |
| 24-h SBP (mm Hg) | 124 ± 1 | 123 ± 1 | 124 ± 1 | 0.4 | 0.3 |
| 24-h DBP (mm Hg) | 79 ± 1 | 78 ± 1 | 80 ± 1 | 0.05 | 0.4 |
| 24-h PP (mm Hg) | 70 ± 1 | 71 ± 1 | 70 ± 1 | 0.3 | 0.1 |
| Daytime SBP (mm Hg) | 129 ± 1 | 128 ± 1 | 129 ± 1 | 0.4 | 0.2 |
| Daytime DBP (mm Hg) | 82 ± 1 | 81 ± 1 | 83 ± 1 | 0.05 | 0.3 |
| Daytime PP (mm Hg) * | 73 ± 1 | 73 ± 1 | 72 ± 1 | 0.3 | 0.05 |
| Nighttime SBP (mm Hg) | 112 ± 2 | 111 ± 1 | 109 ± 2 | 0.4 | 0.5 |
| Nighttime DBP (mm Hg) | 72 ± 1 | 71 ± 1 | 71 ± 1 | >0.9 | 0.8 |
| Nighttime PP (mm Hg) | 63 ± 1 | 64 ± 1 | 64 ± 1 | 0.7 | 0.9 |
| Baseline | Cranberry | Placebo | Treatment p-Value | Period p-Value | |
|---|---|---|---|---|---|
| Glucose (mg/dL) † | 92 ± 1 | 92 ± 1 | 94 ± 1 * | 0.06 | 0.04 |
| Insulin | 6.4 ± 0.6 | 6.6 ± 0.4 | 6.5 ± 0.3 | 0.9 | 0.8 |
| Total Cholesterol (mg/dL) | 193 ± 6 | 192 ± 3 | 192 ± 3 | 0.8 | 0.8 |
| HDL-C (mg/dL) | 48 ± 2 | 48 ± 1 | 48 ± 1 | 0.8 | 0.6 |
| TC:HDL-C (mg/dL) | 4.2 ± 0.2 | 4.2 ± 0.1 | 4.2 ± 0.1 | >0.9 | 0.4 |
| Direct LDL (mg/dL) | 124 ± 5 | 124 ± 2 | 121 ± 3 | 0.3 | 0.7 |
| Non-HDL-C (mg/dL) | 144 ± 6 | 144 ± 2 | 144 ± 3 | 0.8 | 0.6 |
| Triglycerides (mg/dL) | 110 ± 7 | 109 ± 3 | 115 ± 6 | 0.3 | 0.4 |
| CRP (mg/L) | 2.3 ± 0.4 | 2.0 ± 0.3 | 1.7 ± 0.2 | 0.3 | 0.5 |
| Isoprostanes (ng/mL) | 0.03 ± 0.001 | 0.03 ± 0.002 | 0.023 ± 0.002 | 0.7 | 0.5 |
| Baseline | Cranberry | Placebo | Treatment p-Value | Period p-Value | ||
|---|---|---|---|---|---|---|
| VLDL and Chylomicron Particle Concentrations | Total VLDL and Chylomicron Particles (nmol/L) | 44.71 ± 1.72 | 47.40 ± 2.57 | 50.19 ± 2.29 * | 0.3 | 0.9 |
| Large VLDL and Chylomicron Particles (nmol/L) | 4.42 ± 0.39 | 4.59 ± 0.35 | 4.66 ± 0.44 | 0.9 | 0.2 | |
| Medium VLDL Particles (nmol/L) | 17.63 ± 1.35 | 17.23 ± 1.48 | 20.61 ± 1.87 | 0.06 | >0.9 | |
| Small VLDL Particles (nmol/L) | 22.68 ± 1.09 | 25.59 ± 2.00 | 24.94 ± 1.47 | 0.7 | 0.7 | |
| LDL Particle Concentrations | Total LDL Particles (nmol/L) | 1210.15 ± 38.92 | 1180.38 ± 22.80 | 1185.35 ± 30.59 | 0.9 | 0.3 |
| IDL Particles (nmol/L) | 245.3 ± 16.73 | 226.43 ± 16.90 | 228.65 ± 17.81 | 0.9 | 0.5 | |
| Large LDL Particles (nmol/L) | 339 ± 18.00 | 368.53 ± 16.63 | 332.30 ± 17.61 | 0.02 | 0.2 | |
| Small LDL Particles (nmol/L) | 625.83 ± 20.13 | 585.30 ± 20.23 * | 624.33 ± 20.51 | 0.09 | 0.8 | |
| HDL Particle Concentrations | Total HDL Particles (µmol/L) | 31.51 ± 0.52 | 30.87 ± 0.61 | 31.55 ± 0.52 | 0.2 | 0.1 |
| Large HDL Particles (µmol/L) | 6.12 ± 0.32 | 5.96 ± 0.24 | 6.00 ± 0.23 | 0.8 | 0.9 | |
| Medium HDL Particles (µmol/L) | 9.17 ± 0.76 | 8.84 ± 0.49 | 8.65 ± 0.69 | 0.8 | 0.7 | |
| Small HDL Particles (µmol/L) | 16.21 ± 0.70 | 16.13 ± 0.58 | 16.84 ± 0.75 | 0.2 | 0.03 † | |
| Mean Particle Sizes | VLDL Size (nm) | 50.26 ± 0.72 | 49.84 ± 0.78 | 49.31 ± 0.89 | 0.6 | 0.3 |
| LDL Size (nm) | 20.79 ± 0.06 | 20.86 ± 0.04 | 20.72 ± 0.05 | 0.001 | 0.7 | |
| HDL Size (nm) | 9.15 ± 0.05 | 9.16 ± 0.04 | 9.12 ± 0.04 | 0.3 | 0.4 | |
| Calculated Lipids | Total Triglyceride (mg/dL) | 114.6 ± 5.38 | 115.97 ± 5.13 | 121.15 ± 6.58 | 0.4 | 0.5 |
| Total VLDL and Chylomicron Triglyceride (mg/dL) | 76.7 ± 3.85 | 78.88 ± 3.79 | 83.28 ± 4.57 | 0.4 | 0.5 | |
| Total HDL Cholesterol (mg/dL) | 48.55 ± 1.52 | 47.83 ± 1.10 | 47.78 ± 1.1 | >0.9 | 0.3 | |
| Lipoprotein Insulin-Resistance Score (LPIR) | 50.6 ± 2.28 | 49.03 ± 1.80 | 50.10 ± 2.08 | 0.6 | 0.2 |
| Baseline | Cranberry | Placebo | Treatment p-Value | Period p-Value | |
|---|---|---|---|---|---|
| ABCA1 | 2.29 ± 0.12 | 2.86 ± 0.16 * | 2.91 ± 0.15 * | 0.8 | 0.09 |
| Global efflux (+cAMP) | 7.19 ± 0.20 | 8.16 ± 0.23 * | 8.29 ± 0.25 * | 0.7 | 0.8 |
| ABCA1-independent (-cAMP) | 4.89 ± 0.11 | 5.30 ± 0.17 * | 5.38 ± 0.14 * | 0.7 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, C.K.; Skulas-Ray, A.C.; Gaugler, T.L.; Meily, S.; Petersen, K.S.; Kris-Etherton, P.M. Effects of Cranberry Juice Supplementation on Cardiovascular Disease Risk Factors in Adults with Elevated Blood Pressure: A Randomized Controlled Trial. Nutrients 2021, 13, 2618. https://doi.org/10.3390/nu13082618
Richter CK, Skulas-Ray AC, Gaugler TL, Meily S, Petersen KS, Kris-Etherton PM. Effects of Cranberry Juice Supplementation on Cardiovascular Disease Risk Factors in Adults with Elevated Blood Pressure: A Randomized Controlled Trial. Nutrients. 2021; 13(8):2618. https://doi.org/10.3390/nu13082618
Chicago/Turabian StyleRichter, Chesney K., Ann C. Skulas-Ray, Trent L. Gaugler, Stacey Meily, Kristina S. Petersen, and Penny M. Kris-Etherton. 2021. "Effects of Cranberry Juice Supplementation on Cardiovascular Disease Risk Factors in Adults with Elevated Blood Pressure: A Randomized Controlled Trial" Nutrients 13, no. 8: 2618. https://doi.org/10.3390/nu13082618
APA StyleRichter, C. K., Skulas-Ray, A. C., Gaugler, T. L., Meily, S., Petersen, K. S., & Kris-Etherton, P. M. (2021). Effects of Cranberry Juice Supplementation on Cardiovascular Disease Risk Factors in Adults with Elevated Blood Pressure: A Randomized Controlled Trial. Nutrients, 13(8), 2618. https://doi.org/10.3390/nu13082618






