Ketogenic Diet, Physical Activity, and Hypertension—A Narrative Review
Abstract
:1. Methodology of Literature Search
1.1. Data Sources and Search
1.2. Data Analysis
2. Introduction
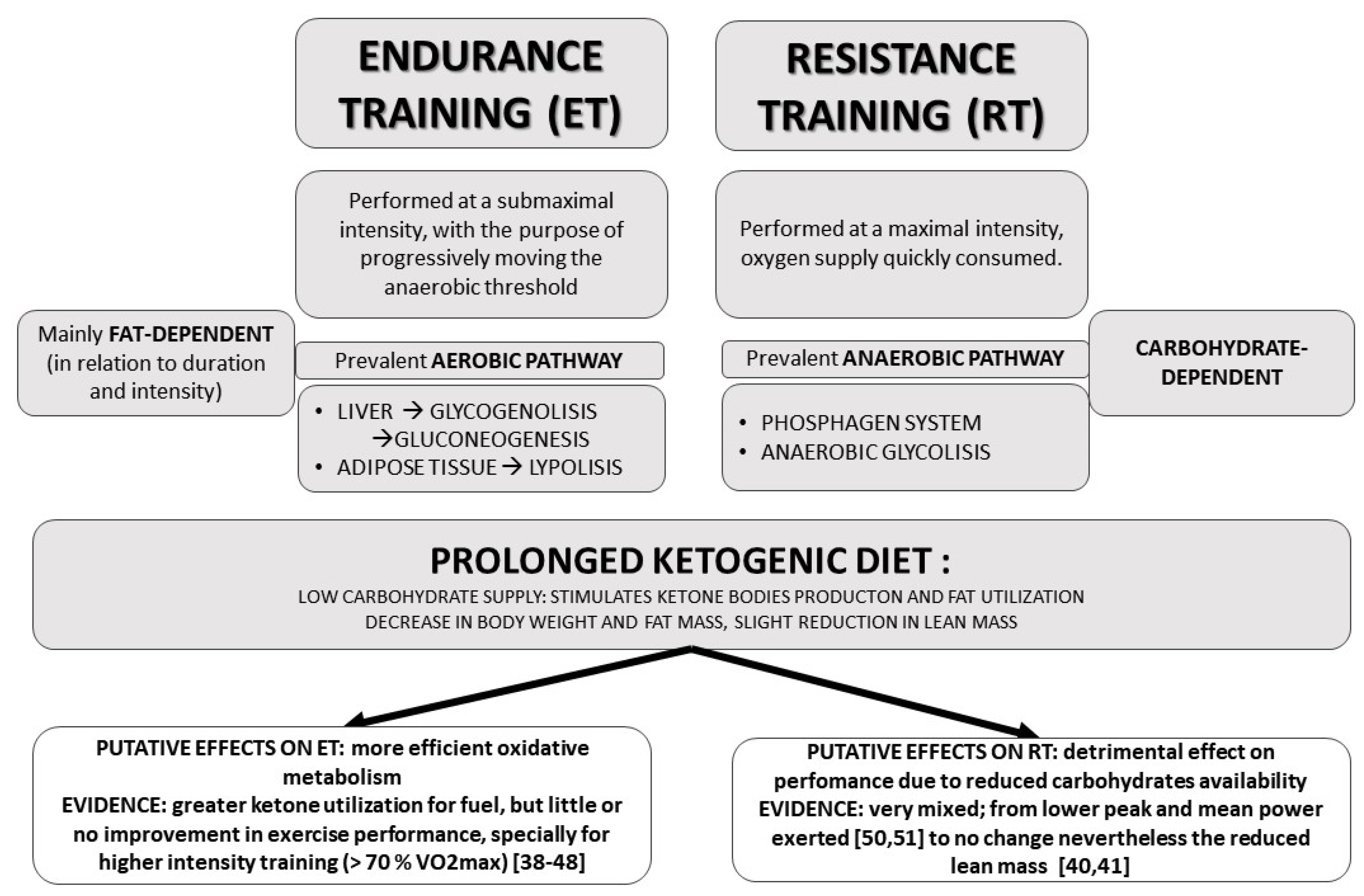
3. Ketogenic Diet and Physical Activity
4. Effects of Ketogenic Diet on Blood Pressure Levels
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.M.; Kossoff, E.H. Ketosis and the ketogenic diet, 2010: Advances in treating epilepsy and other disorders. Adv. Pediatr. 2010, 57, 315–329. [Google Scholar] [CrossRef]
- Freeman, J.M.; Kossoff, E.H.; Hartman, A.L. The ketogenic diet: One decade later. Pediatrics 2007, 119, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, O.; Tasabehji, M.W.; Elseaidy, T.; Tomah, S.; Ashrafzadeh, S.; Mottalib, A. Fat versus carbohydrate-based energy-restricted diets for weight loss in patients with type 2 diabetes. Curr. Diabetes Rep. 2018, 18, 128. [Google Scholar] [CrossRef] [Green Version]
- Buscemi, S.; Verga, S.; Tranchina, M.R.; Cottone, S.; Cerasola, G. Effects of hypocaloric very-low-carbohydrate diet vs. Mediterranean diet on endothelial function in obese women. Eur. J. Clin. Investig. 2009, 39, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F. Overweight and diabetes prevention: Is a low-carbohydrate–high-fat diet recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaspar, M.B.; Austin, K.; Huecker, M.; Sarav, M. Ketogenic Diet: From the Historical Records to Use in Elite Athletes. Curr. Nutr. Rep. 2019, 8, 340–346. [Google Scholar] [CrossRef]
- Ruiz, L.D.; Zuelch, M.L.; Dimitratos, S.M.; Scherr, R.E. Adolescent Obesity: Diet Quality, Psychosocial Health, and Cardiometabolic Risk Factors. Nutrients 2019, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Di Raimondo, D.; Tuttolomondo, A.; Buttà, C.; Casuccio, A.; Giarrusso, L.; Miceli, G.; Licata, G.; Pinto, A. Metabolic and anti-inflammatory effects of a home-based programme of aerobic physical exercise. Int. J. Clin. Pract. 2013, 67, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Kuchkuntla, A.R.; Shah, M.; Velapati, S.; Gershuni, V.M.; Rajjo, T.; Nanda, S.; Hurt, R.T.; Mundi, M.S. Ketogenic Diet: An Endocrinologist Perspective. Curr. Nutr. Rep. 2019, 8, 402–410. [Google Scholar] [CrossRef]
- Roach, P.J.; Depaoli-Roach, A.A.; Hurley, T.D.; Tagliabracci, V.S. Glycogen and its metabolism: Some new developments and old themes. Biochem. J. 2012, 441, 763–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phinney, S.D. Ketogenic diets and physical performance. Nutr. Metab. 2004, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Veech, R.L.; Chance, B.; Kashiwaya, Y.; Lardy, H.A.; Cahill, G.F., Jr. Ketone bodies, potential therapeutic uses. IUBMB Life 2001, 51, 241–247. [Google Scholar] [CrossRef]
- Maalouf, M.; Rho, J.M.; Mattson, M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009, 59, 293–315. [Google Scholar] [CrossRef] [Green Version]
- Stafstrom, C.E.; Rho, J.M. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 2012, 3, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veyrat-Durebex, C.; Reynier, P.; Procaccio, V.; Hergesheimer, R.; Corcia, P.; Andres, C.R.; Blasco, H. How Can a Ketogenic Diet Improve Motor Function? Front. Mol. Neurosci. 2018, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Valenzano, A.; Polito, R.; Trimigno, V.; Di Palma, A.; Moscatelli, F.; Corso, G.; Sessa, F.; Salerno, M.; Montana, A.; Di Nunno, N.; et al. Effects of Very Low Calorie Ketogenic Diet on the Orexinergic System, Visceral Adipose Tissue, and ROS Production. Antioxidants 2019, 8, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, B.; Crujeiras, A.B.; Bellido, D.; Sajoux, I.; Casanueva, F.F. Obesity treatment by very low-calorie-ketogenic diet at two years: Reduction in visceral fat and on the burden of disease. Endocrine 2016, 54, 681–690. [Google Scholar] [CrossRef]
- Boison, D. New insights into the mechanisms of the ketogenic diet. Curr. Opin. Neurol. 2017, 30, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Harvey, C.J.D.C.; Schofield, G.M.; Williden, M. The use of nutritional supplements to induce ketosis and reduce symptoms associated with keto-induction: A narrative review. PeerJ 2018, 6, e4488. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.C.; Khatri, A.; Kifayat, A.; Cho, R.; Aronow, W.S. Starvation Ketoacidosis due to the Ketogenic Diet and Prolonged Fasting—A Possibly Dangerous Diet Trend. Am. J. Case Rep. 2019, 20, 1728–1731. [Google Scholar] [CrossRef]
- Bostock, E.C.S.; Kirkby, K.C.; Taylor, B.V.; Hawrelak, J.A. Consumer Reports of “Keto Flu” Associated With the Ketogenic Diet. Front. Nutr. 2020, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, B.; Raggi, P. The ketogenic diet: Pros and cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.T.; van Dam, R.M.; Hankinson, S.E.; Stampfer, M.; Willett, W.C.; Hu, F.B. Low-carbohydrate diets and all-cause and cause-specific mortality: Two cohort studies. Ann. Intern. Med. 2010, 153, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Bough, K.J.; Wetherington, J.; Hassel, B.; Pare, J.F.; Gawryluk, J.W.; Greene, J.G.; Shaw, R.; Smith, Y.; Geiger, J.D.; Dingledine, R.J. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kashiwaya, Y.; King, M.T.; Baxa, U.; Tam, J.; Niu, G.; Chen, X.; Clarke, K.; Veech, R.L. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 2012, 26, 2351–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gama, G.; Farinatti, P.; Rangel, M.V.D.S.; Mira, P.A.C.; Laterza, M.C.; Crisafulli, A.; Borges, J.P. Muscle metaboreflex adaptations to exercise training in health and disease. Eur. J. Appl Physiol. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Riebe, D., Ehrman, J.K., Liguori, G., Magal, M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2018; ISBN 9781496339065. [Google Scholar]
- Harvey, K.L.; Holcomb, L.E.; Kolwicz, S.C., Jr. Ketogenic Diets and Exercise Performance. Nutrients 2019, 11, 2296. [Google Scholar] [CrossRef] [Green Version]
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Feuerbacher, J.F.; von Schöning, V.; Melcher, J.; Notbohm, H.L.; Freitag, N.; Schumann, M. Short-Term Creatine Loading Improves Total Work and Repetitions to Failure but Not Load-Velocity Characteristics in Strength-Trained Men. Nutrients 2021, 13, 826. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Miceli, G.; Musiari, G.; Tuttolomondo, A.; Pinto, A. New insights about the putative role of myokines in the context of cardiac rehabilitation and secondary cardiovascular prevention. Ann. Transl. Med. 2017, 5, 300. [Google Scholar] [CrossRef] [Green Version]
- Milanović, Z.; Sporiš, G.; Weston, M. Effectiveness of High-Intensity Interval Training (HIT) and Continuous Endurance Training for VO2max Improvements: A Systematic Review and Meta-Analysis of Controlled Trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef]
- Niemeyer, M.; Leithaeuser, R.; Beneke, R. Oxygen uptake plateau occurrence depends on oxygen kinetics and oxygen deficit accumulation. Scand. J. Med. Sci. Sports 2019, 29, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Sansone, M.; Sansone, A.; Borrione, P.; Romanelli, F.; Di Luigi, L.; Sgrò, P. Effects of Ketone Bodies on Endurance Exercise. Curr. Sports Med. Rep. 2018, 17, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; D’Agostino, D.P. Fueling performance: Ketones enter the mix. Cell Metab. 2016, 24, 373–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic Characteristics of Keto-Adapted Ultra-Endurance Runners. Metabolism 2016, 65, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low Carbohydrate, High Fat Diet Impairs Exercise Economy and Negates the Performance Benefit from Intensified Training in Elite Race Walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.J.; Sharma, A.P.; Ross, M.L.; Welvaert, M.; Slater, G.J.; Burke, L.M. Chronic Ketogenic Low Carbohydrate High Fat Diet Has Minimal effects on Acid-Base Status in Elite Athletes. Nutrients 2018, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Cipryan, L.; Plews, D.J.; Ferretti, A.; Ma_etone, P.B.; Laursen, P.B. Effects of a 4-Week Very Low-Carbohydrate Diet on High-Intensity Interval Training Responses. J. Sports Sci. Med. 2018, 17, 259–268. [Google Scholar]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Siedzik, K. Effect of a Four-Week Ketogenic Diet on Exercise Metabolism in Crossfit-Trained Athletes. J. Int. Soc. Sports Nutr. 2019, 16, 16. [Google Scholar] [CrossRef] [Green Version]
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M.; et al. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in Crossfit Trainees: A Pilot Study. Sports 2018, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- McSwiney, F.T.; Wardrop, B.; Hyde, P.N.; Lafountain, R.A.; Volek, J.S.; Doyle, L. Keto-Adaptation Enhances Exercise Performance and Body Composition Responses to Training in Endurance Athletes. Metabolism 2018, 81, 25–34. [Google Scholar] [CrossRef]
- O’Neal, E.K.; Smith, A.F.; Heatherly, A.J.; Killen, L.G.; Waldman, H.S.; Hollingsworth, A.; Koh, Y. Effects of a 3-Week High-Fat-Low-Carbohydrate Diet on Lipid and Glucose Profiles in Experienced, Middle-Age Male Runners. Int. J. Exerc. Sci. 2019, 12, 786–799. [Google Scholar]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Maunder, E.; Dulson, D.K. Effect of a Ketogenic Diet on Submaximal Exercise Capacity and Efficiency in Runners. Med. Sci. Sports Exerc. 2019, 51, 2135–2146. [Google Scholar] [CrossRef]
- Zajac, A.; Poprzecki, S.; Maszczyk, A.; Czuba, M.; Michalczyk, M.; Zydek, G. The Effects of a Ketogenic Diet on Exercise Metabolism and Physical Performance in Off-Road Cyclists. Nutrients 2014, 6, 2493–2508. [Google Scholar] [CrossRef] [PubMed]
- Zinn, C.; Wood, M.; Williden, M.; Chatterton, S.; Maunder, E. Ketogenic Diet Benefits Body Composition and Well-Being but Not Performance in a Pilot Case Study of New Zealand Endurance Athletes. J. Int. Soc. Sports Nutr. 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.P.; Hennessy, E. A review of the ketogenic diet for endurance athletes: Performance enhancer or placebo effect? J. Int. Soc. Sports Nutr. 2020, 17, 33. [Google Scholar] [CrossRef]
- van der Leeden, M.; Stuiver, M.M.; Huijsmans, R.; Geleijn, E.; de Rooij, M.; Dekker, J. Structured clinical reasoning for exercise prescription in patients with comorbidity. Disabil. Rehabil. 2020, 42, 1474–1479. [Google Scholar] [CrossRef] [Green Version]
- Wroble, K.A.; Trott, M.N.; Schweitzer, G.G.; Rahman, R.S.; Kelly, P.V.; Weiss, E.P. Low-carbohydrate, ketogenic diet impairs anaerobic exercise performance in exercise-trained women and men: A randomized-sequence crossover trial. J. Sports Med. Phys. Fit. 2019, 59, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, R.M.; Hamdan, H.; Torisky, D.M.; Akers, J.D. A Low-Carbohydrate Ketogenic Diet Combined with 6-Weeks of Crossfit Training Improves Body Composition and Performance. Int. J. Sports Exerc. Med. 2017, 3, 54. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Tas k Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Wang, X.; Jia, P.; You, Y.; Cheng, Y.; Deng, H.; Luo, S.; Huang, B. Ketogenic diet aggravates hypertension via NF-κB-mediated endothelial dysfunction in spontaneously hypertensive rats. Life Sci. 2020, 258, 118124. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., III; Simons-Morton, D.G.; et al. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Saltzman, E.; Wilson, P.W.F.; Jacques, P.F. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004, 27, 538–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asrih, M.; Jornayvaz, F.R. Diets and nonalcoholic fatty liver disease: The good and the bad. Clin. Nutr. 2014, 33, 186–190. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Packard, C.J.; Borén, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef] [Green Version]
- Volek, J.S.; Feinman, R.D. Carbohydrate restriction improves the features of Metabolic Syndrome. Metabolic Syndrome may be defined by the response to carbohydrate restriction. Nutr. Metab. 2005, 2, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volek, J.S.; Fernandez, M.L.; Feinman, R.D.; Phinney, S.D. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res. 2008, 47, 307–318. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef]
- Samaha, F.F.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, T.; Williams, M.; Gracely, E.J.; Stem, L. A Low-Carbohydrate as Compared with a Low-Fat Diet in Severe Obesity. N. Engl. J. Med. 2003, 348, 2074–2081. [Google Scholar] [CrossRef] [Green Version]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; McGuckin, B.G.; Brill, C.; Mohammed, B.S.; Szapary, P.O.; Rader, D.J.; Edman, J.S.; Klein, S. A Randomized Trial of a Low-Carbohydrate Diet for Obesity. N. Engl. J. Med. 2003, 348, 2082–2090. [Google Scholar] [CrossRef] [Green Version]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef]
- Truby, H.; Baic, S.; deLooy, A.; Fox, K.R.; Livingstone, M.B.E.; Logan, C.M.; Macdonald, I.A.; Morgan, L.M.; Taylor, M.A.; Millward, D.J. Randomised controlled trial of four commercial weight loss programmes in the UK: Initial findings from the BBC “diet trials”. BMJ 2006, 332, 1309–1314. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.D.; Kiazand, A.; Alhassan, S.; Kim, S.; Stafford, R.S.; Balise, R.R.; Kraemer, H.C.; King, A.C. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A TO Z Weight Loss Study: A randomized trial. JAMA 2007, 297, 969–977. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Westman, E.C.; McDuffie, J.R.; Grambow, S.C.; Jeffreys, A.S.; Bolton, J.; Chalecki, A.; Oddone, E.Z. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch. Intern. Med. 2010, 170, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.S.; Noakes, M.; Keogh, J.B.; Clifton, P.M. Long-term effects of a low carbohydrate, low fat or high unsaturated fat diet compared to a no-intervention control. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 599–607. [Google Scholar] [CrossRef]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; Makris, A.P.; Rosenbaum, D.L.; Brill, C.; Stein, R.I.; Mohammed, B.S.; Miller, B.; Rader, D.J.; et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Ann. Intern. Med. 2010, 153, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, G.; Ye, X.; Li, H.; Chen, X.; Tang, L.; Feng, Y.; Shai, I.; Stampfer, M.J.; Hu, F.B.; et al. Effects of a low-carbohydrate diet on weight loss and cardiometabolic profile in Chinese women: A randomised controlled feeding trial. Br. J. Nutr. 2013, 110, 1444–1453. [Google Scholar] [CrossRef] [Green Version]
- Bazzano, L.A.; Hu, T.; Reynolds, K.; Yao, L.; Bunol, C.; Liu, Y.; Chen, C.; Klag, M.J.; Whelton, P.K.; He, J. Effects of low-carbohydrate and low-fat diets: A randomized trial. Ann. Intern. Med. 2014, 161, 309–318. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Benelli, M.; Brancaleoni, M.; Dainelli, G.; Merlini, D.; Negri, R. Middle and Long-Term Impact of a Very Low-Carbohydrate Ketogenic Diet on Cardiometabolic Factors: A Multi-Center, Cross-Sectional, Clinical Study. High Blood Press. Cardiovasc. Prev. 2015, 22, 389–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravata, D.M.; Sanders, L.; Huang, J.; Krumholz, H.M.; Olkin, I.; Gardner, C.D.; Bravata, D.M. Efficacy and safety of low-carbohydrate diets: A systematic review. JAMA 2003, 289, 1837–1850. [Google Scholar] [CrossRef]
- Hession, M.; Rolland, C.; Kulkarni, U.; Wise, A.; Broom, J. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes. Rev. 2009, 10, 36–50. [Google Scholar] [CrossRef]
- Santos, F.L.; Esteves, S.S.; da Costa, P.A.; Yancy, W.S., Jr.; Nunes, J.P. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes. Rev. 2012, 13, 1048–1066. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, D.; Musiari, G.; Miceli, G.; Arnao, V.; Pinto, A. Preventive and Therapeutic Role of Muscle Contraction against Chronic Diseases. Curr. Pharm. Des. 2016, 22, 4686–4699. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Di Raimondo, D.; Casuccio, A.; Guercio, G.; Del Cuore, A.; Puleo, M.G.; Della Corte, V.; Bellia, C.; Caronia, A.; Maida, C.; et al. Endothelial function, adipokine serum levels and white matter hyperintesities in subjects with diabetic foot syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 3920–3930. [Google Scholar] [CrossRef]
- Iacovides, S.; Meiring, R.M. The effect of a ketogenic diet versus a high-carbohydrate, low-fat diet on sleep, cognition, thyroid function, and cardiovascular health independent of weight loss: Study protocol for a randomized controlled trial. Trials 2018, 19, 62. [Google Scholar] [CrossRef] [Green Version]
- Armani, A.; Berry, A.; Cirulli, F.; Caprio, M. Molecular mechanisms underlying metabolic syndrome: The expanding role of the adipocyte. FASEB J. 2017, 31, 4240–4255. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Lopaschuk, G.D. CrossTalk proposal: Ketone bodies are an important metabolic fuel for the heart. J. Physiol. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Ruan, H.B.; Crawford, P.A. Ketone bodies as epigenetic modifiers. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prattichizzo, F.; De Nigris, V.; Micheloni, S.; La Sala, L.; Ceriello, A. Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: Is low-grade inflammation the neglected component? Diabetes Obes. Metab. 2018, 20, 2515–2522. [Google Scholar] [CrossRef]
- Mizuno, Y.; Harada, E.; Nakagawa, H.; Morikawa, Y.; Shono, M.; Kugimiya, F.; Yoshimura, M.; Yasue, H. The diabetic heart utilizes ketone bodies as an energy source. Metabolism 2017, 77, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, J. Ketone Body Supplementation—A Potential New Approach for Heart Disease. JAMA 2021, 326, 17–18. [Google Scholar] [CrossRef] [PubMed]
| Ref | Year | Methodology | Population | Patients | Intervention | Duration | Main Results | Other Results | Adherence |
|---|---|---|---|---|---|---|---|---|---|
| Samaha et al. [62] | 2003 | Randomized controlled trial | Age > 18 years and body mass index at least 35 | 132 | Low-CHO (<30 g/day) vs. low-fat diet | 6 months | Not significant overall or between group changes in BP (mean SBP −1.0 mmHg, mean DBP −3.0 mmHg vs. low-fat diet). | No patient needed change of antihypertensive therapy during the study | Less drop-out in low-CHO group; p = 0.03 at third month |
| Foster et al. [63] | 2003 | Randomized controlled trial | Obese men and women | 63 | Low-CHO (20 g/day), high-protein diet vs. low-calories, high-CHO, low-fat diet (“conventional”) | 12 months | Not significant overall or between group changes in BP (mean SBP −2.7 mmHg, mean DBP −0.1 mmHg vs. “coventional” diet). | Level of adherence reported as “poor” in both groups | |
| Dansinger et al. [64] | 2005 | Randomized Trial | Overweight or obese adults aged 22 to 72 years with known hypertension, dyslipidemia, or fasting hyperglycemia | 160 | Atkins (<20 g/day), Zone (macronutrient balance), Weight Watchers (calorie restriction), or Ornish (fat restriction) diet groups | 12 months | No significant differences in BP found between the groups | Each popular diet modestly reduced body weight and several cardiac risk factors at 1 year. | Overall dietary adherence rates were low |
| Truby et al. [65] | 2006 | Randomized controlled trial | Adults in the United Kingdom | 293 | Dr Atkins’ new diet (<20 g/day), Slim-Fast plan, Weight Watchers pure points programme, and Rosemary Conley’s eat yourself slim diet and fitness plan | 6 months | Low-CHO Diet (Atkins) resulted in SBP reduction at the end of the study of 7.20 mmHg and DBP mean reduction at the end of the study of 4.90. The reduction was not significantly different from the other diets | Regression analysis showed that total weight loss over time had the greatest influence on SBP and DBP | Compliance with each diet varied greatly |
| Gardner et al. [66] | 2009 | Randomized trial | Overweight premenopausal women | 311 | Atkins (<20 g/day) vs. Zone vs. LEARN vs. Ornish diet | 20 months | The decrease in mean BP levels was largest in the Atkins group at all time points (at 20 months SBP mean difference −7.60 mmHg; DBP mean difference −4.40 mmHg) | More favourable control of other CV risk factor for low-CHO diet | Lack of valid assessment of individual compliance |
| Yancy et al. [67] | 2010 | Randomized controlled trial | Overweight or obese outpatients from the Department of Veterans Affairs primary care clinics | 160 | Low-CHO diet (< 20 g/day) vs. low-fat diet plus orlistat | 48 weeks | Low-CHO group had a significantly larger reduction in SBP −5.9 mm Hg (95% CI, −8.8 to −3.1) and DBP −4.5 mm Hg (95% CI, −6.6 to −2.5) than low-fat + orlistat group (p < 0.001) | The 2 interventions resulted in similar weight loss | Reported not relevant |
| Lim et al. [68] | 2010 | Randomized controlled trial | Pts of 47 ± 10 years, BMI 32 ± 6 kg/m2 and one additional cardiovascular risk factor | 113 | Very low carbohydrate (10% of carbs) vs. very low fat (VLF) vs. high unsaturated fat (HUF) vs. control group (no intervention) | 24 months | Decreases in body weight and DBP in the diet groups (−2.9 ± 5.2 mmHg) were significantly different vs. the control group (+0.8 ± 5.0) (p < 0.05) | Equivalent control of the cardiovascular risk factors with the three diets tested | Level of adherence reported as “modest” |
| Foster et al. [69] | 2010 | Randomized controlled trial | Pts of 45.5 ± 9.7 years and mean BMI of 36.1 ± 3.5 kg/m2 | 307 | Low-CHO diet (20 g/day) vs. low-fat diet | 24 months | SBP mean difference at the end of the study: −2.68 mmHg; DBP mean difference at the end of the study: −3.19 mmHg. No differences between groups at any time | 32% of dropout in the low-fat group vs. 42% in the low-CHO | |
| Liu et al. [70] | 2013 | Randomized controlled trial | Chinese women (30–65 years with a BMI = 24 kg/m2) | 50 | Low-CHO non-energy-restricted diet (20 g/day) vs. low-CHO (20 g/day) energy restricted | 12 weeks | SBP mean difference at the end of the study −4.60 mmHg; DBP mean difference at the end of the study −2.70 mmHg; no difference between groups | Adherence reported 96% | |
| Bazzano et al. [71] | 2014 | Randomized controlled trial | Men and women aged 22 to 75 years with a BMI of 30 to 45 kg/m2 without CV disease or diabetes | 148 | Low-CHO (<40 g/day) vs. low-fat diet (<30% fat; <7% saturated fat) | 12 months | SBP mean difference at the end of the study −3.60 mmHg; DBP mean difference not reported. No significant difference between groups | Low-CHO diet was more effective for weight loss and cardiovascular risk factor reduction | Level of adherence reported as “high” |
| Cicero et al. [72] | 2015 | Multi-Center, Cross-Sectional Observational study | Outpatients aged 30–69 years with a BMI of 27–37 kg/m2 and abdominal circumference ≥ 98 cm for men and ≥ 87 cm for women | 377 | Very-low carbohydrate ketogenic diet (carbs 2–6 g/die), low fat, proteins intake 1.2/1.5 g/kg of ideal body weight | 12 months | SBP (−10.5 ± 6.4 mmHg, p < 0.001) and DBP (−2.2 ± 3.1 mmHg, p < 0.001) changed from baseline to three months but no further changes were detected until the end of the study | The max reduction of body weight and CV risk factor levels was found after 12 weeks and only maintained after | 66 patients dropped-out for different reasons, but none of which was a clinical matter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Raimondo, D.; Buscemi, S.; Musiari, G.; Rizzo, G.; Pirera, E.; Corleo, D.; Pinto, A.; Tuttolomondo, A. Ketogenic Diet, Physical Activity, and Hypertension—A Narrative Review. Nutrients 2021, 13, 2567. https://doi.org/10.3390/nu13082567
Di Raimondo D, Buscemi S, Musiari G, Rizzo G, Pirera E, Corleo D, Pinto A, Tuttolomondo A. Ketogenic Diet, Physical Activity, and Hypertension—A Narrative Review. Nutrients. 2021; 13(8):2567. https://doi.org/10.3390/nu13082567
Chicago/Turabian StyleDi Raimondo, Domenico, Silvio Buscemi, Gaia Musiari, Giuliana Rizzo, Edoardo Pirera, Davide Corleo, Antonio Pinto, and Antonino Tuttolomondo. 2021. "Ketogenic Diet, Physical Activity, and Hypertension—A Narrative Review" Nutrients 13, no. 8: 2567. https://doi.org/10.3390/nu13082567
APA StyleDi Raimondo, D., Buscemi, S., Musiari, G., Rizzo, G., Pirera, E., Corleo, D., Pinto, A., & Tuttolomondo, A. (2021). Ketogenic Diet, Physical Activity, and Hypertension—A Narrative Review. Nutrients, 13(8), 2567. https://doi.org/10.3390/nu13082567