Renal Rehabilitation: Exercise Intervention and Nutritional Support in Dialysis Patients
Abstract
:1. Introduction
2. History and Conception of Renal Rehabilitation
3. Guidelines for Renal Rehabilitation
4. Physical Activity in Dialysis Patients
5. Exercise Tolerance in CKD Patients
6. Muscle Energy Metabolism and Nutrients
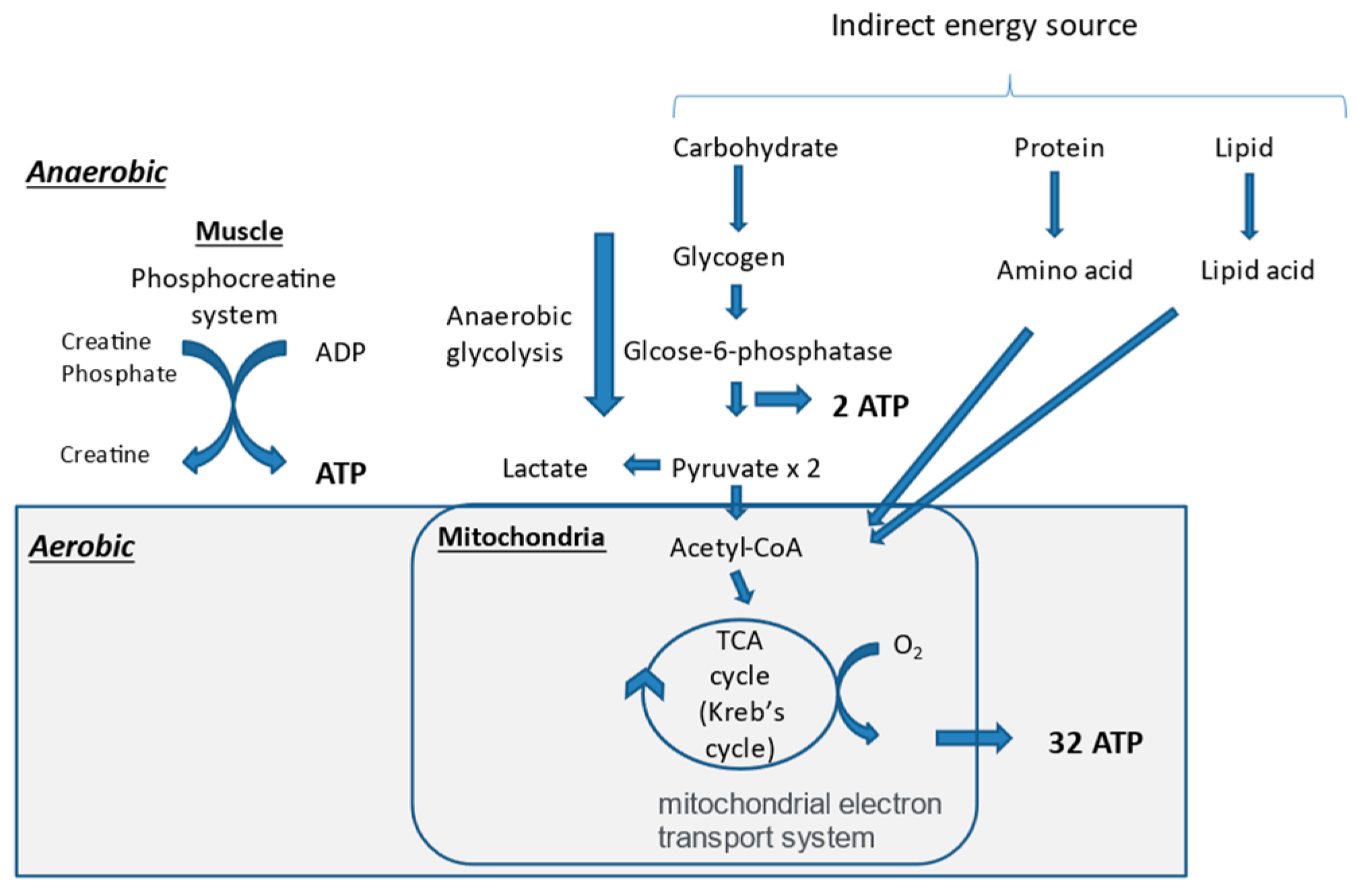
6.1. Mechanisms of Adenosine Triphosphate (ATP) Production
6.2. Synthesis of Skeletal Muscle and Its Related Nutrients
6.3. Muscle Energy Metabolism and Nutrients in CKD
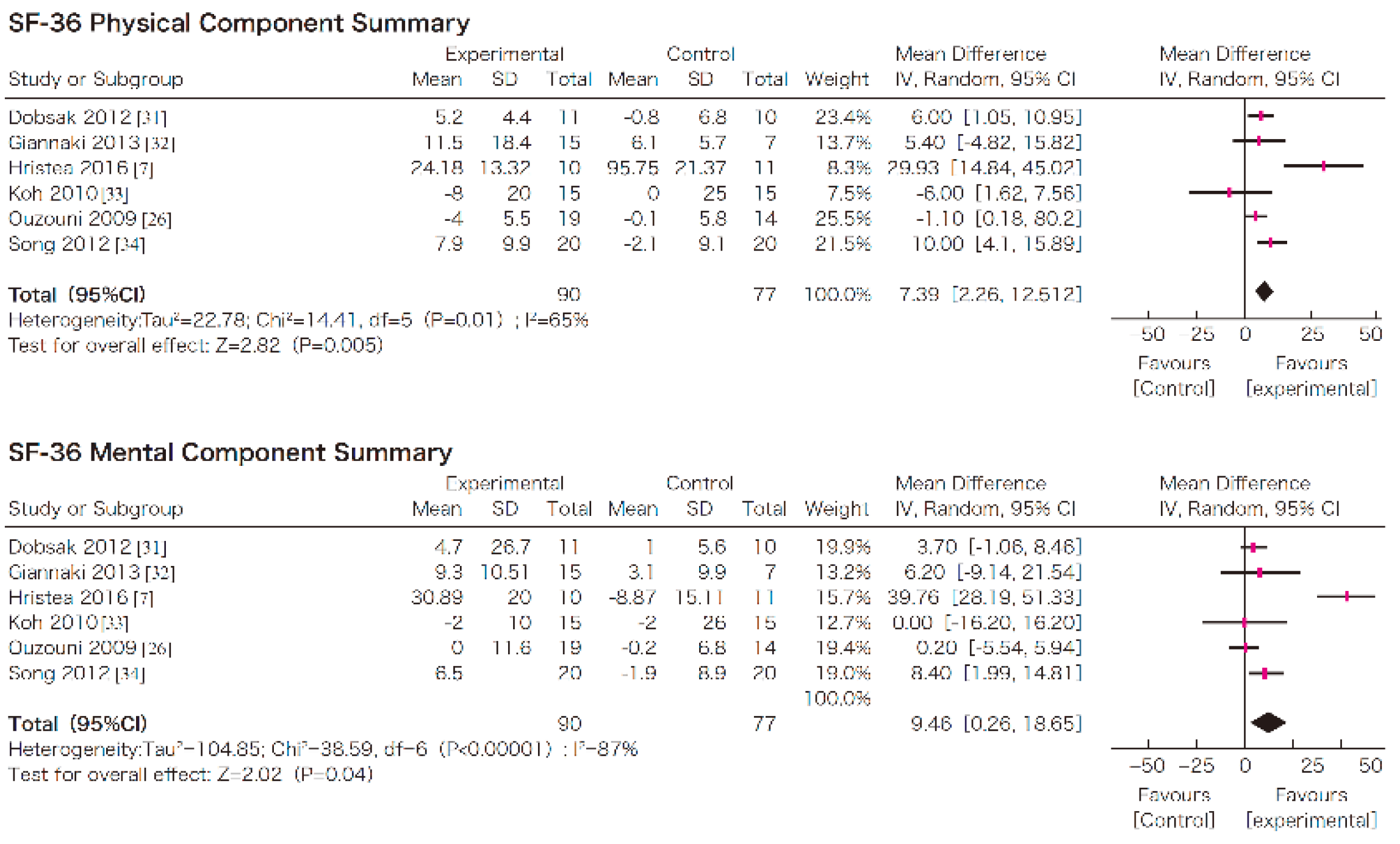
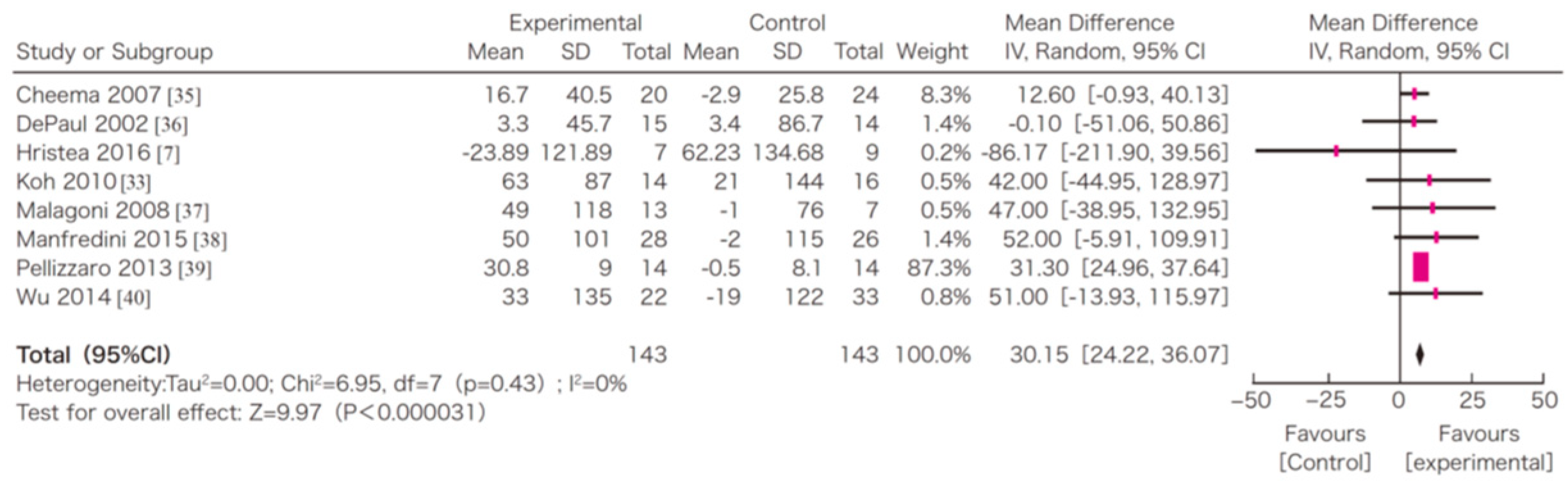
7. Efficacy of Exercise in Dialysis Patients
8. Implementation of Exercise in Dialysis Patients
8.1. Prior Evaluation
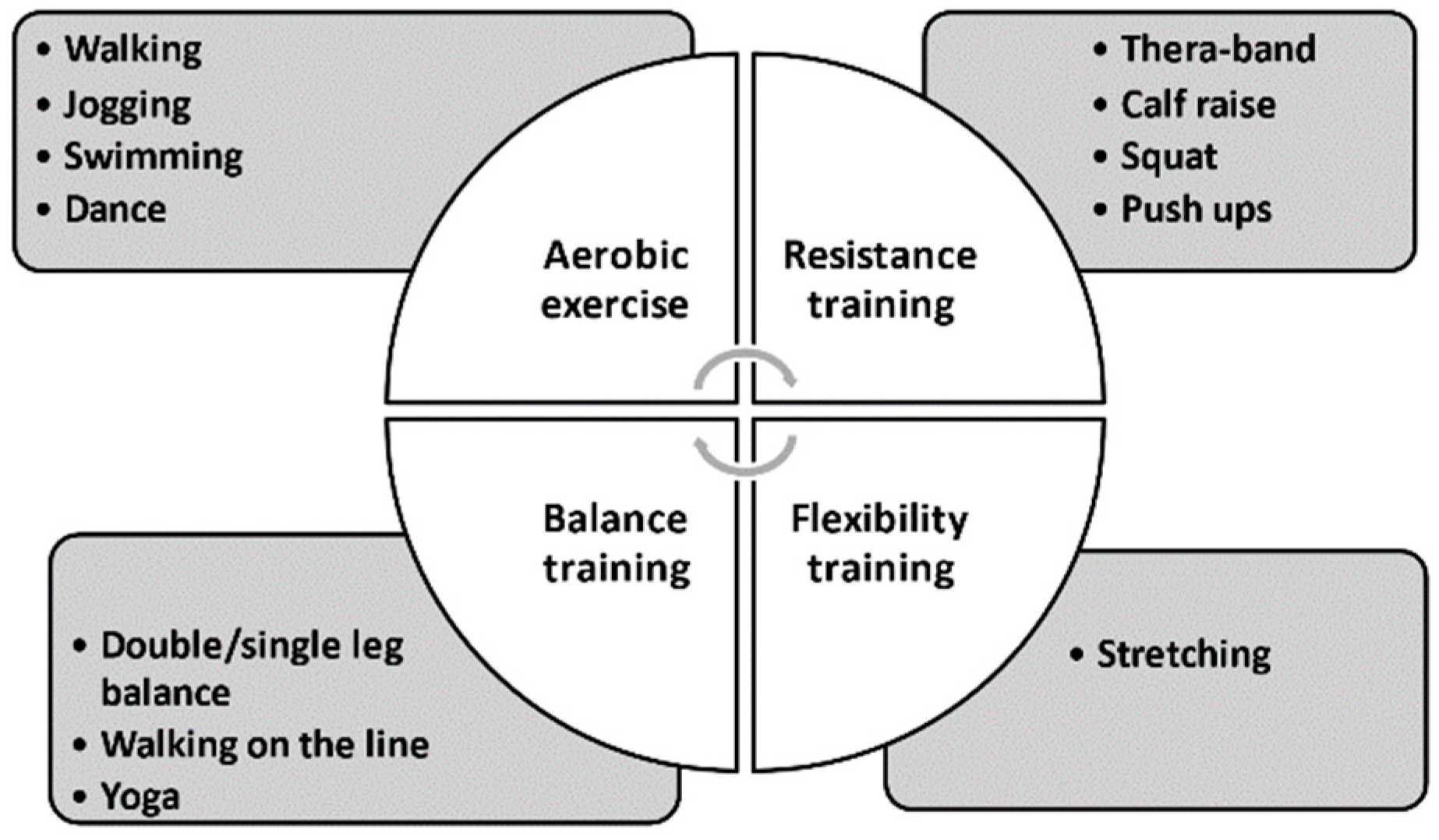
8.2. Four Components of an Exercise Intervention
8.3. Menu of Exercise Intervention
9. Barriers to CKD-Specific Exercise Behavior
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry_of_Health_Labor_and_Welfare. Trends of Life Expectancy and Healthy Life Expectancy in Japan. Available online: https://www.mhlw.go.jp/stf/wp/hakusyo/kousei/19/backdata/01-01-02-06.html (accessed on 16 February 2021). (In Japanese)
- Chapter 11: International Comparisons. Am. J. Kidney Dis. 2019, 73, S549–S594. [CrossRef]
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 41. [Google Scholar] [CrossRef]
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2019, JSDT Renal Data Registry. J. Jpn. Soc. Dial. Ther. 2020, 53, 579–632. [Google Scholar] [CrossRef]
- Hoshino, J.; Yamagata, K.; Nishi, S.; Nakai, S.; Masakane, I.; Iseki, K.; Tsubakihara, Y. Significance of the decreased risk of dialysis-related amyloidosis now proven by results from Japanese nationwide surveys in 1998 and 2010. Nephrol. Dial. Transpl. 2016, 31, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Brahee, D.D.; Guebert, G.M.; Virgin, B. Dialysis-related spondyloarthropathy. J. Manip. Physiol. Ther. 2001, 24, 127–130. [Google Scholar] [CrossRef]
- Charra, B.; Calemard, E.; Uzan, M.; Terrat, J.C.; Vanel, T.; Laurent, G. Carpal tunnel syndrome, shoulder pain and amyloid deposits in long-term haemodialysis patients. In Proceedings of the European Dialysis and Transplant Association-European Renal Association. European Dialysis and Transplant Association-European Renal Association; Congress: Washington, DC, USA, 1985; Volume 21, pp. 291–295. [Google Scholar]
- Hoshino, J.; Yamagata, K.; Nishi, S.; Nakai, S.; Masakane, I.; Iseki, K.; Tsubakihara, Y. Carpal tunnel surgery as proxy for dialysis-related amyloidosis: Results from the Japanese society for dialysis therapy. Am. J. Nephrol. 2014, 39, 449–458. [Google Scholar] [CrossRef]
- Kazama, J.J.; Yamamoto, S.; Takahashi, N.; Ito, Y.; Maruyama, H.; Narita, I.; Gejyo, F. Abeta-2M-amyloidosis and related bone diseases. J. Bone Miner. Metab. 2006, 24, 182–184. [Google Scholar] [CrossRef]
- Labriola, L.; Jadoul, M. Dialysis-related Amyloidosis: Is It Gone or Should It Be? Semin Dial. 2017, 30, 193–196. [Google Scholar] [CrossRef]
- K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42, S1–S201. [CrossRef]
- Jassal, S.V.; Karaboyas, A.; Comment, L.A.; Bieber, B.A.; Morgenstern, H.; Sen, A.; Gillespie, B.W.; De Sequera, P.; Marshall, M.R.; Fukuhara, S.; et al. Functional Dependence and Mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2016, 67, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Johansen, K.L.; Delgado, C.; Bao, Y.; Kurella Tamura, M. Frailty and dialysis initiation. Semin. Dial. 2013, 26, 690–696. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Bolignano, D.; Campo, S.; Perrone, C.; Donato, V.; Fazio, M.R.; Buemi, A.; Sturiale, A.; Buemi, M. Malnutrition in the elderly patient on dialysis. Ren. Fail. 2009, 31, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Moorthi, R.N.; Avin, K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rahman, E.M.; Turgut, F.; Turkmen, K.; Balogun, R.A. Falls in elderly hemodialysis patients. QJM Mon. J. Assoc. Physicians 2011, 104, 829–838. [Google Scholar] [CrossRef]
- Hoshino, J. Renal rehabilitation in CKD patients. Jpn. J. Nephrol. 2020, 62, 730–735. [Google Scholar]
- Eknoyan, G.; Lameire, N.; Eckardt, K.U. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic kidney disease. Kidney Int. Suppl. 2013, 3, 136–150. [Google Scholar]
- Smart, N.A.; Williams, A.D.; Levinger, I.; Selig, S.; Howden, E.; Coombes, J.S.; Fassett, R.G. Exercise & Sports Science Australia (ESSA) position statement on exercise and chronic kidney disease. J. Sci. Med. Sport 2013, 16, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J. Can. Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- Yamagata, K.; Hoshino, J.; Sugiyama, H.; Hanafusa, N.; Shibagaki, Y.; Komatsu, Y.; Konta, T.; Fujii, N.; Kanda, E.; Sofue, T.; et al. Clinical practice guideline for renal rehabilitation: Systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren. Replace. Ther. 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Afsar, B.; Siriopol, D.; Aslan, G.; Eren, O.C.; Dagel, T.; Kilic, U.; Kanbay, A.; Burlacu, A.; Covic, A.; Kanbay, M. The impact of exercise on physical function, cardiovascular outcomes and quality of life in chronic kidney disease patients: A systematic review. Int. Urol. Nephrol. 2018, 50, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, R.; Hoshi, K.; Yoneki, K.; Harada, M.; Watanabe, T.; Shimoda, T.; Yamamoto, S.; Matsunaga, A. Exercise Training in Elderly People Undergoing Hemodialysis: A Systematic Review and Meta-analysis. Kidney Int. Rep. 2017, 2, 1096–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanden Wyngaert, K.; Van Craenenbroeck, A.H.; Van Biesen, W.; Dhondt, A.; Tanghe, A.; Van Ginckel, A.; Celie, B.; Calders, P. The effects of aerobic exercise on eGFR, blood pressure and VO2peak in patients with chronic kidney disease stages 3–4: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0203662. [Google Scholar] [CrossRef]
- Bennett, P.N.; Thompson, S.; Wilund, K.R. An introduction to Exercise and Physical Activity in Dialysis Patients: Preventing the unacceptable journey to physical dysfunction. Semin. Dial. 2019, 32, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Evangelidis, N.; Craig, J.; Bauman, A.; Manera, K.; Saglimbene, V.; Tong, A. Lifestyle behaviour change for preventing the progression of chronic kidney disease: A systematic review. BMJ Open 2019, 9, e031625. [Google Scholar] [CrossRef]
- Pei, G.; Tang, Y.; Tan, L.; Tan, J.; Ge, L.; Qin, W. Aerobic exercise in adults with chronic kidney disease (CKD): A meta-analysis. Int. Urol. Nephrol. 2019, 51, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Wiebe, N.; Padwal, R.S.; Gyenes, G.; Headley, S.A.E.; Radhakrishnan, J.; Graham, M. The effect of exercise on blood pressure in chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2019, 14, e0211032. [Google Scholar] [CrossRef] [Green Version]
- Viana, J.L.; Martins, P.; Parker, K.; Madero, M.; Perez Grovas, H.; Anding, K.; Degenhardt, S.; Gabrys, I.; Raugust, S.; West, C.; et al. Sustained exercise programs for hemodialysis patients: The characteristics of successful approaches in Portugal, Canada, Mexico, and Germany. Semin. Dial. 2019, 32, 320–330. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Xiong, L.; Luo, Y.; Huang, Z.; Yi, B. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: Evidence from a meta-analysis. BMC Nephrol. 2019, 20, 398. [Google Scholar] [CrossRef]
- Ferrari, F.; Helal, L.; Dipp, T.; Soares, D.; Soldatelli, Â.; Mills, A.L.; Paz, C.; Tenório, M.C.C.; Motta, M.T.; Barcellos, F.C.; et al. Intradialytic training in patients with end-stage renal disease: A systematic review and meta-analysis of randomized clinical trials assessing the effects of five different training interventions. J. Nephrol. 2020, 33, 251–266. [Google Scholar] [CrossRef]
- Labib, M.; Bohm, C.; MacRae, J.M.; Bennett, P.N.; Wilund, K.R.; McAdams-DeMarco, M.; Jhamb, M.; Mustata, S.; Thompson, S. An International Delphi Survey on Exercise Priorities in CKD. Kidney Int. Rep. 2020. [Google Scholar] [CrossRef]
- Heiwe, S.; Tollback, A.; Clyne, N. Twelve weeks of exercise training increases muscle function and walking capacity in elderly predialysis patients and healthy subjects. Nephron 2001, 88, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Clyne, N.; Ekholm, J.; Jogestrand, T.; Lins, L.E.; Pehrsson, S.K. Effects of exercise training in predialytic uremic patients. Nephron 1991, 59, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Painter, P.L.; Nelson-Worel, J.N.; Hill, M.M.; Thornbery, D.R.; Shelp, W.R.; Harrington, A.R.; Weinstein, A.B. Effects of exercise training during hemodialysis. Nephron 1986, 43, 87–92. [Google Scholar] [CrossRef]
- Avesani, C.M.; Trolonge, S.; Deleaval, P.; Baria, F.; Mafra, D.; Faxen-Irving, G.; Chauveau, P.; Teta, D.; Kamimura, M.A.; Cuppari, L.; et al. Physical activity and energy expenditure in haemodialysis patients: An international survey. Nephrol. Dial. Transplant. 2012, 27, 2430–2434. [Google Scholar] [CrossRef] [Green Version]
- Heiwe, S.; Clyne, N.; Tollback, A.; Borg, K. Effects of regular resistance training on muscle histopathology and morphometry in elderly patients with chronic kidney disease. Am. J. Phys. Med. Rehabil. 2005, 84, 865–874. [Google Scholar] [CrossRef]
- Tamaki, M.; Miyashita, K.; Wakino, S.; Mitsuishi, M.; Hayashi, K.; Itoh, H. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase. Kidney Int. 2014, 85, 1330–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, M.; Hagiwara, A.; Miyashita, K.; Wakino, S.; Inoue, H.; Fujii, K.; Fujii, C.; Sato, M.; Mitsuishi, M.; Muraki, A.; et al. Improvement of Physical Decline Through Combined Effects of Muscle Enhancement and Mitochondrial Activation by a Gastric Hormone Ghrelin in Male 5/6Nx CKD Model Mice. Endocrinology 2015, 156, 3638–3648. [Google Scholar] [CrossRef] [Green Version]
- Tsubakihara, Y.; Nishi, S.; Akiba, T.; Hirakata, H.; Iseki, K.; Kubota, M.; Kuriyama, S.; Komatsu, Y.; Suzuki, M.; Nakai, S.; et al. 2008 Japanese Society for Dialysis Therapy: Guidelines for renal anemia in chronic kidney disease. Ther. Apher. Dial. 2010, 14, 240–275. [Google Scholar] [CrossRef]
- Ott, S.M. Therapy for patients with CKD and low bone mineral density. Nat. Rev. Nephrol. 2013, 9, 681–692. [Google Scholar] [CrossRef]
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Kurella Tamura, M.; Covinsky, K.E.; Chertow, G.M.; Yaffe, K.; Landefeld, C.S.; McCulloch, C.E. Functional status of elderly adults before and after initiation of dialysis. N. Engl. J. Med. 2009, 361, 1539–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterky, E.; Stegmayr, B.G. Elderly patients on haemodialysis have 50% less functional capacity than gender- and age-matched healthy subjects. Scand. J. Urol. Nephrol. 2005, 39, 423–430. [Google Scholar] [CrossRef]
- Johansen, K.L.; Chertow, G.M.; Jin, C.; Kutner, N.G. Significance of frailty among dialysis patients. J. Am. Soc. Nephrol. 2007, 18, 2960–2967. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satake, S.; Shimada, H.; Yamada, M.; Kim, H.; Yoshida, H.; Gondo, Y.; Matsubayashi, K.; Matsushita, E.; Kuzuya, M.; Kozaki, K.; et al. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the Cardiovascular Health Study criteria. Geriatr. Gerontol. Int. 2017, 17, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, F.; Tang, Z. Social Frailty Is Associated with Physical Functioning, Cognition, and Depression, and Predicts Mortality. J. Nutr. Health Aging 2018, 22, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Santamato, A.; Seripa, D.; Pilotto, A.; Logroscino, G. Cognitive Frailty: A Systematic Review of Epidemiological and Neurobiological Evidence of an Age-Related Clinical Condition. Rejuvenation Res. 2015, 18, 389–412. [Google Scholar] [CrossRef]
- Tentori, F.; Elder, S.J.; Thumma, J.; Pisoni, R.L.; Bommer, J.; Fissell, R.B.; Fukuhara, S.; Jadoul, M.; Keen, M.L.; Saran, R.; et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Correlates and associated outcomes. Nephrol. Dial. Transplant. 2010, 25, 3050–3062. [Google Scholar] [CrossRef]
- Hoshino, J.; Muenz, D.; Zee, J.; Sukul, N.; Speyer, E.; Guedes, M.; Lopes, A.A.; Asahi, K.; van Haalen, H.; James, G.; et al. Associations of Hemoglobin Levels With Health-Related Quality of Life, Physical Activity, and Clinical Outcomes in Persons With Stage 3-5 Nondialysis CKD. J. Ren. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.R.; Wang, S.M.; Liang, C.C.; Kuo, H.L.; Chang, C.T.; Liu, J.H.; Lin, H.H.; Wang, I.K.; Yang, Y.F.; Chou, C.Y.; et al. Association of Walking with Survival and RRT Among Patients with CKD Stages 3–5. Clin. J. Am. Soc. Nephrol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Matsuzawa, R.; Matsunaga, A.; Wang, G.; Kutsuna, T.; Ishii, A.; Abe, Y.; Takagi, Y.; Yoshida, A.; Takahira, N. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2012, 7, 2010–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beddhu, S.; Wei, G.; Marcus, R.L.; Chonchol, M.; Greene, T. Light-intensity physical activities and mortality in the United States general population and CKD subpopulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson-Cohen, C.; Littman, A.J.; Duncan, G.E.; Roshanravan, B.; Ikizler, T.A.; Himmelfarb, J.; Kestenbaum, B.R. Assessment of physical activity in chronic kidney disease. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2013, 23, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, K.; Kessel, A.; Burton, G. Interaction of Physiological Mechanisms During Exercise. J. Appl. Physiol. 1967, 22, 71–85. [Google Scholar] [CrossRef]
- Mezzani, A.; Hamm, L.F.; Jones, A.M.; McBride, P.E.; Moholdt, T.; Stone, J.A.; Urhausen, A.; Williams, M.A. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: A joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation, and the Canadian Association of Cardiac Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2012, 32, 327–350. [Google Scholar] [CrossRef]
- Balady Gary, J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher Gerald, F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to Cardiopulmonary Exercise Testing in Adults. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [Green Version]
- Gulati, M.; Black, H.R.; Arnsdorf, M.F.; Shaw, L.J.; Bakris, G.L. Kidney dysfunction, cardiorespiratory fitness, and the risk of death in women. J. Womens Health 2012, 21, 917–924. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.A.; Stryer, L. Biochemistry, 6th ed.; W. H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Margolis, L.M.; Allen, J.T.; Hatch-McChesney, A.; Pasiakos, S.M. Coingestion of Carbohydrate and Protein on Muscle Glycogen Synthesis after Exercise: A Meta-analysis. Med. Sci. Sports Exerc. 2021, 53, 384–393. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Shubert, T.; Doyle, J.; Soher, B.; Sakkas, G.K.; Kent-Braun, J.A. Muscle atrophy in patients receiving hemodialysis: Effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003, 63, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, P.; Carrero, J.J.; Bover, J.; Chauveau, P.; Mazzaferro, S.; Torres, P.U. Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2017, 8, 686–701. [Google Scholar] [CrossRef]
- Rhee, C.M.; Kalantar-Zadeh, K. Resistance exercise: An effective strategy to reverse muscle wasting in hemodialysis patients? J. Cachexia Sarcopenia Muscle 2014, 5, 177–180. [Google Scholar] [CrossRef]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Sato, Y.; Kobayashi, T.; Kaneko, Y.; Ito, E.; Soma, T.; Okada, H.; Miyamoto, K.; Oya, A.; Matsumoto, M.; et al. Vitamin D protects against immobilization-induced muscle atrophy via neural crest-derived cells in mice. Sci. Rep. 2020, 10, 12242. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.D.; Brownlie, T.t. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–688S, discussion 688S–690S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, E.; Mori, T.; Mishima, E.; Suzuki, A.; Sugawara, S.; Kurasawa, N.; Saigusa, D.; Miura, D.; Morikawa-Ichinose, T.; Saito, R.; et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci. Rep. 2016, 6, 36618. [Google Scholar] [CrossRef]
- Nishikawa, M.; Ishimori, N.; Takada, S.; Saito, A.; Kadoguchi, T.; Furihata, T.; Fukushima, A.; Matsushima, S.; Yokota, T.; Kinugawa, S.; et al. AST-120 ameliorates lowered exercise capacity and mitochondrial biogenesis in the skeletal muscle from mice with chronic kidney disease via reducing oxidative stress. Nephrol. Dial. Transplant. 2015, 30, 934–942. [Google Scholar] [CrossRef] [Green Version]
- Deger, S.M.; Hung, A.M.; Ellis, C.D.; Booker, C.; Bian, A.; Chen, G.; Abumrad, N.N.; Ikizler, T.A. High Dose Omega-3 Fatty Acid Administration and Skeletal Muscle Protein Turnover in Maintenance Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2016, 11, 1227–1235. [Google Scholar] [CrossRef] [Green Version]
- Shinaberger, C.S.; Greenland, S.; Kopple, J.D.; Van Wyck, D.; Mehrotra, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease. Am. J. Clin. Nutr. 2008, 88, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, A.M.; Tawney, K.; Bacchetti, P.; Johansen, K.L. Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. Am. J. Kidney Dis. 2003, 41, 447–454. [Google Scholar] [CrossRef]
- Yurtkuran, M.; Alp, A.; Yurtkuran, M.; Dilek, K. A modified yoga-based exercise program in hemodialysis patients: A randomized controlled study. Complementary Ther. Med. 2007, 15, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Painter, P. Exercise in individuals with CKD. Am. J. Kidney Dis. 2012, 59, 126–134. [Google Scholar] [CrossRef] [Green Version]
- The_Japanese_Society_of_Renal_Rehabilitation. Guideline for Renal Rehabilitation; Nankodo: Tokyo, Japan, 2018. [Google Scholar]
- Sheng, K.; Zhang, P.; Chen, L.; Cheng, J.; Wu, C.; Chen, J. Intradialytic exercise in hemodialysis patients: A systematic review and meta-analysis. Am. J. Nephrol. 2014, 40, 478–490. [Google Scholar] [CrossRef]
- Kornhauser, C.; Malacara, J.-M.; Macías-Cervantes, M.-H.; Rivera-Cisneros, A.-E. Effect of exercise intensity on albuminuria in adolescents with Type 1 diabetes mellitus. Diabet. Med. A J. Br. Diabet. Assoc. 2012, 29, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, Q.; Lou, T.; Qin, J.; Jung, S.; Shetty, V.; Li, F.; Wang, Y.; Feng, X.H.; Mitch, W.E.; et al. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat. Commun. 2017, 8, 1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, D.; Cao, P.; Kakihana, T.; Sato, E.; Suda, C.; Muroya, Y.; Ogawa, Y.; Hu, G.; Ishii, T.; Ito, O.; et al. Chronic Running Exercise Alleviates Early Progression of Nephropathy with Upregulation of Nitric Oxide Synthases and Suppression of Glycation in Zucker Diabetic Rats. PLoS ONE 2015, 10, e0138037. [Google Scholar] [CrossRef]
- Group, J.C.S.J.W. Guidelines for Rehabilitation in Patients With Cardiovascular Disease (JCS 2012)—Digest Version—. Circ. J. 2014, 78, 2022–2093. [Google Scholar] [CrossRef] [Green Version]
- Aging, N. Four Types of Exercise Can Improve Your Health and Physical Ability. Available online: https://www.nia.nih.gov/health/four-types-exercise-can-improve-your-health-and-physical-ability#:~:text=Research%20has%20shown%20that%20it%E2%80%99s%20important%20to%20get,variety%20helps%20reduce%20boredom%20and%20risk%20of%20injury (accessed on 19 April 2021).
- Hellberg, M.; Hoglund, P.; Svensson, P.; Clyne, N. Randomized Controlled Trial of Exercise in CKD-The RENEXC Study. Kidney Int. Rep. 2019, 4, 963–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Hellberg, M.; Hellmark, T.; Hoglund, P.; Clyne, N. Muscle mass and plasma myostatin after exercise training: A substudy of Renal Exercise (RENEXC)-a randomized controlled trial. Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef]
- Clarke, A.L.; Young, H.M.; Hull, K.L.; Hudson, N.; Burton, J.O.; Smith, A.C. Motivations and barriers to exercise in chronic kidney disease: A qualitative study. Nephrol. Dial. Transplant. 2015, 30, 1885–1892. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Kader, K.; Unruh, M.L.; Weisbord, S.D. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1057–1064. [Google Scholar] [CrossRef]
- Tong, A.; Manns, B.; Wang, A.Y.M.; Hemmelgarn, B.; Wheeler, D.C.; Gill, J.; Tugwell, P.; Pecoits-Filho, R.; Crowe, S.; Harris, T.; et al. Implementing core outcomes in kidney disease: Report of the Standardized Outcomes in Nephrology (SONG) implementation workshop. Kidney Int. 2018, 94, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Moorman, D.; Suri, R.; Hiremath, S.; Jegatheswaran, J.; Kumar, T.; Bugeja, A.; Zimmerman, D. Benefits and Barriers to and Desired Outcomes with Exercise in Patients with ESKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshino, J. Renal Rehabilitation: Exercise Intervention and Nutritional Support in Dialysis Patients. Nutrients 2021, 13, 1444. https://doi.org/10.3390/nu13051444
Hoshino J. Renal Rehabilitation: Exercise Intervention and Nutritional Support in Dialysis Patients. Nutrients. 2021; 13(5):1444. https://doi.org/10.3390/nu13051444
Chicago/Turabian StyleHoshino, Junichi. 2021. "Renal Rehabilitation: Exercise Intervention and Nutritional Support in Dialysis Patients" Nutrients 13, no. 5: 1444. https://doi.org/10.3390/nu13051444





