The Prevalence of Sarcopenia in Chinese Older Adults: Meta-Analysis and Meta-Regression
Abstract
:1. Introduction
2. Methods
2.1. Search Methods
2.2. Data Extraction
2.3. Critical Appraisal
2.4. Statistical Analysis
3. Results
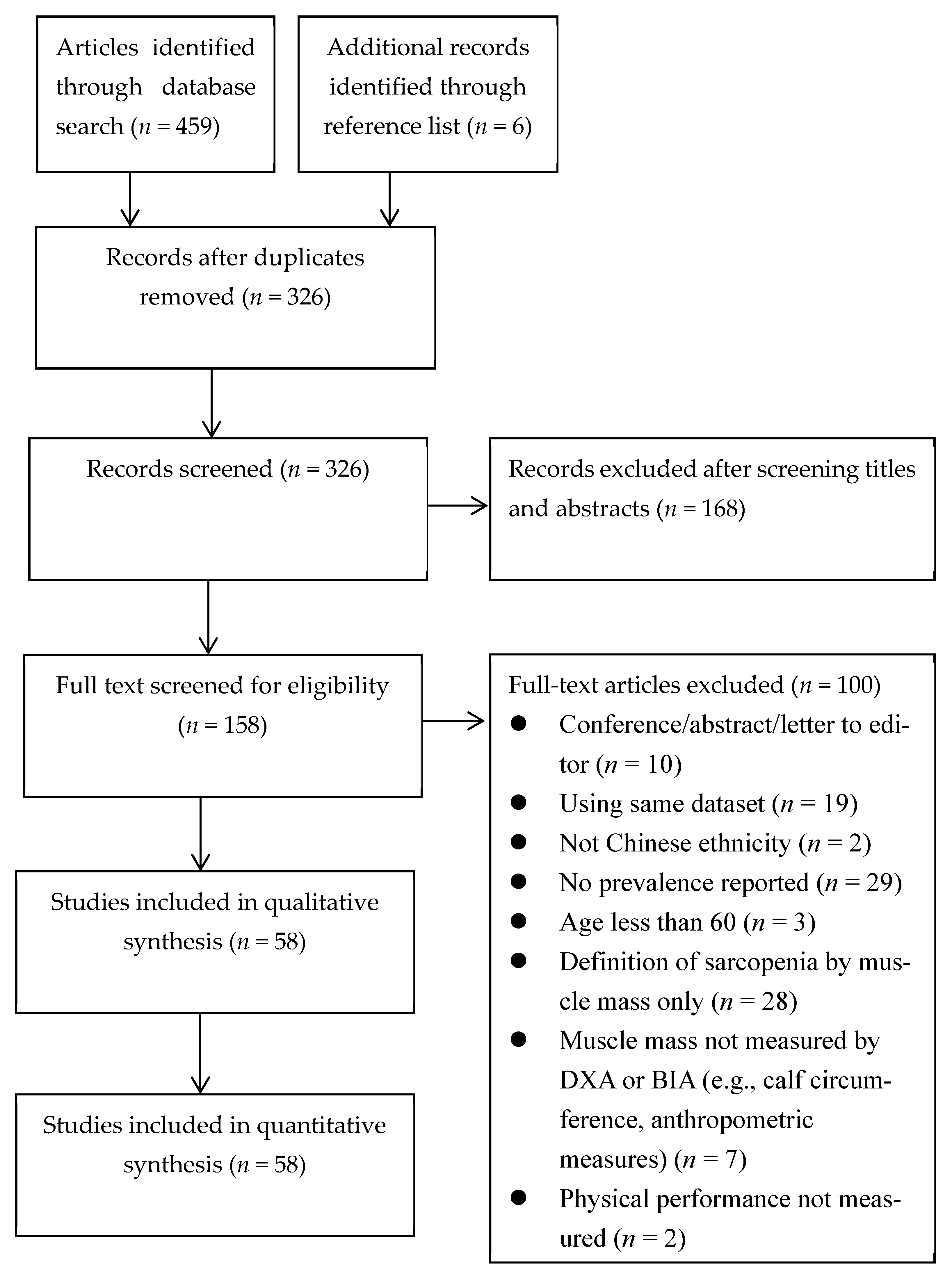
3.1. Search Outcomes
3.2. Prevalence in Older Men
3.3. Prevalence in Older Women
3.4. Meta-Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 13 May 2020).
- National Bureau of Statistics. 2019 China Statistical Yearbook. Available online: http://www.stats.gov.cn/tjsj/ndsj/2019/indexeh.htm (accessed on 17 August 2020).
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A. Sarcopenic obesity and endocrinal adaptation with age. Int. J. Endocrinol. 2013, 2013, 204164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Hida, A.; Mankowski, R.; Layne, A.; Solberg, L.M.; Mainous, A.G.; Buford, T. Nutrition and Exercise in Sarcopenia. Curr. Protein Pept. Sci. 2018, 19, 649–667. [Google Scholar] [CrossRef]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet Quality and Sarcopenia in Older Adults: A Systematic Review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Tessier, A.J.; Chevalier, S. An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline. Nutrients 2018, 10, 1099. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.-Y.; Bruyère, O. Health outcomes of sarcopenia: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef] [Green Version]
- Pamoukdjian, F.; Bouillet, T.; Lévy, V.; Soussan, M.; Zelek, L.; Paillaud, E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin. Nutr. 2018, 37, 1101–1113. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, L.; Xia, X.; Liu, Y.; Zuo, Z.; Zhang, Y.; Zhao, W.; Hao, Q.; Yue, J.; Dong, B. Prevalence of sarcopenia in multi ethnics adults and the association with cognitive impairment: Findings from West-China health and aging trend study. BMC Geriatr. 2020, 20, 63. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Reginster, J.Y.; Slomian, J.; Buckinx, F.; Dardenne, N.; Quabron, A.; Slangen, C.; Gillain, S.; Petermans, J.; Bruyère, O. Estimation of sarcopenia prevalence using various assessment tools. Exp. Gerontol. 2015, 61, 31–37. [Google Scholar] [CrossRef]
- Bruyère, O.; Beaudart, C.; Reginster, J.Y.; Buckinx, F.; Schoene, D.; Hirani, V.; Cooper, C.; Kanis, J.A.; Rizzoli, R.; McCloskey, E.; et al. Assessment of muscle mass, muscle strength and physical performance in clinical practice: An international survey. Eur. Geriatr. Med. 2016, 7, 243–246. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Tsintavis, P.; Potsaki, P.; Papandreou, D. Differences in the Prevalence of Sarcopenia in Community-Dwelling, Nursing Home and Hospitalized Individuals. A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2020, 24, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Leung, J.; Woo, J. A prospective cohort study to examine the association between dietary patterns and sarcopenia in Chinese community-dwelling older people in Hong Kong. J. Am. Med. Dir. Assoc. 2016, 17, 336–342. [Google Scholar] [CrossRef]
- Huang, C.Y.; Hwang, A.C.; Liu, L.K.; Lee, W.J.; Chen, L.Y.; Peng, L.N.; Lin, M.H.; Chen, L.K. Association of dynapenia, sarcopenia, and cognitive impairment among community-dwelling older Taiwanese. Rejuvenation Res. 2016, 19, 71–78. [Google Scholar] [CrossRef]
- Chen, X.; Guo, J.; Han, P.; Fu, L.; Jia, L.; Yu, H.; Yu, X.; Hou, L.; Wang, L.; Zhang, W.; et al. Twelve-month incidence of depressive symptoms in suburb-dwelling Chinese older adults: Role of sarcopenia. J. Am. Med. Dir. Assoc. 2019, 20, 64–69. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Xie, H.; Zheng, S.; Wu, X.; Zhu, X.; Zhang, X.; Xue, S.; Li, H.; Hong, W.; et al. Sex differences in the prevalence and adverse outcomes of sarcopenia and sarcopenic obesity in community dwelling elderly in East China using the AWGS criteria. BMC Endocr. Disord. 2019, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hai, S.; Liu, Y.; Cao, L.; Liu, Y.; Liu, P.; Zhou, J.; Yang, Y.; Dong, B. Association between depressive symptoms and sarcopenia in older Chinese community-dwelling individuals. Clin. Interv. Aging 2018, 13, 1605–1611. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.J.; Li, J.X. Prevalence of sarcopenia in the community-dwelling elder people in China: A systematic review and meta-analysis. Modern Prev. Med. 2019, 46, 18. (In Chinese) [Google Scholar]
- Tian, S.; Xu, Y.; Han, F. Prevalence of sarcopenia in the community-dwelling, elderly Chinese population: A systematic review and meta-analysis. Lancet 2017, 390, S35. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, J.; Chen, X.; Hou, L.; Lin, X.; Yang, M. Prevalence and Associated Factors of Sarcopenia in Nursing Home Residents: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Mogire, R.M.; Mutua, A.; Kimita, W.; Kamau, A.; Bejon, P.; Pettifor, J.M.; Adeyemo, A.; Williams, T.N.; Atkinson, S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e134–e142. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 15; StataCorp LP: College Station, TX, USA, 2017. [Google Scholar]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Easterbrook, P.J.; Berlin, J.A.; Gopalan, R.; Matthews, D.R. Publication bias in clinical research. Lancet 1991, 337, 867–872. [Google Scholar] [CrossRef]
- Meng, N.H.; Li, C.I.; Liu, C.S.; Lin, C.H.; Lin, W.Y.; Chang, C.K.; Li, T.C.; Lin, C.C. Comparison of height- and weight-adjusted sarcopenia in a Taiwanese metropolitan older population. Geriatr. Gerontol. Int. 2015, 15, 45–53. [Google Scholar] [CrossRef]
- Wen, X.; An, P.; Chen, W.C.; Lv, Y.; Fu, Q. Comparisions of sarcopenia prevalence based on different disgnostic criteria in Chinese older adults. J. Nutr. Health Aging 2015, 19, 342–347. [Google Scholar] [CrossRef]
- Xia, Z.W.; Meng, L.C.; Man, Q.Q.; Li, L.X.; Song, P.K.; Li, Y.Q.; Gao, Y.X.; Jia, S.S.; Zhang, J. Analysis of the dietary factors on sarcopenia in elderly in Beijing. J. Hyg. Res. 2016, 45, 388–393. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.T.; Huang, X.X.; Zhang, Z.H.; Bai, J.J.; Zhang, M.; Huang, Y.Q.; Chen, J.; Wang, J.F.; Bao, Z.J. Prevalence of Sarcopenia and the Associated Risk Factors in Community Elderly in Shanghai. Geriatr. Health Care 2018, 24, 608–613. (In Chinese) [Google Scholar]
- Liu, J.L.; Cai, Y.Y. Investigation of prevalence among community-dwelling older adults in Shanghai. China Health Vis. 2016, 248–249. (In Chinese) [Google Scholar] [CrossRef]
- Xu, H.Q.; Shi, J.P.; Shen, C.; Liu, Y.; Liu, J.M.; Zheng, X.Y. Sarcopenia-related features and factors associated with low muscle mass, weak muscle strength, and reduced function in Chinese rural residents: A cross-sectional study. Arch. Osteoporos. 2018, 14, 2. [Google Scholar] [CrossRef]
- Wang, Z.T. Correlation Analysis of Coronary Heart Disease and Sarcopenia in Elderly Inpatients. Master’s Thesis, Southern Medical University, Guangzhou, China, 2019. [Google Scholar]
- Fung, F.Y.; Koh, Y.L.E.; Malhotra, R.; Ostbye, T.; Lee, P.Y.; Shariff Ghazali, S.; Tan, N.C. Prevalence of and factors associated with sarcopenia among multi-ethnic ambulatory older Asians with type 2 diabetes mellitus in a primary care setting. BMC Geriatr. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Wu, Z.L.; Wang, Y.B.; Su, F.Q.; Song, B.; Li, G.; Zhu, H.M. Epidemiological investigation of sarcopenia in the nursing homes in Fengxian District and analysis of their risk factors. Shanghai Med. Pharm. J. 2017, 38, 37–40. (In Chinese) [Google Scholar]
- Yang, L.J. Research on Sarcopenia and Its Relative factors in Elderly Population in Suzhou. Master’s Thesis, Nanjing Medical University, Suzhou, China, 2018. [Google Scholar]
- Meng, P.; Hu, Y.X.; Fan, L.; Zhang, Y.; Zhang, M.X.; Sun, J.; Liu, Y.; Li, M.; Yang, Y.; Wang, L.H.; et al. Sarcopenia and sarcopenic obesity among men aged 80 years and older in Beijing: Prevalence and its association with functional performance. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 29–35. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.H.; Chen, K.T.; Hou, M.T.; Chang, Y.F.; Chang, C.S.; Liu, P.Y.; Wu, S.J.; Chiu, C.J.; Jou, I.M.; Chen, C.Y. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: The Tianliao Old People study 04. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 69–75. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hu, Y.X.; Fan, L.; Zhang, M.X.; Sun, J.; Han, X.Q.; Ma, X.N.; Dong, H.Y.; Li, M. Relationship between site-specific loss of bodr skeletal muscle mass and gait performance in very old men in Beijing. Chin. J. Health Care Med. 2014, 16, 421–425. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, Y.; Zhan, J.K.; Tang, Z.Y.; He, J.Y.; Tan, P.; Deng, H.Q.; Huang, W.; Liu, Y.S. Sarco-Osteoporosis: Prevalence and Association with Frailty in Chinese Community-Dwelling Older Adults. Int. J. Endocrinol. 2015, 2015, 482940. [Google Scholar] [CrossRef] [Green Version]
- Han, D.S.; Chang, K.V.; Li, C.M.; Lin, Y.H.; Kao, T.W.; Tsai, K.S.; Wang, T.G.; Yang, W.S. Skeletal muscle mass adjusted by height correlated better with muscular functions than that adjusted by body weight in defining sarcopenia. Sci. Rep. 2016, 6, 19457. [Google Scholar] [CrossRef]
- Han, P.; Kang, L.; Guo, Q.; Wang, J.; Zhang, W.; Shen, S.; Wang, X.; Dong, R.; Ma, Y.; Shi, Y.; et al. Prevalence and Factors Associated With Sarcopenia in Suburb-dwelling Older Chinese Using the Asian Working Group for Sarcopenia Definition. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 529–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Hai, S.; Cao, L.; Zhou, J.; Liu, P.; Dong, B.R. Estimation of prevalence of sarcopenia by using a new bioelectrical impedance analysis in Chinese community-dwelling elderly people. BMC Geriatr. 2016, 16, 216. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Feng, X.; Zhou, J.; Gong, H.; Xia, S.; Wei, Q.; Hu, X.; Tao, R.; Li, L.; Qian, F.; et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci. Rep. 2016, 6, 38937. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Pan, L.; Chen, L.; Chen, J.Y.; Peng, Y.D.; Gu, W.S.; You, L. Sarcopenia screening for older women with low body-weight and low handgrip strength is more urgently. Chin. J. Endocrinol. Metab. 2017, 33, 1043–1046. (In Chinese) [Google Scholar]
- Hai, S.; Cao, L.; Wang, H.; Zhou, J.; Liu, P.; Yang, Y.; Hao, Q.; Dong, B. Association between sarcopenia and nutritional status and physical activity among community-dwelling Chinese adults aged 60 years and older. Geriatr. Gerontol. Int. 2017, 17, 1959–1966. [Google Scholar] [CrossRef]
- Hua, C.; Chen, G.L.; Wen, X.L.; Liu, C.; Zhang, J. HMB intervention of muscle loss in community-dwelling elders with malnutrition. Electron. J. Metab. Nutr. Cancer 2017, 4, 72–77. (In Chinese) [Google Scholar] [CrossRef]
- Meng, L.; Shi, J.; Zou, C.S.; Tan, X.; Zhou, B.Y.; Duan, C.B.; Shi, H.; Xi, H. Correlation of frailty severity with muscle mass and physical function in Chinese older adults:preliminary findings. Chin. J. Geriatr. 2017, 36, 1313–1317. (In Chinese) [Google Scholar] [CrossRef]
- Chu, X.J. Osteopenia and Sarcopenia in Chinese Elderly. Master’s Thesis, Jiangsu University, Suzhou, China, 2018. [Google Scholar]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. Validation of the Chinese version of the Mini Sarcopenia Risk Assessment questionnaire in community-dwelling older adults. Medicine 2018, 97, e12426. [Google Scholar] [CrossRef]
- Liu, L.L. Prevalence and Risk Factors of Sarcopenia in Meddle-Aged and Elderly Population in Urban Area of Chongqing, China. Master’s Thesis, Chongqing Medical University, Chongqing, China, 2019. [Google Scholar]
- Wang, H.; Hai, S.; Liu, Y.X.; Cao, L.; Liu, Y.; Liu, P.; Yang, Y.; Dong, B.R. Associations between Sarcopenic Obesity and Cognitive Impairment in Elderly Chinese Community-Dwelling Individuals. J. Nutr. Health Aging 2019, 23, 14–20. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Q.; Feng, B.L.; Wang, C.Y.; Han, P.P.; Hu, J.; Sun, X.D.; Zeng, W.F.; Zheng, Z.X.; Li, H.S.; et al. A Cross-Sectional Study of the Association between Arterial Stiffness and Sarcopenia in Chinese Community-Dwelling Elderly Using the Asian Working Group for Sarcopenia Criteria. J. Nutr. Health Aging 2019, 23, 195–201. [Google Scholar] [CrossRef]
- Rong, Y.D.; Bian, A.L.; Hu, H.Y.; Ma, Y.; Zhou, X.Z. A cross-sectional study of the relationships between different components of sarcopenia and brachial ankle pulse wave velocity in community-dwelling elderly. BMC Geriatr. 2020, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, T.; Cai, Y.; Hu, Y.; Fan, L.; Wu, C. Sarcopenia in Community-Dwelling Oldest Old Is Associated with Disability and Poor Physical Function. J. Nutr. Health Aging 2020, 24, 339–345. [Google Scholar] [CrossRef]
- Yang, L.; Yao, X.; Shen, J.; Sun, G.; Sun, Q.; Tian, X.; Li, X.; Li, X.; Ye, L.; Zhang, Z.; et al. Comparison of revised EWGSOP criteria and four other diagnostic criteria of sarcopenia in Chinese community-dwelling elderly residents. Exp. Gerontol. 2020, 130, 110798. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.Z. Clinical Characteristics of Sarcopenia among Patients with Type2 Diabetes. Master’s Thesis, Jilin University, Changchun, China, 2018. [Google Scholar]
- Zhai, Y.; Xiao, Q.; Miao, J. The Relationship between NAFLD and Sarcopenia in Elderly Patients. Can. J. Gastroenterol. Hepatol. 2018, 2018, 5016091. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Hao, Q.; Ding, Y.; Dong, B. The Association between Sarcopenia and Prealbumin Levels among Elderly Chinese Inpatients. J. Nutr. Health Aging 2019, 23, 122–127. [Google Scholar] [CrossRef]
- Yao, S.H. Prevalence of Sarcopenia and Osteoporosis and Analysis of Their Risk Factors and Correlation in Elderly Hospitalized Patients. Master’s Thesis, Jishou University, Jishou, China, 2019. [Google Scholar]
- Yi, H.W.; Li, W.L.; Yu, Y.L.; Ma, D.B. The relative factors analysis of malnutrition status and sarcopenia in elderly patients. Electron. J. Metab. Nutr. Cancer 2019, 6. (In Chinese) [Google Scholar] [CrossRef]
- Tan, Z.Q. Study on the Influencing Factors of Sarcopenia in Elderly Patients with HFp EF, HFmr EF, and HFr EF. Master’s Thesis, Chengdu Medical College, Chengdu, China, 2019. [Google Scholar]
- Zhang, N.; Zhu, W.L.; Liu, X.H.; Chen, W.; Zhu, M.L.; Kang, L.; Tian, R. Prevalence and prognostic implications of sarcopenia in older patients with coronary heart disease. J. Geriatr. Cardiol. 2019, 16, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Gang, X.; Wang, G.; Xiao, X.; Li, Z.; Jiang, Z.; Wang, G. A cross-sectional study: Associations between sarcopenia and clinical characteristics of patients with type 2 diabetes. Medicine 2020, 99, e18708. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.L.; Liu, J.; Wang, J.T. Related factors for sarcopenia in elderly hospitalized patients with chronic diseases. Chin. General. Practic. 2020, 23, 611–616. (In Chinese) [Google Scholar] [CrossRef]
- Li, M.; Hu, Y.X.; Dong, H.Y.; Zhang, Y.; Fan, L.; Zhang, M.X.; Sun, J.; Han, X.Q.; Liu, Y.X.; Ma, X.N. Compare different measurement for prevalence of sarcopenia in a cohort of healthy community-dwelling older men in Beijing area. Chin. J. Health Care Med. 2014, 16, 426–429. (In Chinese) [Google Scholar] [CrossRef]
- Wang, R.; Hu, Y.X.; Fan, L.; Cui, H.; Gao, L.G.; Zhang, Y.; Gao, D.W.; Cao, J.; Gong, W.Q. Effect of sarcopenia on the rate of rehospitalization in elderly male patients. Chin. J. Health Care Med. 2016, 18, 106–109. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.N.; Song, H.L.; Gu, Y.H.; Xu, J.L. Influencing factors of sarcopenia among older adults with type2 diabetes. Prev. Med. 2019, 31, 582–585. (In Chinese) [Google Scholar] [CrossRef]
- Hsu, Y.H.; Liang, C.K.; Chou, M.Y.; Liao, M.C.; Lin, Y.T.; Chen, L.K.; Lo, Y.K. Association of cognitive impairment, depressive symptoms and sarcopenia among healthy older men in the veterans retirement community in southern Taiwan: A cross-sectional study. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 102–108. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.W. Prevalence and Risk Factors of Sarcopenia among Older Adults Living in Nursing Homes in Chongqing. Master’s Thesis, Chongqing Medical University, Chongqing, China, 2018. [Google Scholar]
- Zeng, Y.; Hu, X.; Xie, L.; Han, Z.; Zuo, Y.; Yang, M. The Prevalence of Sarcopenia in Chinese Elderly Nursing Home Residents: A Comparison of 4 Diagnostic Criteria. J. Am. Med. Dir. Assoc. 2018, 19, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Wu, G.H.; Yang, Y.L.; Wu, Y.H.; Zhang, L.; Wang, M.H.; Mo, L.Y.; Xue, G.; Wang, C.Z.; Weng, X.F. Nutrition, Physical Exercise, and the Prevalence of Sarcopenia in Elderly Residents in Nursing Homes in China. Med. Sci. Monit. 2019, 25, 4390–4399. [Google Scholar] [CrossRef]
- Chen, J.M. The Associated Factors of Traditional Chinese Medicine Syndromes in Older Adults with Sarcopenia. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2018. [Google Scholar]
- Feng, X. The Occurrence of Sarcopenia and Related Factors Analysis in Elderly Patients with Type 2 Diabetes Mellitus. Master’s Thesis, Jiangsu Univeristy, Suzhou, China, 2016. [Google Scholar]
- Ma, Y. The Occurence and Associated Factors of Sarcopenic Obesity in Older Adults. Master’s Thesis, Tianjin University of Traditional Chinese Medicine, Tianjin, China, 2017. [Google Scholar]
- Zhou, X.L.; Wang, S.; Xu, T.Y. Correlation between chronic atrophic gastritis and sarcopenia in elderly population. Med. J. Natl. Defending Forces Southwest China 2018, 28, 329–331. [Google Scholar] [CrossRef]
- Zhang, T.; Gu, Y.H. Effect of abdominal obesity on sarcopenia and osteoporosis in elderly people with normal body mass index. Chin. J. Clin. Med. 2019, 26, 754–758. [Google Scholar] [CrossRef]
- Yang, P.P. Investigation on the Current Status of Sarcopenia in Middle-Aged and Elderly Patients with T2DM. Master’s Thesis, Nanchang University, Nanchang, China, 2019. [Google Scholar]
- Beaudart, C.; Reginster, J.Y.; Slomian, J.; Buckinx, F.; Locquet, M.; Bruyère, O. Prevalence of sarcopenia: The impact of different diagnostic cut-off limits. J. Musculoskelet. Neuronal. Interact. 2014, 14, 425–431. [Google Scholar] [CrossRef]
- Von Hippel, P.T. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chen, S.; Jiang, F.; Zhou, C.; Tang, S. Malnutrition and Physical Frailty among Nursing Home Residents: A Cross-Sectional Study in China. J. Nutr. Health Aging 2020, 24, 500–506. [Google Scholar] [CrossRef]
- Pigłowska, M.; Guligowska, A.; Kostka, T. Nutritional Status Plays More Important Role in Determining Functional State in Older People Living in the Community than in Nursing Home Residents. Nutrients 2020, 12, 2042. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, O.; Bahat, G. Suggestions for assessment of muscle mass in primary care setting. Aging Male 2017, 20, 168–169. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Tyrovolas, S.; Koyanagi, A.; Olaya, B.; Ayuso-Mateos, J.L.; Miret, M.; Chatterji, S.; Tobiasz-Adamczyk, B.; Koskinen, S.; Leonardi, M.; Haro, J.M. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: A multi-continent study. J. Cachexia Sarcopenia Muscle 2016, 7, 312–321. [Google Scholar] [CrossRef]
| Study | Language | Region | Design | Sample Size | Diagnostic Criteria | Assessment | Prevalence n (%) | Risk of Bias | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle Mass | Muscle Strength | Physical Performance | |||||||||||||
| Total | Male | Female | Distance (m) | Gait Speed | Total | Male | Female | ||||||||
| Community (n = 32) | |||||||||||||||
| Meng et al., 2014 [45] | English | Mainland | Cross-sectional | 101 | 101 | — | EWGSOP | DXA | Dynamometer | 6 | Usual | 46 (45.7) | 46 (45.7) | — | Moderate |
| Wu et al., 2014 [46] | English | Taiwan | Cross-sectional | 549 | 285 | 264 | EWGSOP | BIA | Dynamometer | 5 | — | 39 (7.1) | 11 (3.9) | 28 (10.6) | Moderate |
| Zhang et al., 2014 [47] | Chinese | Mainland | Cross-sectional | 116 | — | — | EWGSOP | DXA | Dynamometer | 6 | Usual | 48 (41.4) | — | — | Moderate |
| Meng et al., 2015 [35] | English | Taiwan | Cross-sectional | 771 | 412 | 359 | EWGSOP | DXA | Dynamometer | 5 | Usual | 44 (5.7) | 35 (8.4) | 9 (2.6) | Moderate |
| Wang et al., 2015 [48] | English | Mainland | Cross-sectional | 316 | 164 | 152 | AWGS | BIA | Dynamometer | 4 | Usual | 94 (29.4) | 43 (26.2) | 51 (33.6) | Moderate |
| Wen et al., 2015 [36] | English | Mainland | Cross-sectional | 286 | 136 | 150 | IWGS | DXA | Dynamometer | 6 | Usual | 17 (5.9) | 10 (7.4) | 7 (4.7) | Moderate |
| EWGSOP | 1 (0.3) | 1 (0.8) | — | ||||||||||||
| AWGS | 9 (3.1) | 8 (5.9) | 1 (0.7) | ||||||||||||
| Chan et al., 2016 [19] | English | HK | Cross-sectional | 3957 | 1979 | 1878 | AWGS | DXA | Dynamometer | 6 | Usual | 290 (7.3) | 185 (9.3) | 105 (5.6) | Low |
| Han et al., 2016 [49] | English | Taiwan | Cross-sectional | 878 | 402 | 476 | EWGSOP | BIA | Dynamometer | 7 | Usual | 29 (3.3) | 27 (6.7) | 2 (0.4) | Moderate |
| Han et al., 2016 [50] | English | Mainland | Cross-sectional | 1069 | 467 | 602 | AWGS | BIA | Dynamometer | 4 | Usual | 99 (9.3) | 30 (6.4) | 69 (11.5) | Moderate |
| Huang et al., 2016 [20] | English | Taiwan | Cross-sectional | 731 | 386 | 345 | AWGS | DXA | Dynamometer | 6 | — | 50 (6.8) | 36 (9.3) | 14 (4.1) | Low |
| Wang et al., 2016 [51] | English | Mainland | Cross-sectional | 944 | 462 | 482 | AWGS | BIA | Dynamometer | 6 | Usual | 98 (10.4) | 38 (8.2) | 60 (12.5) | Moderate |
| Wang et al., 2016 [52] | English | Mainland | Cross-sectional | 854 | 404 | 450 | AWGS | BIA | Dynamometer | 4 | Usual | 96 (11.2) | 53 (13.1) | 43 (9.6) | Low |
| Xia et al., 2016 [37] | Chinese | Mainland | Cross-sectional | 683 | 239 | 444 | AWGS | BIA | Dynamometer | 4 | — | 137 (20.1) | 41 (17.2) | 96 (21.6) | Moderate |
| Fang et al., 2017 [53] | Chinese | Mainland | Cross-sectional | 106 | — | 106 | AWGS | DXA | Dynamometer | 6 | Usual | 13 (12.2) | — | 13 (12.2) | Moderate |
| Hai et al., 2017 [54] | English | Mainland | Cross-sectional | 836 | 415 | 421 | AWGS | BIA | Dynamometer | 6 | Usual | 88 (10.5) | 47 (11.3) | 41 (9.7) | Moderate |
| Hua et al., 2017 [55] | Chinese | Mainland | Cross-sectional | 300 | 168 | 132 | AWGS | BIA | Dynamometer | 6 | Usual | 54 (18.0) | 38 (22.6) | 16 (12.1) | Moderate |
| Meng et al., 2017 [56] | Chinese | Mainland | Cross-sectional | 106 | 101 | 5 | AWGS | BIA | Dynamometer | — | — | 29 (27.4) | — | — | Moderate |
| Chu 2018 [57] | Chinese | Mainland | Cross-sectional | 191 | 69 | 122 | AWGS | BIA | Dynamometer | 4 | Maximal | 28 (14.7) | 8 (11.6) | 20 (16.4) | Moderate |
| Wang et al., 2018 [23] | English | Mainland | Cross-sectional | 865 | 427 | 438 | AWGS | BIA | Dynamometer | 6 | Usual | 71 (7.1) | 28 (6.6) | 33 (7.5) | Moderate |
| Yang et al., 2018 [58] | English | Mainland | Cross-sectional | 384 | 160 | 224 | EWGSOP | BIA | Dynamometer | 4 | Usual | 45 (11.72) | 17 (10.6) | 28 (12.5) | Moderate |
| Zhang et al., 2018 [38] | Chinese | Mainland | Cross-sectional | 1148 | 368 | 780 | AWGS | BIA | Dynamometer | 6 | Usual | 164 (14.3) | 55 (14.9) | 109 (14.0) | Low |
| Chen et al., 2019 [21] | English | Mainland | Prospective | 691 | 304 | 387 | AWGS | BIA | Dynamometer | 4 | — | 55 (8.0) | — | — | Moderate |
| Du et al., 2019 [22] | English | Mainland | Cross-sectional | 631 | 213 | 418 | AWGS | BIA | Dynamometer | 6 | Usual | 77 (12.2) | 41 (19.2) | 36 (8.6) | Moderate |
| Liu et al., 2019 [39] | Chinese | Mainland | Cross-sectional | 1723 | 915 | 808 | AWGS | BIA | Dynamometer | 6 | Usual | 121 (7.0) | 96 (10.5) | 25 (3.1) | Moderate |
| Liu 2019 [59] | Chinese | Mainland | Cross-sectional | 769 | 416 | 353 | AWGS | BIA | Dynamometer | 6 | Usual | 32 (4.16) | 12 (2.9) | 20 (5.7) | Moderate |
| Wang et al., 2019 [60] | English | Mainland | Cross-sectional | 945 | 465 | 480 | AWGS | BIA | Dynamometer | 6 | Usual | 276 (29.2) | 123 (26.5) | 153 (55.4) | Moderate |
| Xu et al., 2019 [40] | English | Mainland | Cross-sectional | 2412 | 1012 | 1400 | AWGS | BIA | Dynamometer | 6 | Usual | 156 (6.5) | 58 (5.7) | 98 (7.0) | Moderate |
| Zhang et al., 2019 [61] | English | Mainland | Cross-sectional | 1002 | 420 | 582 | AWGS | BIA | Dynamometer | 4 | — | 107 (10.7) | 37 (8.8) | 70 (12.0) | Moderate |
| Liu et al., 2020 [15] | English | Mainland | Cross-sectional | 1712 | — | — | AWGS | BIA | Dynamometer | 4 | Usual | 556 (32.5) | — | — | Moderate |
| Rong et al., 2020 [62] | English | Mainland | Cross-sectional | 450 | 266 | 184 | AWGS | BIA | Dynamometer | 6 | Usual | 89 (19.7) | 50 (18.8) | 39 (21.2) | Moderate |
| Xu et al., 2020 [63] | English | Mainland | Cross-sectional | 582 | 246 | 336 | AWGS | BIA | Dynamometer | 6 | Usual | 15 (526.6) | 82 (33.3) | 73 (21.7) | Moderate |
| Yang et al., 2020 [64] | English | Mainland | Cross-sectional | 483 | 184 | 299 | FNIH | BIA | Dynamometer | 4 | Usual | 16 (3.3) | 11 (6.0) | 5 (1.7) | Moderate |
| IWGS | 78 (16.1) | 45 (24.5) | 33 (11.0) | ||||||||||||
| AWGS | 44 (9.1) | 20 (10.9) | 24 (8.0) | ||||||||||||
| EWGSOP1 | 76 (15.7) | 41 (22.3) | 35 (11.7) | ||||||||||||
| EWGSOP2 | 22 (4.6) | 12 (6.5) | 10 (3.3) | ||||||||||||
| Hospitals (n = 11) | |||||||||||||||
| Wang et al., 2016 [52] † | English | Mainland | Cross-sectional | 236 | 116 | 120 | AWGS | BIA | Dynamometer | 4 | Usual | 35 (14.8) | 20 (17.2) | 15 (12.5) | Low |
| Cui 2018 [65] | Chinese | Mainland | Cross-sectional | 132 | 59 | 73 | AWGS | DXA | Dynamometer | 6 | Usual | 38 (28.8) | 21 (35.6) | 17 (23.3) | Moderate |
| Zhai et al., 2018 [66] | English | Mainland | Cross-sectional | 494 | 216 | 278 | AWGS | DXA | Dynamometer | 6 | — | 158 (32.0) | 87 (40.3) | 71 (25.5) | Moderate |
| Chen et al., 2019 [67] | English | Mainland | Cross-sectional | 118 | 92 | 26 | AWGS | DXA | Dynamometer | 6 | Usual | 71 (60.17) | 65 (70.65) | 6 (23.08) | Moderate |
| Wang 2019 [41] | Chinese | Mainland | Cross-sectional | 119 | 64 | 55 | AWGS | BIA | Dynamometer | — | — | 26 (21.8) | 17 (26.6) | 9 (16.3) | Moderate |
| Yao 2019 [68] | Chinese | Mainland | Cross-sectional | 378 | 153 | 225 | AWGS | BIA | Dynamometer | 6 | Usual | 47 (12.4) | 15 (9.8) | 32 (14.2) | Moderate |
| Yi et al., 2019 [69] | Chinese | Mainland | Cross-sectional | 200 | — | — | AWGS | BIA | Dynamometer | 6 | — | 98 (49) | — | — | Moderate |
| Tan 2019 [70] | Chinese | Mainland | Cross-sectional | 734 | — | — | AWGS | BIA | Dynamometer | 4 | — | 258 (35.1) | — | — | Moderate |
| Zhang et al., 2019 [71] | English | Mainland | Prospective | 345 | 208 | 137 | AWGS | BIA | Dynamometer | 6 | — | 78 (22.6) | 32 (15.4) | 46 (33.6) | Moderate |
| Cui et al., 2020 [72] | English | Mainland | Cross-sectional | 132 | 59 | 73 | AWGS | DXA | Dynamometer | 6 | Usual | 38 (28.8) | 21 (55.3) | 17 (44.7) | Moderate |
| Wang et al., 2020 [73] | Chinese | Mainland | Cross-sectional | 236 | 144 | 92 | AWGS | BIA | Dynamometer | 6 | — | 63 (26.7) | 28 (19.4) | 35 (38.0) | Moderate |
| Outpatient Services (n = 4) | |||||||||||||||
| Li et al., 2014 [74] | Chinese | Mainland | Cross-sectional | 169 | 169 | — | IWGS | DXA | Dynamometer | 6 | Usual | 106 (62.9) | 106 (62.9) | — | Moderate |
| EWGSOP | Usual | 56 (33.3) | 56 (33.3) | — | |||||||||||
| Wang et al., 2016 [75] | Chinese | Mainland | Cross-sectional | 410 | — | — | EWGSOP | DXA | Dynamometer | 6 | Usual | 80 (19.5) | — | — | Moderate |
| Fung et al., 2019 [42] | English | Singapore | Cross-sectional | 266 | — | — | AWGS | BIA | Dynamometer | 6 | Usual | 70 (26.3) | — | — | low |
| Wang et al., 2019 [76] | Chinese | Mainland | Cross-sectional | 430 | 191 | 239 | EWGSOP | BIA | Dynamometer | 6 | Usual | 95 (22.1) | 32 (16.8) | 63 (26.4) | Moderate |
| Nursing Home (n = 5) | |||||||||||||||
| Hsu et al., 2014 [77] | English | Taiwan | Cross-sectional | 353 | 353 | — | EWGSOP | BIA | Dynamometer | 6 | Usual | 109 (30.9) | 109 (30.9) | — | Moderate |
| Wu et al., 2017 [43] | Chinese | Mainland | Cross-sectional | 786 | 320 | 466 | EWGSOP | BIA | Dynamometer | 4 | — | 199 (25.3) | 64 (20.0) | 135 (29.0) | Moderate |
| Liao 2018 [78] | Chinese | Mainland | Cross-sectional | 225 | 63 | 162 | AWGS | BIA | Dynamometer | 6 | Usual | 86 (38.2) | 26 (41.3) | 60 (37.0) | Moderate |
| Zeng et al., 2018 [79] | English | Mainland | Cross-sectional | 277 | 83 | 194 | FNIH | BIA | Dynamometer | 4 | Usual | 87 (31.4) | 19 (22.9) | 68 (35.1) | Moderate |
| Yang et al., 2019 [80] | English | Mainland | Cross-sectional | 316 | 112 | 204 | AWGS | BIA | Dynamometer | 4 | — | 91 (28.8) | 34 (30.4) | 57 (27.9) | Moderate |
| Mixed Settings: Communities and Nursing Homes (n = 2) | |||||||||||||||
| Chen 2018 [81] | Chinese | Mainland | Cross-sectional | 158 | 43 | 115 | AWGS | BIA | Dynamometer | 6 | Usual | 34 (21.5) | 5 (11.4) | 29 (25.4) | Moderate |
| Yang 2018 [44] | Chinese | Mainland | Cross-sectional | 316 | 112 | 204 | AWGS | BIA | Dynamometer | 4 | Usual | 91 (28.8) | 34 (30.4) | 57 (27.9) | Low |
| Mixed Settings: Hospital and Outpatient Services (n = 5) | |||||||||||||||
| Feng 2016 [82] | Chinese | Mainland | Cross-sectional | 330 | 157 | 173 | AWGS | BIA | Dynamometer | 4 | Maximal | 35 (10.6) | 21 (13.4) | 14 (8.1) | Moderate |
| Ma 2017 [83] | Chinese | Mainland | Cross-sectional | 764 | 550 | 214 | AWGS | BIA | Dynamometer | 4 | Usual | 138 (18.1) | 82 (14.9) | 56 (26.2) | Moderate |
| Zhou et al., 2018 [84] | Chinese | Mainland | Cross-sectional | 163 | 100 | 63 | IWGS | DXA | Dynamometer | 3 | Maximal | 26 (16.0) | — | — | Moderate |
| Zhang et al., 2019 [85] | Chinese | Mainland | Cross-sectional | 223 | — | — | AWGS | BIA | Dynamometer | 6 | Usual | 49 (22.0) | — | — | Moderate |
| Yang 2019 [86] | Chinese | Mainland | Cross-sectional | 102 | 51 | 51 | AWGS | BIA | Dynamometer | 4 | Maximal | 17 (16.0) | — | — | Moderate |
| Covariates | Males (n = 43) | Females (n = 41) | ||||
|---|---|---|---|---|---|---|
| Exp (β) | 95% CI | p-Value | Exp (β) | 95% CI | p-Value | |
| Populations | ||||||
| Community-dwelling (ref) | 1.00 | 1.00 | ||||
| Outpatients | 1.29 | (0.52, 3.17) | 0.570 | 2.28 | (0.67, 7.73) | 0.180 |
| Hospitalized people | 1.69 | (1.01, 2.86) | 0.047 | 2.10 | (1.17, 3.78) | 0.015 |
| Nursing-home residents | 2.50 | (1.35, 4.66) | 0.005 | 2.73 | (1.38, 5.38) | 0.005 |
| Diagnosis criteria | ||||||
| AWGS (ref) | 1.00 | 1.00 | ||||
| EWGSOP | 1.23 | (0.67, 2.27) | 0.490 | 0.92 | (0.39, 2.15) | 0.840 |
| Assessment of muscle mass | ||||||
| DXA (ref) | 1.00 | 1.00 | ||||
| BIA | 0.58 | (0.35, 0.98) | 0.044 | 1.17 | (0.60, 2.29) | 0.640 |
| Area | ||||||
| Mainland (ref) | 1.00 | 1.00 | ||||
| Out of mainland | 0.47 | (0.22, 0.98) | 0.045 | 0.51 | (0.18, 1.43) | 0.190 |
| Walk distance | ||||||
| 6 m (ref) | 1.00 | 1.00 | ||||
| 4 m | 0.84 | (0.53, 1.32) | 0.440 | 1.12 | (0.68, 1.83) | 0.650 |
| Others | 0.81 | (0.34, 1.93) | 0.630 | 0.73 | (0.24, 2.25) | 0.580 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Li, W.-Y.; Ho, M.; Chau, P.-H. The Prevalence of Sarcopenia in Chinese Older Adults: Meta-Analysis and Meta-Regression. Nutrients 2021, 13, 1441. https://doi.org/10.3390/nu13051441
Chen Z, Li W-Y, Ho M, Chau P-H. The Prevalence of Sarcopenia in Chinese Older Adults: Meta-Analysis and Meta-Regression. Nutrients. 2021; 13(5):1441. https://doi.org/10.3390/nu13051441
Chicago/Turabian StyleChen, Zi, Wei-Ying Li, Mandy Ho, and Pui-Hing Chau. 2021. "The Prevalence of Sarcopenia in Chinese Older Adults: Meta-Analysis and Meta-Regression" Nutrients 13, no. 5: 1441. https://doi.org/10.3390/nu13051441






