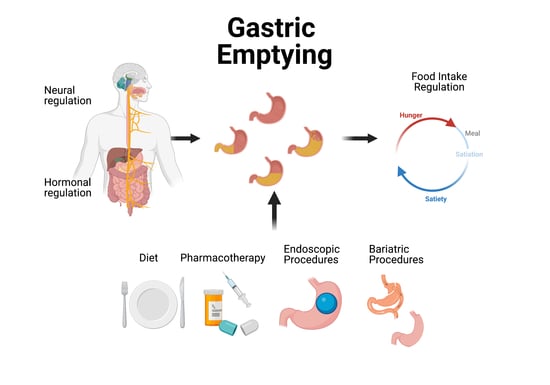
Gastric Sensory and Motor Functions and Energy Intake in Health and Obesity—Therapeutic Implications
Abstract
1. Introduction
2. Regulation of Gastric Emptying and Gastric Accommodation
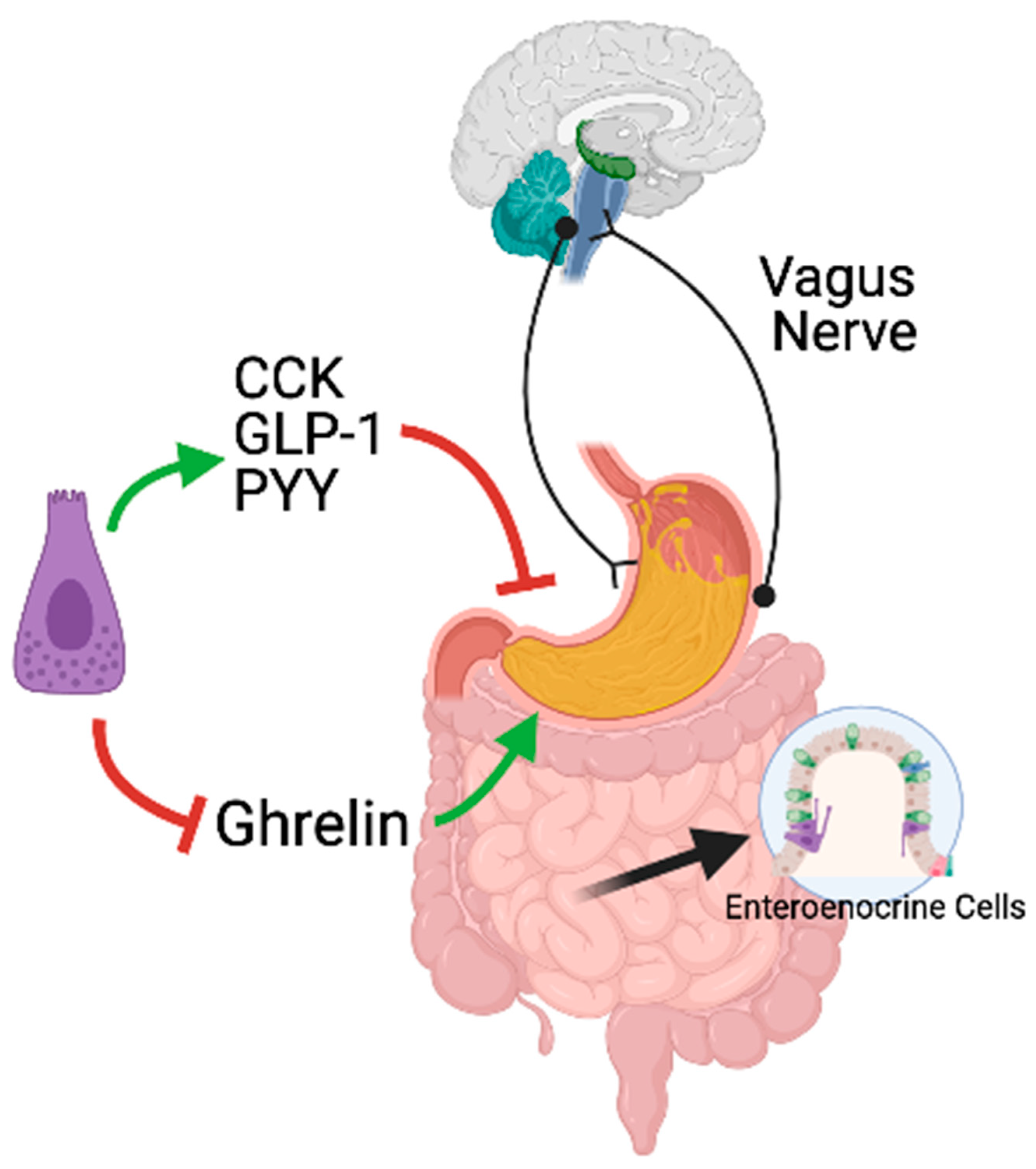
2.1. Neural Regulation
2.2. Hormonal Regulation
2.3. Measurement of Gastric Motor Functions
3. Gastric Sensory and Motor Functions and Food Intake
Gastric Sensory and Motor Functions in Obesity
4. Treatments of Obesity and Their Effects on Gastric Sensory and Motor Functions
4.1. Lifestyle Interventions
4.2. Pharmacological Treatment
4.3. Endoscopic Bariatric Procedures
4.4. Bariatric Surgery
| Study Design/ Intervention | Methods | Effect on GI Motor Function | Weight Loss BMI, kg/m2 | Energy Intake Kcal/Day | Appetite Sensations, VAS | Ref. |
|---|---|---|---|---|---|---|
| RCT, SB in obesity (BMI 37.4 ± 4.0 kg/m2) (n = 42) Diet: ETEE-600 kcal (minimum1200 kcal), exercise and behavioral modification. | Scinti-graphic GE and VAS: Pre and 1-month post-Rx | GE T1/2 liquids min: baseline 21.8 ± 10.1 vs.1-month 24.4 ± 8.7; ns GE solids %/h: baseline 30.3 ± 15.2 vs. 1-month 26.2 ± 15.2; ns | Baseline 37.4 ± 3.9 1-month 36.7 ± 3.9, Δ−0.8 (95% CI [−1.0, −0.5]; p < 0.001). | Δ−599.9 (95% CI [−885.6, −315.2]; p < 0.001). | Decrease in: Desire to eat AUC, Hunger AUC, and Fullness AUC | [179] |
| RCT, DB in obesity (BMI 37.4 ± 4.0 kg/m2) (n = 14) Placebo vs. diet Diet: ETEE-600 kcal (minimum 1200 kcal). | Scinti-graphic GE and VAS: Pre, 1- and 12 months post-Rx | GE T1/2 liquids min: baseline 25.5 ± 10.7; 1-month 19.3 ± 9.0; 12-months 21.8 ± 10.3; ns GE rate %/h solids: baseline 26.6 ± 16.3 1 month: 35.8 ± 13.8; ns 12-months 17.6 ± 13.0; p < 0.05 vs baseline | Baseline: 37.6 ± 3.9; 1-month: 37.2 ± 3.9; 12 months: 34.2 ± 5.4; p < 0.05 | No effect | No effect | [179] |
| Case-control study Obese (BMI, kg/m2: 48 [IQR 45.3–53.8) (n = 10). 4 months of low-calorie diet (800–1000 kcal) | Scinti-graphic GE and VAS: Pre vs. 1-month post-Rx | GE rate for solids %/min: Δ 0.80 (IQR: 0.60–1.15); p < 0.01 | Weight loss: Δ 9% (0.7–23.66); p < 0.05 | NA | No effect | [129] |
| Case-control study 19 Obese (mean BMI = 38.7 kg/m2): Diet-induced wt loss. Low-calorie diet (1000 kcal/day) for 8 weeks, then energy-restricted (1500 kcal/day) for 8 weeks, then maintenance diet for 8 weeks (ETEE286 kcal) | Scinti-graphic GE: Pre and 24 weeks post-Rx | GE solids % at 30 min: Baseline 24.0 (95% CI (18.4, 29.5)), 24-weeks: 18.3 (95% CI (14.0, 22.6)); p < 0.02) GE solids AUC0–60 min %min: Baseline 4903 (95% CI (4678, 5127)), 24-wks 4651 (95% CI (4404, 4897)); p < 0.03) | Baseline 38.7 (95% CI [37.2, 40.1]) vs. 24 weeks 33.0, Δ−14.7 (95% CI [30.9, 35.0]); p < 0.001) | NA | NA | [223] |
| Cross-sectional study 8 Obese (Weight: 148.9 kg [IQR 81–240) 3 to 4 weeks of a very-low calorie diet. | Scintigraphic GE: Pre- vs. 1-month post-Rx | GE for liquids, min: Baseline 41.0 ± 7.8 vs. 1-month 48.5 ± 8.7; ns GE for solids, min: Baseline 93.0 ± 11.2 vs. 1-month 100.7 ± 12.6; ns | Weight loss (mean 8.3 kg) | NA | NA | [147] |
| Study Design/ Intervention | Methods | Effect on GI Motor Function | Δ Weight, kg | Energy Intake Ad Libitum meal | Ref. |
|---|---|---|---|---|---|
| PC, DB, RCT. 20 obese (BMI 33.9 ± 1 kg/m2) with accelerated GE at baseline Exenatide SQ, 5 μg BID (n = 10) or placebo (n = 10) | Scintigraphic GE test: Pre vs. 1-month post-Rx | GE % 1 h: Exenatide 12.4% (IQR 8–18.5) vs. placebo 38.2% (IQR 26.6–42.1); p < 0.001 GE T1/2, min: Exenatide 187 (IQR 141–240) vs. placebo 86 (IQR 73–125); p < 0.001 | Exenatide −0.95 (IQR −0.7–2.1) vs. placebo 0.55 (IQR 0.3–2.1); p = 0.23 | No effect | [182] |
| RCT Healthy participants (BMI 29.6 ± 0.6 kg/ m2 vs. 29.5 ± 1.0 kg/m2) Exenatide 2.0 mg SQ weekly (n = 16) vs. placebo (n = 16) | Scintigraphic GE test: Pre vs. 2-months post-Rx | GE for solids AUC0–120min: Exenatide slowed GE vs. placebo; p = 0.046 GE for liquids AUC0–120min: Exenatide slowed GE vs. placebo; p = 0.01 | Exenatide −2.1 ± 0.5 vs. placebo 0.2 ± 0.5; p = 0.001 | NA | [186] |
| PC, DB, RCT. 40 obese (BMI: 34.6 kg/m2 vs. 37.2 kg/m2) Liraglutide group (n = 19) vs. placebo (n = 21) Liraglutide or placebo dose: 0.6 mg increments to 3.0 mg daily. | Scintigraphic GE test and VAS: Pre vs. 16-weeks post-Rx | GE T1/216 weeks, min: Liraglutide 142 (IQR 120–177) vs. placebo 113 min (IQR 101–133); ns GE T1/216 weeks vs baseline, min: Liraglutide 30.5 (IQR -11–54) vs. placebo 1 min (IQR −19–7); p = 0.025 | Liraglutide 5.3 (IQR 5.2–6.8) vs. placebo 2.5 (IQR 0.1–4.2); p = 0.0009 | No effect | [82,224] |
| OL, three-arm, RCT 142 Participants with T2DM (BMI 30.8 ± 0.34 kg/m2) Rx + insulin glargine for 8 wks: a) Lixisenatide 20 μg SQ daily b) Liraglutide 1.2 mg SQ daily c) Liraglutide 1.8 mg SQ daily | 13C-sodium-octanoic acid GE test: Pre vs. 2-months post-Rx | GE T1/28 weeks vs. baseline, min: Lixisenatide 20 μg 453.6 ± 58.2; p < 0.001 Liraglutide 1.2 mg 175.3 ± 58; p < 0.05 Liraglutide 1.8 mg 130.5 ± 60.3; p < 0.05 | Lixisenatide 20 μg −1.6 ± 0.5; p < 0.05 Liraglutide 1.2 mg −1.8 ± 0.5; p < 0.05 Liraglutide 1.8 mg −2.4 ± 0.5; p < 0.001 | NA | [225] |
| OL, parallel-group, RCT Participants with T2DM Lixisenatide of 20 µg SQ daily (n = 69) vs. Sitagliptin 50 mg oral daily (n = 67) Lixisenatide weekly 5 µg increments: from 10 µg to 20 µg daily. | 13C-sodium-octanoic acid GE test: Pre vs. 1-month post-Rx. | Lixisenatide vs. sitagliptin GE AUC0–240min mean change from baseline, ng/mL: −4.8 ± 0.47 vs. 0.9 ± 0.48 (−5.8 [−7.10, −4.44]; p < 0.0001) | Lixisenatide −0.41 vs. sitagliptin +0.39 (descriptive statistics only) | NA | [226] |
| DB, X-O, RCT 8 obese (BMI 30.3 ± 1.0 kg/m2) with T2DM Lixisenatide 10-µg SQ for 14 days and 20-µg for additional 14 days | 13C-octanoate GE test: 1-month post-Rx | GE AUC1–8h: reduced after lixisenatide compared with after placebo; p = 0.048 | Lixisenatide −2.4 ± 4.73 vs. placebo −1.5 ± 4.24; ns | NA | [227] |
| PC, DB, RCT. 30 patients with T2DM (BMI 32.1 ± 5.1 kg/m2) Lixisenatide (n = 19) vs. placebo (n = 21) Lixisenatide or placebo dose was double weekly from 0.5 μg until a dose of 20 μg daily was reached. | Scintigraphic GE test: Pre- vs. 8-weeks. post-Rx | Gastric retention post-Rx AUC0–240min: Adjusted geometric means for lixisenatide vs. placebo 2.19 (95% CI 1.82, 2.64; p < 0.001) | Lixisenatide −1.20 ± 5.22 vs. placebo −1.0 ± 6.22; ns | NA | [228] |
| DB, PC, RCT. 24 obese (BMI 30.3 ± 1.0 kg/m2) Phentermine/topiramate 3.75 mg and 23 mg, respectively, for the first 5 days, and 7.5 mg and 46 mg for 10 days | Scintigraphic GE test: Pre- vs. 2-weeks. post-Rx | Phentermine/topiramate vs. placebo GE T1/2 Phentermine/Topiramate vs. placebo, min: 109.0 ± 7 vs. 88 ± 7; p = 0.05 | Phentermine/topiramate −1.42 ± 0.4 vs. placebo −0.23 ± 0.4; p = 0.03 | Phentermine-topiramate vs. placebo Δ-260 (95% CI [−491.6, −28.3]; p < 0.05). | [14] |
| Study Design/ Intervention | Methods | Effect on GI Motor Function | Weight Loss | Ref. |
|---|---|---|---|---|
| 10 subjects (BMI 32.4 ± 1.53 kg/m2) IGB filled with 200–229 mL of air for 12 weeks. | Scintigraphic GE test: pre- and 5-wks. post-Tx | GE T1/2 for solids, min: baseline 57 ± 27.8 vs. 5-weeks. Post-Tx. 67 ± 27.5; p < 0.05 | Δ3 months—BL, kg: −2.4 ± 1.04 | [229] |
| 15 subjects (BMI 34.4 ± 0.7 kg/m2) IGB filled with 600 mL of saline for 24 weeks. | 13C-octanoate GE test: pre- and 16-wks. post-Tx | GE T1/2 for solids, min: BL 92 ± 45 vs. 16-weeks. post-Tx 157 ± 70; p = 0.052 | Body weight loss, %: 9.4 ± 1.8 | [201] |
| 3 subjects (BMI 40.93 ± 8.8 kg/m2) IGB filled with 500 cc of saline for 24 weeks. | Scintigraphic GE test: pre- and 3-months post-Tx | GE T1/2 for solids, min: BL 114 ± 18.5 vs.12-weeks. post-Tx 375.3 ± 207; p = 0.02 | Δ6 months—BL, kg: −14.67 ± 4.33 | [202] |
| 7 subjects (BMI 33.76 ± 1.78 kg/m2) IGB filled with 500 cc of saline for 24 weeks. | Scintigraphic GE test: pre- and 3-months post-Tx | GE T1/2 for liquids, min: BL 38.71 ± 15.91 vs. 12-wks. 318.71 ± 168.07; p = 0.001 | Δ6 months—BL, kg: −13.14 ± 2.5 | [202] |
| 15 subjects (BMI 34.7 ± 3.42 kg/m2) vs. 14 controls (BMI 35.6 ± 2.84 kg/m2) IGB + lifestyle (1000–1500 kcal) vs. lifestyle intervention alone IGB filled with 550 cc of saline for 24 weeks. Lifestyle intervention: Diet: (1000–1500 kcal), exercise, behavioral Rx | Scintigraphic GE test: pre- and 8-, 16-wks. post-IGB | Gastric retention at 120 min after 8 weeks, %: IGB 61.4 ± 23.2 vs. controls 25.7 ± 18; p = 0.003 Gastric retention at 120 min after 16 weeks, %: IGB 62.1 ± 16.4 vs. controls 18.7 ± 15.6; p < 0.001 | Δ26 wks—BL, %TBW: IGB −14 ± 7.8 vs. controls −5.4 ± 4; p = 0.003 | [153] |
| 24 subjects (BMI 35.58 ± 2.79kg/m2) IGB filled with 600 cc of saline for 24 weeks. | Scintigraphic GE test: pre- and 2-months post-IGB | GE T1/2 for solids, min: BL 117.92 ± 150.23 vs. 12-weeks. post-Tx 281.48 ± 206.49; p = 0.004 | Δ6 months—BL, kg: −17.09 ± 3.34; p < 0.001 | [230] |
| 20 subjects (BMI 51.7 kg/m2) BPD-DS | Scintigraphic GE test: 3.5y postoperatively | GE T1/2 for solids, min: BPD-DS 28 ± 16 vs. laboratory control (n = 160) 91 ± 20 | Δ 3.5 years—BL BMI, kg/m2: 51.7 vs. 31.3 (IQR 21.8–46.3) | [231] |
| 16 subjects (BMI 47.8 ± 1.7 kg/m2) Gastric banding | Scintigraphic GE test: pre- and 6-months post-GB | GE rate, %/h: BL 42 (IQR 23.3–59) vs. 24-weeks. post-Tx 38 (IQR 31–71); ns Fundus emptying rate, %/h: BL 59 (IQR 37–91) vs. 24-weeks. post-Tx 70 (IQR 53–89); ns | Δ6 months—BL BMI, kg/m2: −6.1 ± 0.66; p < 0.001 | [232] |
| 33 subjects (weight 76 ± 4.0 kg) Gastric banding (n = 12) vs. controls (n = 11) | Scintigraphic GE test: 12-months post-GB | GE T1/2 for liquids, min: GB 7 ± 3 vs. controls 15 ± 2; (p < 0.005) GE T1/2 for solids: 8 patients showed slower GE T1/2 for solids (147 ± 25 min) vs. controls (70 ± 7 min) | Δ6 months—BL, kg: 28 ± 3 | [149] |
| 29 subjects Jejunoileal Bypass | Scintigraphic GE test: 2- and 12-months post-surgical | GE T60 min, %: 2 months 70 ± 24 vs. 12 months 89 ± 7; p < 0.05 | Δ12 months—BL, kg: 42.3 ± 10.9; p < 0.001 | [233] |
| 11 subjects, BMI 46.8 kg/m2 (IQR 35.8–62.5) Laparoscopic Gastric Sleeve | Scintigraphic GE test: pre- and 6-months post-LSG | GE T1/2, min: BL 94.3 ± 15.4 vs. 6 months 47.6 ± 23.2; p < 0.01 GE at 90 min, %: BL 49.2 ± 8.7 vs. 6 months 75.4 ± 14.9; p < 0.01 | Δ6 months—BL, kg: 42.3 ± 10.9; p < 0.001 | [234] |
| 21 subjects (BMI 45.09 ± 6.2 kg/m2) Laparoscopic Gastric Sleeve | Scintigraphic GE test: pre- and 3-months post-LSG | GE T1/2, min: BL 62.39 ± 19.83 vs. 3 months 56.79 ± 18.72 (p = 0.36, t = −0.92, ns) | Δ3 months—BL, kg: −7.29 ± 1.87; p <0.001 | [235] |
| 20 subjects underwent LSG (BMI 38.3 kg/m2 [IQR 34.5–48.3]) vs. 18 controls (BMI 19.8–23.5 kg/m2) | Scintigraphic GE test: 3-months post-LSG | GE T1/2 for liquids: control vs post-surgical, min: 34.9 ± 24.6 vs. 13.6 ± 11.9; p < 0.01 GE T1/2 for solids: control vs post-surgical, min: 78 ± 15.01 vs. 38.3 ± 18.77; p < 0.01 | Weight lost at the first month after surgery was 11.1 ± 2.2 kg and 45.5 ± 5.2 kg in the sixth months | [236] |
| 23 subjects underwent LSG (BMI 40.7 ± 6.6 kg/m2) vs. 44 controls, 24 lean (BMI 22.2 ± 2.89 kg/m2) and 20 obese (BMI 37.7 ± 5.4 kg/m2) | Scintigraphic GE test: 2 years post-LSG | GE T1/2 for solids, min: lean 72.8 ± 29.6 vs. post-surgical 52.8 ± 13.5; p = 0.025 GE T1/2 for solids, min: obese controls 73.7 ± 29.0 vs. post-surgical 52.8 ± 13.5; p = 0.01 | Δ12 months—BL, kg: −26.80 ± 5.75; p < 0.001 | [152] |
| 4 subjects underwent LSG, BMI 41.9 kg/m2(IQR 38–44.3) | Scintigraphic GE test: pre- and 3 months post-LSG | GE T1/2, min: BL 57.5 ± 12.7 vs. 3-months 32.25 ± 17.3; p = 0.016 GE at 90 min, %: BL 20.5 vs 3-months 9.5; p = 0.073 | Δ3 months—BL, kg: −7.29 ± 1.87; p < 0.001 | [237] |
| 45 subjects underwent LSG (BMI 49.5 kg/ m2) | Scintigraphic GE test: pre- and 3 months post-LSG | GE T1/2, min: BL 80.4 ± 33.1 vs. 3-months 64.3 ± 40; p = 0.06 | Pre-surgical vs. 12 months BMI, kg/m2: 48.5 vs. 36.8; p < 0.05 | [238] |
| 21 subjects underwent LSG, BMI 46.8 kg/ m2 (IQR 35.8–62.5) | Scintigraphic GE test: pre- and 4 months post LSG | GE T1/2, min: BL 61.7 (IQR 37.0–94.3) vs. 4-months 49.1 (IQR 22.4–92.1); p < 0.05 | Pre-surgical vs. 6 months BMI, kg/m2: 46.8 (35.8–62.5) vs. 37.4 (28.2–53.2) (p < 0.05) | [239] |
| 20 subjects underwent LSG, BMI 48.7 ± 3.3 kg/ m2 | Scintigraphic GE test: pre- and 1–4 weeks post-LSG for liquids. | GE T1/2 for liquids, min: BL 25.3 ± 4.4 vs. 1-months 11.8 ± 3.0; p < 0.001 | Δ1 month—BL BMI, kg/m2: −8.20 ± 1.03; p < 0.001 | [240] |
| 20 subjects underwent LSG, BMI 49.1 ± 7.1 kg/ m2 | Scintigraphic GE test: Pre- and 4–6 weeks post-LSG for solids | GE T1/2for solids, min: BL 74.9 ± 7.1 vs. 6-weeks 28.4 ± 8.3; p < 0.001 | Δ6 weeks—BL BMI, kg/m2: −11.40 ± 1.86; p < 0.001 | [240] |
| 20 subjects underwent LSG, dichotomize according to postprandial symptoms. Low symptoms score, BMI 45.5 ± 10.7 kg/ m2 (n = 13) vs high symptom score, BMI, 40.5 ± 4.5 kg/m2 (n = 7) | Scintigraphic GE test: 2 years post-LSG. | GE T1/2 for liquids, min: Low symptoms 10.4 ± 2.9 vs. high symptom 10.6 ± 4.3; p = 0.27 GE T1/2 for solids, min: Low symptoms 40.6 ± 10.0 vs. high symptom 34.4 ± 9.3; p = 0.90 | Group I Δ24 months-BL BMI, kg/m2: −13.00 ± 3.27; p < 0.05 Group II Δ24 months-BL BMI, kg/m2: −10.50 ± 1.37; p < 0.05 | [241] |
| 20 subjects underwent LSG, BMI 33.4 ± 1.2 kg/ m2 | Scintigraphic GE test: pre- and 3-, 6-, 12- and 24-months post-LSG | GE T1/2, min: BL 38.4 ± 13 vs. 3- months 20.3 ± 7.6 vs. 6-months 20.7 ± 9.5 vs. 12- months 20.6 ± 4.4; p < 0.05 | Δ3 months—BL BMI, kg/m2: −5.5 ± 1.9; p < 0.05 | [242] |
| 30 subjects underwent LSG, BMI 50.96 ± 5.18 kg/ m2 | Scintigraphic GE test: pre- and 6- and 12-months post-LSG | GE T1/2, min: BL 96.5 ± 78.9 vs. 6-months 44.3 ± 21.1 vs. 12-months 36.1 ± 10.2; p < 0.001 | Δ12 months—BL BMI, kg/m2: −17.28 ± 6.76; p < 0.05 | [243] |
| 30 subjects underwent LSG, BMI 51.27 ± 7.20 kg/ m2 | Scintigraphic GE test: pre- and 6- and 12-months post-LSG | GE T1/2, min: BL 99.9 ± 71.4 vs. 6-months 48,1 ± 21.6 vs. 12-months 44.4 ± 15.9; p < 0.001 | Δ12 months—BL BMI, kg/m2: −16.79 ± 8.35; p < 0.05 | [243] |
| 50 subjects underwent LSG, BMI 44.5 ± 8.1 kg/ m2 | Scintigraphic GE test: pre- and 3-months post-LSG | GE T1/2 for liquids, min: BL 26.7 ± 23 vs. 3-months 15.2 ± 13; p < 0.05 GE T1/2 for solids, min: BL 68.7 ± 25 vs. 3-months 15.2 ± 13; p < 0.05 | %EWL after 3 months (n = 26): 24.6 ± 12.1 %EWL after 3 months (n = 26): 25.1 ± 10.9 | [244] |
| 38 subjects underwent LSG, 12 with antrum resection-2 cm from the pylorus vs. 13 with antrum preservation-5 cm from the pylorus | Scintigraphic GE test: pre- and 2-months and 1-year post-LSG | AR pre vs. 2 months post LSG GE60-min for semi-solids, %: 55.8 ± 22 vs. 69.7 ± 18; ns AR pre vs. 12 months post LSG GE60-min for semi-solids, %: 55.8 ± 22 vs. 66.5 ± 21; ns AP pre vs. 2 months post LSG GE60-min for semi-solids, %: 52.7 ± 24 vs. 72.8 ± 20; p = 0.024 AP pre vs. 12 months post LSG GE60-min for semi-solids, %: 52.7 ± 24 vs. 74.2 ± 16; p = 0.010 | AR pre vs. 12 months post-LSG BMI, kg/m2: 43.01 vs. 31.43 AP pre vs. 12 months post-LSG BMI, kg/m2: 45.3 vs. 31.88 | [214] |
| 23 subjects underwent LSG, BMI 41.9 ± 5.3 kg/ m2 | Scintigraphic GE test: pre- and 3-months post-LSG | GE T1/2 for solids, min: BL 52.7 ± 20.5 vs. 3-months 33.6 ± 3.0; p < 0.001 | Δ3 months—BL BMI, kg/m2: −7 ± 7.35; p < 0.001 | [245] |
| 21 subjects underwent LSG, BMI 38.89 ± 7.55kg/ m2 | Scintigraphic GE test: pre- and 3-months post-LSG | GE T1/2 for solids, min: BL 67.1 ± 33.43 vs. 3-months 20.71 ± 12.81; p < 0.05 | Δ3 months—BL BMI, kg/m2: −8 ± 9.80; p < 0.05 | [246] |
| 100 subjects underwent LSG, BMI 43.43 ± 3.8 kg/ m2 | Scintigraphic GE test: pre- and 3-months post-LSG | Retention, %: 1 h: 64 ± 13 vs. 54.5 ± 15; p < 0.0001 2 h: 45 ± 12 vs. 35.5 ± 13); p < 0.0001 4 h: 6 ± 3 vs. 4 ± 2; p < 0.0001 | Δ3 months—BL BMI, kg/m2: −8.83 ± 4.54; p < 0.001 | [247] |
| 23 subjects underwent LSG, BMI 42.4 ± 5.8 kg/ m2 | MRI GE test: pre- and after 40% of EBW loss post-LSG | Total gastric volume, mL: BL 467 (95% CI (455, 585)) vs. post-Tx 139 (95% CI (121, 185); p < 0.0001) Early-phase GE, mL/min: 1.9 (95% CI (1.1, 4.0)) vs. 2.69 (95% CI [1.6, 3.4]; p = 0.001) Late-phase GE, mL/min: 2.5 (95% CI (2.0, 2.9)) vs. 1.4 (95% CI (1.1, 1.7); p = 0.001) | Δ7 months—BL BMI, kg/m2: −9.6 ± 7.28; p < 0.001 | [213] |
| 26 subjects underwent LSG, BMI 47.5 ± 6.6 kg/ m2 | Scintigraphic GE test: after >20% TBWL post-LSG | GE T1/2 for solids, min: BL 24.4 ± 11.4 vs. post-Tx 75.80 ± 45.19; p < 0.001 | Δ8 months—BL BMI, kg/m2: −12.60 ± 9.99; p < 0.01 | [248] |
| 10 SG (BMI 33.4 ± 2.4 kg/ m2), 10 RYGB (BMI 33.5 ± 2.1 kg/ m2), and 10 controls (BMI 33.4 ± 1.7 kg/ m2) | Scintigraphic GE test: SG vs. RYGB vs. controls | GE T1/2 for solids, min: RYGB 11 ± 2; SG 56 ± 11; controls 113 ± 8; p < 0.01 | BMI loss %: SG: 60 ± 8 vs. RYGB: 61 ± 7 | [249] |
| 17 RYGB (BMI 45.8 ± 4.7 kg/ m2), and 9 controls (BMI 23.5 ± 1.9 kg/ m2) | Scintigraphic GE test: Between 15- and 21-months post-RYGB | Emptying of pouch or stomach (fraction of total meal x hours): Liquid marker, RYGB vs. controls: 0.19 (IQR 0.07–0.26) vs. 0.49 (IQR 0.47–0.64); p < 0.001 Solid marker, RYGB vs. controls: 0.45 (IQR 0.31–1.04) vs. 1.33 (IQR 1.15–1.65); p = 0.004 | Δ18 months—BL BMI, kg/m2: −11.20 ± 5.32; p = 0.04 | [250] |
| 10 RYGB (BMI 29.9 ± 1.9 kg/ m2), and 10 controls (BMI 24.3 ± 0.9 kg/ m2) | Scintigraphic GE test: 5 years post-surgical | RYGB vs. controls Pouch/GE T1/2, min: faster un RYGB; p < 0.001 RYGB Sitting vs. supine position Pouch/GE T1/2, min: 2.5 ± 0.7 vs. 16.6 ± 5.3 min; p = 0.02 | Δ18 months—BL BMI, kg/m2: −12.9 ± 3.4 kg/m2 | [221] |
| 8 RYGB, and 24 controls (12 lean controls vs. 12 obese controls) | 13C-acetate breath test GE test: RYGB 10 weeks post-surgical | Gastric emptying in lean controls and obese controls was significantly slower vs. RYGB; p < 0.001) | Post-surgical BMI, kg/m2: 38.6 ± 1.7 | [251] |
| 10 RYGB, divided according TBWL: poor weight loss (< 25%) (n = 5) vs. and Successful weight loss (> 25%) (n = 5) | Scintigraphic GE test: 2 years post-surgical | Poor weight loss vs. Successful weight loss Pouch/GE T1/2, min: 5.1 ± 1.3 vs. 34 ± 32 (p = 0.12) Poor weight loss vs. Successful weight loss PERmax, %/min: 17 ± 4.7 vs. 5.6 ± 3.4; p = 0.002 | Poor weight loss vs. Successful weight loss pre-surgical BMI, kg/m2: 43 ± 4.3 vs. 45 ± 3.8 Poor weight loss vs. Successful weight loss at scintigraphy, %: 17 ± 4.1 vs. 44 ± 5.7 | [219] |
| 94 subjects underwent surgery: 47 RYGB BMI 42.4 kg/ m2 (IQR 36.0–54.9) and 47 BRYGB BMI 44.3 kg/ m2 (IQR 21.8–52.5) | Scintigraphic GE test: Between 6 months and 2 years post-surgical | GE T1/2 for solids, min: RYGB 65.9 (IQR 40.6–183.0) vs. BRYGB 79.4 (IQR 41.1–390.9); p = 0.031 | RYGB BMI, kg/m2: 42.4 (IQR 36.0–54.9) vs. 30.9 (IQR 23.7–43.8) BRYGB BMI, kg/m2: 44.3 (IQR 37.5–60.8) vs. 29.8 (IQR 21.8–52.5) | [146] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- WHO. Obesity and Overweight. Fact Sheet No 311; WHO Media Centre: 2015. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 24 December 2015).
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Dayyeh, B.K.A.; Port, J.D.; Camilleri, M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut 2014, 63, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Alhadeff, A.L.; Rupprecht, L.E.; Hayes, M.R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012, 153, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.M.; Finlayson, G.; Byrne, N.M.; King, N.A. Food reward in active compared to inactive men: Roles for gastric emptying and body fat. Physiol. Behav. 2016, 160, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L. Neural integration of satiation and food reward: Role of GLP-1 and orexin pathways. Physiol. Behav. 2014, 136, 194–199. [Google Scholar] [CrossRef]
- Rinaman, L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010, 1350, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Peripheral mechanisms in appetite regulation. Gastroenterology 2015, 148, 1219–1233. [Google Scholar] [CrossRef]
- Hussain, S.S.; Bloom, S.R. The regulation of food intake by the gut-brain axis: Implications for obesity. Int. J. Obes. 2012, 37, 625–633. [Google Scholar] [CrossRef]
- Hunt, J.; Cash, R.; Newland, P. Energy density of food, gastric emptying, and obesity. Lancet 1975, 306, 905–906. [Google Scholar] [CrossRef]
- Park, M.I.; Camilleri, M. Gastric motor and sensory functions in obesity. Obes. Res. 2005, 13, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Camilleri, M.; Shin, A.; Vazquez-Roque, M.I.; Iturrino, J.; Burton, D.; O’Neill, J.; Eckert, D.; Zinsmeister, A.R. Quantitative Gastrointestinal and Psychological Traits Associated With Obesity and Response to Weight-Loss Therapy. Gastroenterol. 2015, 148, 537–546.e4. [Google Scholar] [CrossRef] [PubMed]
- Jahnberg, T.; Martinson, J.; Hultén, L.; Fasth, S. Dynamic Gastric Response to Expansion before and after Vagotomy. Scand. J. Gastroenterol. 1975, 10, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, S.D.; Vergeer, M.; Heisterkamp, S.H.; Tytgat, G.N.J.; Boeckxstaens, G.E.E. Role of nitric oxide in gastric motor and sensory functions in healthy subjects. Gut 2002, 51, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Malagelada, J.R.; Brown, M.L.; Becker, G.; Zinsmeister, A.R. Relation between antral motility and gastric emptying of solids and liquids in humans. Am. J. Physiol. Liver Physiol. 1985, 249, G580–G585. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.; Read, N.; Heddle, R.; Horowitz, M.; Collins, P.; Chatterton, B.; Dent, J. Relationship of the motor activity of the antrum, pylorus, and duodenum to gastric emptying of a solid-liquid mixed meal. Gastroenterol. 1988, 94, 1285–1291. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Wang, X.-Y.; Chen, D.; Huizinga, J.D. Role of interstitial cells of Cajal in the generation and modulation of motor activity induced by cholinergic neurotransmission in the stomach. Neurogastroenterol. Motil. 2011, 23, e356–e371. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Sanders, K.M.; Hirst, G.D.S. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol. Motil. 2004, 16, 112–117. [Google Scholar] [CrossRef]
- Sanders, K.M.; Koh, S.D.; Ro, S.; Ward, S.M. Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 633–645. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R. Disintegration of Solid Foods in Human Stomach. J. Food Sci. 2008, 73, R67–R80. [Google Scholar] [CrossRef]
- Meyer, J.; Ohashi, H.; Jehn, D.; Thomson, J. Size of liver particles emptied from the human stomach. Gastroenterol. 1981, 80, 1489–1496. [Google Scholar] [CrossRef]
- Goyal, R.K.; Hirano, I. The Enteric Nervous System. New Engl. J. Med. 1996, 334, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.H.; Camilleri, M.; E Crowe, S.; Elomar, E.M.; Fox, J.G.; Kuipers, E.J.; Malfertheiner, P.; McColl, K.E.L.; Pritchard, D.; Rugge, M.; et al. The stomach in health and disease. Gut 2015, 64, 1650–1668. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, T.; Luhovyy, B.L.; Brown, P.H.; E Cho, C.; Anderson, G.H. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin. Nutr. 2010, 91, 966–975. [Google Scholar] [CrossRef]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroenterol. Motil. 2019, 31, e13546. [Google Scholar] [CrossRef]
- Powley, T.L.; Jaffey, D.M.; McAdams, J.; Baronowsky, E.A.; Black, D.; Chesney, L.; Evans, C.; Phillips, R.J. Vagal innervation of the stomach reassessed: Brain−gut connectome uses smart terminals. Ann. N. Y. Acad. Sci. 2019, 1454, 14–30. [Google Scholar] [CrossRef]
- Burton, M.; Rolls, E.; Mora, F. Effects of hunger on the responses of neurons in the lateral hypothalamus to the sight and taste of food. Exp. Neurol. 1976, 51, 668–677. [Google Scholar] [CrossRef]
- Matzinger, D.; Degen, L.; Drewe, J.; Meuli, J.; Duebendorfer, R.; Ruckstuhl, N.; D’Amato, M.; Rovati, L.; Beglinger, C. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut 2000, 46, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Beglinger, C. Effect of Cholecystokinin on Gastric Motility in Humans. Ann. N. Y. Acad. Sci. 1994, 713, 219–225. [Google Scholar] [CrossRef] [PubMed]
- A Liddle, R.; Morita, E.T.; Conrad, C.K.; A Williams, J. Regulation of gastric emptying in humans by cholecystokinin. J. Clin. Investig. 1986, 77, 992–996. [Google Scholar] [CrossRef]
- Chey, W.; Hitanant, S.; Hendricks, J.; Lorber, S. Effect of Secretin and Cholecystokinin on Gastric Emptying and Gastric Secretion in Man. Gastroenterology 1970, 58, 820–827. [Google Scholar] [CrossRef]
- Fried, M.; Erlacher, U.; Schwizer, W.; Löchner, C.; Koerfer, J.; Beglinger, C.; Jansen, J.B.; Lamers, C.B.; Harder, F.; Bischof-Delaloye, A. Role of cholecystokinin in the regulation of gastric emptying and pancreatic enzyme secretion in humans: Studies with the cholecystokinin-receptor antagonist loxiglumide. Gastroenterology 1991, 101, 503–511. [Google Scholar] [CrossRef]
- Holst, J.J.; Ørskov, C.; Hartmann, B.; Deacon, C.F. Posttranslational Processing of Proglucagon and Postsecretory Fate of Proglucagon Products. Front. Diabetes 1997, 13, 24–48. [Google Scholar] [CrossRef]
- Williams, D.L.; Baskin, D.G.; Schwartz, M.W. Evidence that Intestinal Glucagon-Like Peptide-1 Plays a Physiological Role in Satiety. Endocrinol. 2008, 150, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- I˙meryüz, N.; Yeğen, B.C.; Bozkurt, A.; Coşkun, T.; Villanueva-Peñacarrillo, M.L.; Ulusoy, N.B. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 273, G920–G927. [Google Scholar] [CrossRef] [PubMed]
- Deane, A.M.; Nguyen, N.Q.; Stevens, J.E.; Fraser, R.J.L.; Holloway, R.H.; Besanko, L.K.; Burgstad, C.; Jones, K.L.; Chapman, M.J.; Rayner, C.K.; et al. Endogenous Glucagon-Like Peptide-1 Slows Gastric Emptying in Healthy Subjects, Attenuating Postprandial Glycemia. J. Clin. Endocrinol. Metab. 2010, 95, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Halland, M.; Burton, D.; Desai, A.; Neja, B.; Low, P.; Singer, W.; Camilleri, M.; Zinsmeister, A.R.; E Bharucha, A. GI Dysfunctions in Diabetic Gastroenteropathy, Their Relationships With Symptoms, and Effects of a GLP-1 Antagonist. J. Clin. Endocrinol. Metab. 2018, 104, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Näslund, E.; Gutniak, M.; Skogar, S.; Rössner, S.; Hellström, P.M. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am. J. Clin. Nutr. 1998, 68, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Niedereichholz, U.; Ettler, R.; Holst, J.J.; Ørskov, C.; Ritzel, R.; Schmiegel, W.H. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. Metab. 1997, 273, E981–E988. [Google Scholar] [CrossRef] [PubMed]
- Nagell, C.; Wettergren, A.; Pedersen, J.F.; Mortensen, D.; Holst, J.J. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scand. J. Gastroenterol. 2004, 39, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Minami, K.; Shinozaki, H.; Matsumura, K.; Saraya, A.; Ikeda, H.; Yamada, Y.; Holst, J.J.; Seino, S. Distinct Effects of Glucose-Dependent Insulinotropic Polypeptide and Glucagon-Like Peptide-1 on Insulin Secretion and Gut Motility. Diabetes 2005, 54, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Edholm, T.; Degerblad, M.; Grybäck, P.; Hilsted, L.; Holst, J.J.; Jacobsson, H.; Efendic, S.; Schmidt, P.; Hellström, P.M. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol. Motil. 2010, 22, 1191-e315. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Jones, K.L.; Horowitz, M.; Chapman, M.J.; Deane, A.M. Measurement of gastric emptying in the critically ill. Clin. Nutr. 2015, 34, 557–564. [Google Scholar] [CrossRef]
- Adrian, T.; Ferri, G.-L.; Bacarese-Hamilton, A.; Fuessl, H.; Polak, J.; Bloom, S. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985, 89, 1070–1077. [Google Scholar] [CrossRef]
- Pironi, L.; Stanghellini, V.; Miglioli, M.; Corinaldesi, R.; de Giorgio, R.; Ruggeri, E.; Tosetti, C.; Poggioli, G.; Morselli^labate, A.M.; Monetti, N.; et al. Fat-induced heal brake in humans: A dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology 1993, 105, 733–739. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nat. Cell Biol. 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Samsom, M.; Szarka, L.A.; Camilleri, M.; Vella, A.; Zinsmeister, A.R.; Rizza, R.A. Pramlintide, an amylin analog, selectively delays gastric emptying: Potential role of vagal inhibition. Am. J. Physiol. Liver Physiol. 2000, 278, G946–G951. [Google Scholar] [CrossRef] [PubMed]
- Bado, A.; Levasseur, S.; Attoub, S.; Kermorgant, S.; Laigneau, J.-P.; Bortoluzzi, M.-N.; Moizo, L.; Lehy, T.; Guerre-Millo, M.; Le Marchand-Brustel, Y.; et al. The stomach is a source of leptin. Nat. Cell Biol. 1998, 394, 790–793. [Google Scholar] [CrossRef]
- Martínez, V.; Barrachina, M.-D.; Wang, L.; Taché, Y. Intracerebroventricular leptin inhibits gastric emptying of a solid nutrient meal in rats. Neuro Rep. 1999, 10, 3217–3221. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Kasimay, O.; Devseren, E.; Yeğen, B.C. Leptin inhibits gastric emptying in rats: Role of CCK receptors and vagal afferent fibers. Physiol. Res. 2006, 56, 315–322. [Google Scholar] [PubMed]
- Sanger, G.J.; Hellström, P.M.; Näslund, E. The hungry stomach: Physiology, disease, and drug development opportunities. Front. Pharmacol. 2011, 1, 145. [Google Scholar] [CrossRef] [PubMed]
- Druce, M.R.; Wren, A.M.; Park, A.J.; E Milton, J.; Patterson, M.; Frost, G.; A Ghatei, M.; Small, C.; Bloom, S.R. Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. 2005, 29, 1130–1136. [Google Scholar] [CrossRef]
- Meleine, M.; Mounien, L.; Atmani, K.; Ouelaa, W.; Bôle-Feysot, C.; Guérin, C.; Depoortere, I.; Gourcerol, G. Ghrelin inhibits autonomic response to gastric distension in rats by acting on vagal pathway. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Camilleri, M.; McCallum, R.W.; Tack, J.; Spence, S.C.; Gottesdiener, K.; Fiedorek, F.T. Efficacy and Safety of Relamorelin in Diabetics With Symptoms of Gastroparesis: A Randomized, Placebo-Controlled Study. Gastroenterology 2017, 153, 1240–1250.e2. [Google Scholar] [CrossRef]
- Lembo, A.; Camilleri, M.; McCallum, R.; Sastre, R.; Breton, C.; Spence, S.; White, J.; Currie, M.; Gottesdiener, K.; Stoner, E. Relamorelin Reduces Vomiting Frequency and Severity and Accelerates Gastric Emptying in Adults With Diabetic Gastroparesis. Gastroenterology 2016, 151, 87–96.e6. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.D.; Camilleri, M.; Acosta, A.; Busciglio, I.; Nord, S.L.; Boldingh, A.; Rhoten, D.; Ryks, M.; Burton, D. Effects of ghrelin receptor agonist, relamorelin, on gastric motor functions and satiation in healthy volunteers. Neurogastroenterol. Motil. 2016, 28, 1705–1713. [Google Scholar] [CrossRef]
- James, J.; Mair, S.; Doll, W.; Sandefer, E.; Wurtman, D.; Maurer, A.; Deane, A.M.; Harris, M.S. The effects of ulimorelin, a ghrelin agonist, on liquid gastric emptying and colonic transit in humans. Neurogastroenterol. Motil. 2019, 32, e13784. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Burton, D.D.; Breen-Lyles, M.; Camilleri, M. Gastric Accommodation Influences Proximal Gastric and Total Gastric Emptying in Concurrent Measurements Conducted in Healthy Volunteers. Am. J. Physiol. Liver Physiol. 2021. [Google Scholar] [CrossRef]
- Schwizer, W.; Maecke, H.; Michael, F. Measurement of gastric emptying by magnetic resonance imaging in humans. Gastroenterology 1992, 103, 369–376. [Google Scholar] [CrossRef]
- Abell, T.L.; Camilleri, M.; Donohoe, K.; Hasler, W.L.; Lin, H.C.; Maurer, A.H.; McCallum, R.W.; Nowak, T.; Nusynowitz, M.L.; Parkman, H.P.; et al. Consensus Recommendations for Gastric Emptying Scintigraphy: A Joint Report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J. Nucl. Med. Technol. 2008, 36, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Iturrino, J.; Bharucha, A.E.; Burton, D.; Shin, A.; Jeong, I.-D.; Zinsmeister, A.R. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol. Motil. 2012, 24, 1076-e562. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Zinsmeister, A.R.; Greydanus, M.P.; Brown, M.L.; Proano, M. Towards a less costly but accurate test of gastric emptying and small bowel transit. Dig. Dis. Sci. 1991, 36, 609–615. [Google Scholar] [CrossRef]
- Wise, J.L.; Vazquez-Roque, M.I.; McKinney, C.J.; Zickella, M.A.; Crowell, M.D.; Lacy, B.E. Gastric Emptying Scans: Poor Adherence to National Guidelines. Dig. Dis. Sci. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hveem, K.; Jones, K.L.; E Chatterton, B.; Horowitz, M. Scintigraphic measurement of gastric emptying and ultrasonographic assessment of antral area: Relation to appetite. Gut 1996, 38, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Gilja, O.H.; Hausken, T.; Ødegaard, S.; Berstad, A. Gastric emptying measured by ultrasonography. World J. Gastroenterol. 1999, 5, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Gilja, O.H.; Lunding, J.; Hausken, T.; Gregersen, H. Gastric accommodation assessed by ultrasonography. World J. Gastroenterol. 2006, 12, 2825–2829. [Google Scholar] [CrossRef]
- Gilja, O.; Detmer, P.R.; Jong, J.M.; Leotta, D.F.; Li, X.N.; Beach, K.; Martin, R.; Strandness Jr, D. Intragastric distribution and gastric emptying assessed by three-dimensional ultrasonography. Gastroenterology 1997, 113, 38–49. [Google Scholar] [CrossRef]
- Stevens, J.E.; Gilja, O.H.; Gentilcore, D.; Hausken, T.; Horowitz, M.; Jones, K.L. Measurement of gastric emptying of a high-nutrient liquid by 3D ultrasonography in diabetic gastroparesis. Neurogastroenterol. Motil. 2010, 23, 220-e114. [Google Scholar] [CrossRef]
- Tefera, S.; Gilja, O.H.; Olafsdottir, E.; Hausken, T.; Hatlebakk, J.G.; Berstad, A. Intragastric maldistribution of a liquid meal in patients with reflux oesophagitis assessed by three dimensional ultrasonography. Gut 2002, 50, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Hausken, T.; Horowitz, M.; Jones, K. Measurements of gastric emptying of low-and high-nutrient liquids using 3D ultrasonography and scintigraphy in healthy subjects. Neurogastroenterol. Motil. 2006, 18, 1062–1068. [Google Scholar] [CrossRef]
- Medhus, A.W.; Lofthus, C.M.; Bredesen, J.; Husebye, E. Gastric emptying: The validity of the paracetamol absorption test adjusted for individual pharmacokinetics. Neurogastroenterol. Motil. 2001, 13, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Szarka, L.A.; Camilleri, M.; Vella, A.; Burton, D.; Baxter, K.; Simonson, J.; Zinsmeister, A.R. A Stable Isotope Breath Test With a Standard Meal for Abnormal Gastric Emptying of Solids in the Clinic and in Research. Clin. Gastroenterol. Hepatol. 2008, 6, 635–643.e1. [Google Scholar] [CrossRef] [PubMed]
- Bluemel, S.; Menne, D.; Fried, M.; Schwizer, W.; Steingoetter, A. On the validity of the13C-acetate breath test for comparing gastric emptying of different liquid test meals: A validation study using magnetic resonance imaging. Neurogastroenterol. Motil. 2015, 27, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Heading, R.C.; Nimmo, J.; Prescott, L.F.; Tothill, P. The dependence of paracetamol absorption on the rate of gastric emptying. Br. J. Pharmacol. 1973, 47, 415–421. [Google Scholar] [CrossRef]
- Willems, M.; Quartero, A.O.; Numans, M.E. How Useful Is Paracetamol Absorption as a Marker of Gastric Emptying? A Systematic Literature Study. Dig. Dis. Sci. 2001, 46, 2256–2262. [Google Scholar] [CrossRef]
- Bartholome, R.; Salden, B.; Vrolijk, M.F.; Troost, F.J.; Masclee, A.; Bast, A.; Haenen, G.R. Paracetamol as a Post Prandial Marker for Gastric Emptying, A Food-Drug Interaction on Absorption. PLoS ONE 2015, 10, e0136618. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Rayner, C.K.; Marathe, C.S.; Wu, T.; Jones, K.L. Glucagon-like peptide-1 receptor agonists and the appropriate measurement of gastric emptying. Diabetes, Obes. Metab. 2020, 22, 2504–2506. [Google Scholar] [CrossRef] [PubMed]
- Van Can, J.; Sloth, B.; Jensen, C.B.; Flint, A.; E Blaak, E.; Saris, W.H.M. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 784–793. [Google Scholar] [CrossRef]
- Halawi, H.; Khemani, D.; Eckert, D.; O’Neill, J.; Kadouh, H.; Grothe, K.; Clark, M.M.; Burton, D.D.; Vella, A.; Acosta, A.; et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: A randomised, placebo-controlled pilot trial. Lancet Gastroenterol. Hepatol. 2017, 2, 890–899. [Google Scholar] [CrossRef]
- Maqbool, S.; Parkman, H.P.; Friedenberg, F.K. Wireless Capsule Motility: Comparison of the SmartPill® GI Monitoring System with Scintigraphy for Measuring Whole Gut Transit. Dig. Dis. Sci. 2009, 54, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Schwizer, W.; Steingotter, A.; Fox, M.; Zur, T.; Thumshirn, M.; Bosiger, P.; Fried, M. Non-invasive measurement of gastric accommodation in humans. Gut 2002, 51, i59–i62. [Google Scholar] [CrossRef] [PubMed]
- Chial, H.J.; Camilleri, M.; Delgado-Aros, S.; Burton, D.; Thomforde, G.; Ferber, I. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: Effects of gender, body mass index and age in health. Neurogastroenterol. Motil. 2002, 14, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Kindt, S.; Coulie, B.; Wajs, E.; Janssens, J.; Tack, J. Reproducibility and symptomatic predictors of a slow nutrient drinking test in health and in functional dyspepsia. Neurogastroenterol. Motil. 2008, 20, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Caenepeel, P.; Piessevaux, H.; Cuomo, R.; Janssens, J. Assessment of meal induced gastric accommodation by a satiety drinking test in health and in severe functional dyspepsia. Gut 2003, 52, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Vijayvargiya, P.; Chedid, V.; Wang, X.J.; Atieh, J.; Maselli, D.; Burton, D.D.; Clark, M.M.; Acosta, A.; Camilleri, M. Associations of gastric volumes, ingestive behavior, calorie and volume intake, and fullness in obesity. Am. J. Physiol. Liver Physiol. 2020, 319, G238–G244. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Halford, J.C.G. Regulation of nutrient supply: The brain and appetite control. Proc. Nutr. Soc. 1994, 53, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Halawi, H.; Camilleri, M.; Acosta, A.; Vazquez-Roque, M.; Oduyebo, I.; Burton, D.; Busciglio, I.; Zinsmeister, A.R. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am. J. Physiol. Liver Physiol. 2017, 313, G442–G447. [Google Scholar] [CrossRef]
- Cannon, W.B.; Washburn, A.L. An Explanation of Hunger 1. Obes. Res. 1993, 1, 494–500. [Google Scholar] [CrossRef]
- Cannon, W.B. The Mechanical Factors of Digestion; Longmans, Green & Company: New York, NY, USA, 1911. [Google Scholar]
- Delgado-Aros, S.; Cremonini, F.; Castillo, J.E.; Chial, H.J.; Burton, D.D.; Ferber, I.; Camilleri, M. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology 2004, 126, 432–440. [Google Scholar] [CrossRef]
- Park, S.-Y.; Acosta, A.; Camilleri, M.; Burton, D.; Harmsen, S.W.; Fox, J.; A Szarka, L. Gastric Motor Dysfunction in Patients With Functional Gastroduodenal Symptoms. Am. J. Gastroenterol. 2017, 112, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Piessevaux, H.; Coulie, B.; Caenepeel, P.; Janssens, J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 1998, 115, 1346–1352. [Google Scholar] [CrossRef]
- Blundell, J.E.; Gillett, A. Control of Food Intake in the Obese. Obes. Res. 2001, 9, 263S–270S. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Rehfeld, J.; Holst, J.; Astrup, A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int. J. Obes. 2000, 24, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Chapman, I.M.; A Goble, E.; A Wittert, G.; Horowitz, M. Effects of small-intestinal fat and carbohydrate infusions on appetite and food intake in obese and nonobese men. Am. J. Clin. Nutr. 1999, 69, 6–12. [Google Scholar] [CrossRef]
- Feinle-Bisset, C.; Christen, M.; Grundy, D.; Faas, H.; Meier, O.; Otto, B.; Fried, M. Effects of duodenal fat, protein or mixed-nutrient infusions on epigastric sensations during sustained gastric distension in healthy humans. Neurogastroenterol. Motil. 2002, 14, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, D.; Gutzwiller, J.-P.; Drewe, J.; Orban, A.; Engel, R.; D’Amato, M.; Rovati, L.; Beglinger, C. Inhibition of food intake in response to intestinal lipid is mediated by cholecystokinin in humans. Am. J. Physiol. Content 1999, 277, R1718–R1724. [Google Scholar] [CrossRef]
- Corvilain, B.; Abramowicz, M.; Féry, F.; Schoutens, A.; Verlinden, M.; Balasse, E.; Horowitz, M. Effect of short-term starvation on gastric emptying in humans: Relationship to oral glucose tolerance. Am. J. Physiol. Liver Physiol. 1995, 269, G512–G517. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a Protein Preload on Gastric Emptying, Glycemia, and Gut Hormones After a Carbohydrate Meal in Diet-Controlled Type 2 Diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.E.; Phillips, L.K.; Wu, T.; Bound, M.J.; Checklin, H.; Grivell, J.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Title: Differentiating the effects of whey protein and guar gum preloads on postprandial glycemia in type 2 diabetes. Clin. Nutr. 2019, 38, 2827–2832. [Google Scholar] [CrossRef]
- Watson, L.E.; Phillips, L.K.; Wu, T.; Bound, M.J.; Checklin, H.L.; Grivell, J.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. A whey/guar “preload” improves postprandial glycaemia and glycated haemoglobin levels in type 2 diabetes: A 12-week, single-blind, randomized, placebo-controlled trial. Diabetes Obes. Metab. 2019, 21, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jesudason, D.R.; Stevens, J.E.; Keogh, J.B.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Sustained effects of a protein ‘preload’on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: A randomized clinical trial. Diabetes Res. Clin. Pract. 2015, 108, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.; Carter, D.; Tothill, P.; Heading, R.; Prescott, L. Effect of gel fibre on gastric emptying and absorption of glucose and paracetamol. Lancet 1979, 313, 636–639. [Google Scholar] [CrossRef]
- French, S.J.; Read, N.W. Effect of guar gum on hunger and satiety after meals of differing fat content: Relationship with gastric emptying. Am. J. Clin. Nutr. 1994, 59, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Benini, L.; Castellani, G.; Brighenti, F.; Heaton, K.W.; Brentegani, M.T.; Casiraghi, M.C.; Sembenini, C.; Pellegrini, N.; Fioretta, A.; Minniti, G. Gastric emptying of a solid meal is accelerated by the removal of dietary fibre naturally present in food. Gut 1995, 36, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Fedoroff, I.C.; Guthrie, J.F. Gender differences in eating behavior and body weight regulation. Health Psychol. 1991, 10, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Luscombe-Marsh, N.D.; Hutchison, A.T.; Lange, K.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Effect of gender on the acute effects of whey protein ingestion on energy intake, appetite, gastric emptying and gut hormone responses in healthy young adults. Nutr. Diabetes 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Giezenaar, C.; Lange, K.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of Age on Acute Appetite-Related Responses to Whey-Protein Drinks, Including Energy Intake, Gastric Emptying, Blood Glucose, and Plasma Gut Hormone Concentrations—A Randomized Controlled Trial. Nutrients 2020, 12, 1008. [Google Scholar] [CrossRef]
- Gonenne, J.; Esfandyari, T.; Camilleri, M.; Burton, D.D.; Stephens, D.A.; Baxter, K.L.; Zinsmeister, A.R.; Bharucha, A.E. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Neurogastroenterol. Motil. 2006, 18, 911–918. [Google Scholar] [CrossRef]
- Hellmig, S.; Von Schöning, F.; Gadow, C.; Katsoulis, S.; Hedderich, J.; Fölsch, U.R.; Stüber, E. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: Influence of age, sex and body mass index. J. Gastroenterol. Hepatol. 2006, 21, 1832–1838. [Google Scholar] [CrossRef]
- Moore, J.G.; Tweedy, C.; Christian, P.E.; Datz, F.L. Effect of age on gastric emptying of liquid-solid meals in man. Dig. Dis. Sci. 1983, 28, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Weindruch, R.; Sohal, R.S. Caloric Intake and Aging. N. Engl. J. Med. 1997, 337, 986–994. [Google Scholar] [CrossRef]
- Schwartz, J.G.; Mcmahan, C.A.; Green, G.M.; Phillips, W.T. Gastric emptying in Mexican Americans compared to non-Hispanic whites. Dig. Dis. Sci. 1995, 40, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Salman, U.; Mcmahan, C.; Phillips, W. Gastric emptying of beer in Mexican-Americans compared with non-hispanic whites. Metabolism 1996, 45, 1174–1178. [Google Scholar] [CrossRef]
- Cohen, M.P.; Stern, E.; Rusecki, Y.; Zeidler, A. High prevalence of diabetes in young adult Ethiopian immigrants to Israel. Diabetes 1988, 37, 824–828. [Google Scholar] [CrossRef]
- Howard, B.V.; Bogardus, C.; Ravussin, E.; E Foley, J.; Lillioja, S.; Mott, D.M.; Bennett, P.H.; Knowler, W.C. Studies of the etiology of obesity in Pima Indians. Am. J. Clin. Nutr. 1991, 53, 1577S–1585S. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, C.; Marathe, C.S.; Malbert, C.-H.; Horowitz, M.; Jones, K.L.; Rayner, C.K.; Sun, Z.; Wu, T. Disparities in gastric emptying and postprandial glycaemia between Han Chinese and Caucasians with type 2 diabetes. Diabetes Res. Clin. Pract. 2020, 159, 107951. [Google Scholar] [CrossRef] [PubMed]
- Carrio, I.; Estorch, M.; Serra-Grima, R.; Ginjaume, M.; Notivol, R.; Calabuig, R.; Vilardell, F. Gastric emptying in marathon runners. Gut 1989, 30, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Camilleri, M.; Eckert, D.; Burton, D.; Joyner, M.; Acosta, A. Physical activity is associated with accelerated gastric emptying and increased ghrelin in obesity. Neurogastroenterol. Motil. 2020, 32, e13879. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.M.; Schubert, M.M.; Desbrow, B.; Byrne, N.M.; King, N.A. Acute Exercise and Gastric Emptying: A Meta-Analysis and Implications for Appetite Control. Sports Med. 2015, 45, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Mateos, A.M.C.; Roa-Colomo, A.; Vílchez, B.V. Changes in gastric emptying of digestible solids in professional cyclists: Relationship with exercise intensity. Revista Española de Enfermedades Digestivas 2020. [Google Scholar] [CrossRef]
- Delgado-Aros, S.; Camilleri, M.; Castillo, E.J.; Cremonini, F.; Stephens, D.; Ferber, I.; Baxter, K.; Burton, D.; Zinsmeister, A.R. Effect of Gastric Volume or Emptying on Meal-Related Symptoms After Liquid Nutrients in Obesity: A Pharmacologic Study. Clin. Gastroenterol. Hepatol. 2005, 3, 997–1006. [Google Scholar] [CrossRef]
- Roque, M.I.V.; Camilleri, M.; Stephens, D.A.; Jensen, M.D.; Burton, D.D.; Baxter, K.L.; Zinsmeister, A.R. Gastric Sensorimotor Functions and Hormone Profile in Normal Weight, Overweight, and Obese People. Gastroenterology 2006, 131, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.A.; Krinsky, S.; Fleeman, C.; Trujillo, J.; Teague, E. Gastric emptying and obesity. Gastroenterology 1983, 84, 747–751. [Google Scholar] [CrossRef]
- Tosetti, C.; Corinaldesi, R.; Stanghellini, V.; Pasquali, R.; Corbelli, C.; Zoccoli, G.; Di Febo, G.; Monetti, N.; Barbara, L. Gastric emptying of solids in morbid obesity. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1996, 20, 200. [Google Scholar]
- Maddox, A.; Horowitz, M.; Wishart, J.; Collins, P. Gastric and Oesophageal Emptying in Obesity. Scand. J. Gastroenterol. 1989, 24, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Leahy, F.E.; McGowan, A.A.; Bluck, L.J.C.; Coward, W.A.; Jebb, S.A. Delayed gastric emptying in the obese: An assessment using the non-invasive 13C-octanoic acid breath test. Diabetes Obes. Metab. 2004, 6, 264–270. [Google Scholar] [CrossRef]
- Horowitz, M.; Collins, P.J.; Cook, D.J.; E Harding, P.; Shearman, D.J. Abnormalities of gastric emptying in obese patients. Int. J. Obes. 1983, 7, 415–421. [Google Scholar] [CrossRef]
- Phillips, L.K.; Deane, A.M.; Jones, K.L.; Rayner, C.K.; Horowitz, M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat. Rev. Endocrinol. 2015, 11, 112–128. [Google Scholar] [CrossRef]
- Parkman, H.P.; Urbain, J.-L.C.; Knight, L.C.; Brown, K.L.; Trate, D.M.; A Miller, M.; Maurer, A.H.; Fisher, R.S. Effect of gastric acid suppressants on human gastric motility. Gut 1998, 42, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.M.; Daly, J.; Horowitz, M.; Read, N.W. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut 1991, 32, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Palmer, K.R.; Smith, B.; Ferrington, C.; Merrick, M.V. Smoking delays gastric emptying of solids. Gut 1989, 30, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Ekelund, K. Relation between body weight and the gastric and intestinal handling of an oral caloric load. Gut 1976, 17, 456–462. [Google Scholar] [CrossRef]
- Pajot, G.; Camilleri, M.; Calderon, G.; Davis, J.; Eckert, D.; Burton, D.; Acosta, A. Association between gastrointestinal phenotypes and weight gain in younger adults: A prospective 4-year cohort study. Int. J. Obes. 2020, 44, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Geliebter, A.; Schachter, S.; Lohmann-Walter, C.; Feldman, H.; A Hashim, S. Reduced stomach capacity in obese subjects after dieting. Am. J. Clin. Nutr. 1996, 63, 170–173. [Google Scholar] [CrossRef][Green Version]
- Granström, L.; Backman, L. Stomach distension in extremely obese and in normal subjects. Acta Chir. Scand. 1985, 151, 367–370. [Google Scholar]
- Geliebter, A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol. Behav. 1988, 44, 665–668. [Google Scholar] [CrossRef]
- Bagger, J.I.; Holst, J.J.; Hartmann, B.; Andersen, B.; Knop, F.K.; Vilsbøll, T. Effect of Oxyntomodulin, Glucagon, GLP-1, and Combined Glucagon +GLP-1 Infusion on Food Intake, Appetite, and Resting Energy Expenditure. J. Clin. Endocrinol. Metab. 2015, 100, 4541–4552. [Google Scholar] [CrossRef]
- Schjoldager, B.; Mortensen, P.E.; Myhre, J.; Christiansen, J.; Holst, J.J. Oxyntomodulin from distal gut. Dig. Dis. Sci. 1989, 34, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Naslund, E.; Grybäck, P.; Bäckman, L.; Jacobsson, H.; Holst, J.J.; Theodorsson, H.E.; Hellstrom, P.M.; Juul, J. Distal Small Bowel Hormones (Correlation with Fasting Antroduodenal Motility and Gastric Emptying). Dig. Dis. Sci. 1998, 43, 945–952. [Google Scholar] [CrossRef]
- Bonazzi, P.; Petrelli, M.D.; Lorenzini, I.; Peruzzi, E.; Nicolai, A.; Galeazzi, R. Gastric emptying and intragastric balloon in obese patients. Eur. Rev. Med Pharmacol. Sci. 2006, 9, 15–21. [Google Scholar]
- Reis, G.M.F.; Malheiros, C.A.; Savassi-Rocha, P.R.; Júnior, O.L.C.; Thuler, F.R.; Faria, M.L.; Filho, V.G. Gastric Emptying and Food Tolerance Following Banded and Non-banded Roux-en-Y Gastric Bypass. Obes. Surg. 2018, 29, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Hutson, W.R.; Wald, A. Obesity and weight reduction do not influence gastric emptying and antral motility. Am. J. Gastroenterol. 1993, 88, 88. [Google Scholar]
- Torra, S.; Ilzarbe, L.; Malagelada, J.R.; Negre, M.; Mestre-Fusco, A.; Aguadé-Bruix, S.; Florensa, E.; Suñé, P.; Gras, B.; Hernandez, J.J.; et al. Meal size can be decreased in obese subjects through pharmacological acceleration of gastric emptying (The OBERYTH trial). Int. J. Obes. 2010, 35, 829–837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horowitz, M.; Cook, D.J.; Collins, P.J.; Harding, P.E.; Hooper, M.J.; Walsh, J.F.; Shearman, D.J.C. Measurement of gastric emptying after gastric bypass surgery using radionuclides. BJS 1982, 69, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Morínigo, R.; Moizé, V.; Musri, M.; Lacy, A.M.; Navarro, S.; Marín, J.L.; Delgado, S.; Casamitjana, R.; Vidal, J. Glucagon-Like Peptide-1, Peptide YY, Hunger, and Satiety after Gastric Bypass Surgery in Morbidly Obese Subjects. J. Clin. Endocrinol. Metab. 2006, 91, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Falkén, Y.; Hellström, P.M.; Holst, J.J.; Näslund, E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: Role of gut peptides. J. Clin. Endocrinol. Metab. 2011, 96, 2227–2235. [Google Scholar] [CrossRef]
- Shah, S.; Shah, P.; Todkar, J.; Gagner, M.; Sonar, S.; Solav, S. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg. Obes. Relat. Dis. 2010, 6, 152–157. [Google Scholar] [CrossRef]
- Gómez, V.; Woodman, G.; Abu Dayyeh, B.K. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: Results of a prospective study. Obesity 2016, 24, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, M.; Breitschaft, A.; Tadayon, S.; Wizert, A.; Skovgaard, D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in subjects with obesity. Diabetes Obes. Metab. 2021, 23, 754–762. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Jaruvongvanich, V.; Storm, A.C.; Maselli, D.B.; Bautista-Castaño, I.; Vargas, E.J.; Matar, R.; Acosta, A.; Abu Dayyeh, B.K. Personalization of Endoscopic Bariatric and Metabolic Therapies Based on Physiology: A Prospective Feasibility Study with a Single Fluid-Filled Intragastric Balloon. Obes. Surg. 2020, 30, 3347–3353. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Loos, R.J. Genetic determinants of common obesity and their value in prediction. Best Pr. Res. Clin. Endocrinol. Metab. 2012, 26, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; O’Rahilly, S. Monogenic Obesity in Humans. Annu. Rev. Med. 2005, 56, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Haqq, A.M.; Farooqi, I.S.; O’Rahilly, S.; Stadler, D.D.; Rosenfeld, R.G.; Pratt, K.L.; LaFranchi, S.H.; Purnell, J.Q. Serum Ghrelin Levels Are Inversely Correlated with Body Mass Index, Age, and Insulin Concentrations in Normal Children and Are Markedly Increased in Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Arenz, T.; Schwarzer, A.; Pfluger, T.; Koletzko, S.; Schmidt, H. Delayed gastric emptying in patients with Prader Willi Syndrome. J. Pediatr. Endocrinol. Metab. 2010, 23, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.H.; Jin, D.-K.; Kim, S.E.; Song, S.Y.; Paik, K.H.; Park, H.Y.; Oh, Y.J.; Kim, A.H.; Kim, J.S.; Kim, C.W.; et al. Hyperghrelinemia Does Not Accelerate Gastric Emptying in Prader-Willi Syndrome Patients. J. Clin. Endocrinol. Metab. 2005, 90, 3367–3370. [Google Scholar] [CrossRef][Green Version]
- Yau, A.M.W.; McLaughlin, J.; Maughan, R.J.; Gilmore, W.; Ashworth, J.J.; Evans, G.H. A Pilot Study Investigating the Influence of Glucagon-Like Peptide-1 Receptor Single Nucleotide Polymorphisms on Gastric Emptying Rate in Caucasian Men. Front. Physiol. 2018, 9, 1331. [Google Scholar] [CrossRef]
- Acosta, A.; Camilleri, M.; Shin, A.; Carlson, P.; Burton, D.; O’Neill, J.; Eckert, D.; Zinsmeister, A.R. Association of melanocortin 4 receptor gene variation with satiation and gastric emptying in overweight and obese adults. Genes Nutr. 2014, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, F.; Camilleri, M.; McKinzie, S.; Carlson, P.; E Camilleri, C.; Burton, D.D.; Thomforde, G.M.; Urrutia, R.; Zinsmeister, A.R. Effect of CCK-1 Antagonist, Dexloxiglumide, in Female Patients with Irritable Bowel Syndrome: A Pharmacodynamic and Pharmacogenomic Study. Am. J. Gastroenterol. 2005, 100, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Carlson, P.; Laurenti, M.; Vella, A.; Camilleri, M.; Desai, A.; Feuerhak, K.; Bharucha, A.E. Association between allelic variants in the glucagon-like peptide 1 and cholecystokinin receptor genes with gastric emptying and glucose tolerance. Neurogastroenterol. Motil. 2020, 32, e13724. [Google Scholar] [CrossRef] [PubMed]
- Chedid, V.; Vijayvargiya, P.; Carlson, P.; Van Malderen, K.; Acosta, A.; Zinsmeister, A.; Camilleri, M. Allelic variant in the glucagon-like peptide 1 receptor gene associated with greater effect of liraglutide and exenatide on gastric emptying: A pilot pharmacogenetics study. Neurogastroenterol. Motil. 2018, 30, e13313. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Yockey, S.R. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr. Obes. Rep. 2017, 6, 187–194. [Google Scholar] [CrossRef]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of Modest Weight Loss in Improving Cardiovascular Risk Factors in Overweight and Obese Individuals With Type 2 Diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef]
- A King, N.; Hopkins, M.; Caudwell, P.; Stubbs, R.J.; E Blundell, J. Beneficial effects of exercise: Shifting the focus from body weight to other markers of health. Br. J. Sports Med. 2009, 43, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Maddox, A.; Wishart, J.; Vernon-Roberts, J.; Chatterton, B.; Shearman, D. Effect of dexfenfluramine on gastric emptying of a mixed solid-liquid meal in obese subjects. Br. J. Nutr. 1990, 63, 447–455. [Google Scholar] [CrossRef]
- Pilitsi, E.; Farr, O.M.; Polyzos, S.A.; Perakakis, N.; Nolen-Doerr, E.; Papathanasiou, A.-E.; Mantzoros, C.S. Pharmacotherapy of obesity: Available medications and drugs under investigation. Metabolism 2019, 92, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Czepiel, K.S.; Perez, N.P.; Reyes, K.J.C.; Sabharwal, S.; Stanford, F.C. Pharmacotherapy for the Treatment of Overweight and Obesity in Children, Adolescents, and Young Adults in a Large Health System in the US. Front. Endocrinol. 2020, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.S.; Auerbach, P.; Barrientos-Perez, M.; Gies, I.; Hale, P.M.; Marcus, C.; Mastrandrea, L.D.; Prabhu, N.; Arslanian, S. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N. Engl. J. Med. 2020, 382, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Schwizer, W.; Asal, K.; Kreiss, C.; Mettraux, C.; Borovicka, J.; Remy, B.; Guzelhan, C.; Hartmann, D.; Fried, M. Role of lipase in the regulation of upper gastrointestinal function in humans. Am. J. Physiol. Content 1997, 273, G612–G620. [Google Scholar] [CrossRef] [PubMed]
- Borovicka, J.; Schwizer, W.; Guttmann, G.; Hartmann, D.; Kosinski, M.; Wastiel, C.; Bischof-Delaloye, A.; Fried, M. Role of lipase in the regulation of postprandial gastric acid secretion and emptying of fat in humans: A study with orlistat, a highly specific lipase inhibitor. Gut 2000, 46, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Pilichiewicz, A.; O’Donovan, D.; Feinle, C.; Lei, Y.; Wishart, J.M.; Bryant, L.; Meyer, J.H.; Horowitz, M.; Jones, K.L. Effect of Lipase Inhibition on Gastric Emptying of, and the Glycemic and Incretin Responses to, an Oil/Aqueous Drink in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 3829–3834. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, D.; Horowitz, M.; Russo, A.; Feinle-Bisset, C.; Murolo, N.; Gentilcore, D.; Wishart, J.M.; Morris, H.A.; Jones, K.L. Effects of lipase inhibition on gastric emptying of, and on the glycaemic, insulin and cardiovascular responses to, a high-fat/carbohydrate meal in type 2 diabetes. Diabetologia 2004, 47, 2208–2214. [Google Scholar] [CrossRef]
- Mathus-Vliegen, E.M.H.; Leeuwen, M.L.V.I.-V.; Bennink, R.J. Influences of fat restriction and lipase inhibition on gastric emptying in obesity. Int. J. Obes. 2006, 30, 1203–1210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flint, A.; Raben, A.; Ersbøll, A.; Holst, J.; Astrup, A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int. J. Obes. 2001, 25, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Clapper, J.R.; Athanacio, J.; Wittmer, C.; Griffin, P.S.; D’Souza, L.; Parkes, D.G.; Roth, J.D. Effects of amylin and bupropion/naltrexone on food intake and body weight are interactive in rodent models. Eur. J. Pharmacol. 2013, 698, 292–298. [Google Scholar] [CrossRef]
- Acosta, A.; Camilleri, M.; Burton, D.; O’Neill, J.; Eckert, D.; Carlson, P.; Zinsmeister, A.R. Exenatide in obesity with accelerated gastric emptying: A randomized, pharmacodynamics study. Physiol. Rep. 2015, 3, 12610. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef]
- Hjerpsted, J.B.; Flint, A.; Brooks, A.; Axelsen, M.B.; Kvist, T.; Blundell, J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes. Metab. 2017, 20, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Linnebjerg, H.; Park, S.; Kothare, P.A.; Trautmann, M.E.; Mace, K.; Fineman, M.; Wilding, I.; Nauck, M.; Horowitz, M. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul. Pept. 2008, 151, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Huynh, L.Q.; Hatzinikolas, S.; Rigda, R.S.; Phillips, L.K.; Pham, H.T.; Marathe, C.S.; Wu, T.; Malbert, C.H.; Stevens, J.E.; et al. Exenatide once weekly slows gastric emptying of solids and liquids in healthy, overweight people at steady-state concentrations. Diabetes, Obes. Metab. 2020, 22, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Maselli, D.B.; Camilleri, M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv. Exp. Med. Biol. 2021, 1307, 171–192. [Google Scholar] [PubMed]
- Horowitz, M.; Aroda, V.R.; Han, J.; Hardy, E.; Rayner, C.K. Upper and/or lower gastrointestinal adverse events with glucagon-like peptide-1 receptor agonists: Incidence and consequences. Diabetes Obes. Metab. 2017, 19, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Hansen, T.; Macura, S.; Marre, M.; A Nauck, M.; De La Rosa, R.; Woo, V.; Yildirim, E.; Wilding, J. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res. Care 2020, 8, e001706. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.; Brooks, A.; Almazedi, F.; Hoff, S.T.; Boschini, C.; Bækdal, T.A. Oral semaglutide improves postprandial glucose and lipid metabolism, and delays gastric emptying, in subjects with type 2 diabetes. Diabetes Obes. Metab. 2021. [Google Scholar] [CrossRef]
- Holst, J.J. Incretin hormones and the satiation signal. Int. J. Obes. 2013, 37, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Sofopoulos, M.; Tsoukas, M.A.; Dincer, F.; Thakkar, B.; Sahin-Efe, A.; Filippaios, A.; Bowers, J.; Srnka, A.; Gavrieli, A.; et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover, randomised, placebo-controlled trial. Diabetologia 2016, 59, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Jalleh, R.; Pham, H.; Marathe, C.S.; Wu, T.; Buttfield, M.D.; Hatzinikolas, S.; Malbert, C.H.; Rigda, R.S.; Lange, K.; Trahair, L.G.; et al. Acute Effects of Lixisenatide on Energy Intake in Healthy Subjects and Patients with Type 2 Diabetes: Relationship to Gastric Emptying and Intragastric Distribution. Nutrients 2020, 12, 1962. [Google Scholar] [CrossRef]
- Smith, S.R.; Aronne, L.J.; Burns, C.M.; Kesty, N.C.; Halseth, A.E.; Weyer, C. Sustained Weight Loss Following 12-Month Pramlintide Treatment as an Adjunct to Lifestyle Intervention in Obesity. Diabetes Care 2008, 31, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.V.M.; Sinezia, C.; Sisnande, T.; Lima, L.M.T.; Lacativa, P.G. BZ043, a novel long-acting amylin analog, reduces gastric emptying, food intake, glycemia and insulin requirement in streptozotocin-induced diabetic rats. Peptides 2019, 114, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of Food Intake in Obese Subjects by Peptide YY3–36. N. Engl. J. Med. 2003, 349, 941–948. [Google Scholar] [CrossRef] [PubMed]
- CllinicalTrials.gov. A Research Study of NNC0165-1562 and Semaglutide in People Who Are Overweight or Obese. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03574584 (accessed on 22 December 2020).
- R Mattin, L.; J McIver, V.; Yau, A.M.W.; J James, L.; H Evans, G. A comparison of intermittent and continuous exercise bouts at different intensities on appetite and postprandial metabolic responses in healthy men. Nutrients 2020, 12, 2370. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.-B.; Grybäck, P.; Holst, J.J.; Hilsted, L.; Hellström, P.M.; Jacobsson, H.; Schmidt, P. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regul. Pept. 2009, 158, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Izundegui, D.G.; Singh, S.; Acosta, A. Food intake regulation: Relevance to bariatric and metabolic endoscopic therapies. Tech. Innov. Gastrointest. Endosc. 2020, 22, 100–108. [Google Scholar] [CrossRef]
- Mion, F.; Napoléon, B.; Roman, S.; Malvoisin, E.; Trepo, F.; Pujol, B.; Lefort, C.; Bory, R.-M. Effects of Intragastric Balloon on Gastric Emptying and Plasma Ghrelin Levels in Non-morbid Obese Patients. Obes. Surg. 2005, 15, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-J.; Kao, C.-H.; Chen, W.-C.; Chang, T.-T.; Lin, C.-Y. Effect of Intragastric Balloon on Gastric Emptying Time in Humans for Weight Control. Clin. Nucl. Med. 2013, 38, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.J.; Bazerbachi, F.; Calderon, G.; Prokop, L.J.; Gomez, V.; Murad, M.H.; Acosta, A.; Camilleri, M.; Abu Dayyeh, B.K. Changes in Time of Gastric Emptying After Surgical and Endoscopic Bariatrics and Weight Loss: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 57–68.e5. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Rajan, E.; Gostout, C.J. Endoscopic sleeve gastroplasty: A potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest. Endosc. 2013, 78, 530–535. [Google Scholar] [CrossRef] [PubMed]
- James, A.N.; Ryan, J.P.; Parkman, H.P. Inhibitory effects of botulinum toxin on pyloric and antral smooth muscle. Am. J. Physiol. Liver Physiol. 2003, 285, G291–G297. [Google Scholar] [CrossRef] [PubMed]
- Topazian, M.; Camilleri, M.; De La Mora-Levy, J.; Enders, F.B.; Foxx-Orenstein, A.E.; Levy, M.J.; Nehra, V.; Talley, N.J. Endoscopic ultrasound-guided gastric botulinum toxin injections in obese subjects: A pilot study. Obes. Surg. 2008, 18, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Topazian, M.; Camilleri, M.; Enders, F.T.; Clain, J.E.; Gleeson, F.C.; Levy, M.J.; Rajan, E.; Nehra, V.; Dierkhising, R.A.; Collazo–Clavell, M.L.; et al. Gastric antral injections of botulinum toxin delay gastric emptying but do not reduce body weight. Clin. Gastroenterol. Hepatol. 2013, 11, 145–150.e1. [Google Scholar] [CrossRef] [PubMed]
- Trostler, N.; Mann, A.; Zilberbush, N.; Avinoach, E.; Charuzi, I. Weight Loss and Food Intake 18 Months following Vertical Banded Gastroplasty or Gastric Bypass for Severe Obesity. Obes. Surg. 1995, 5, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma Ghrelin Levels after Diet-Induced Weight Loss or Gastric Bypass Surgery. N. Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Aylwin, S.J.B.; Batterham, R.L.; Borg, C.M.; Coyle, F.; Prasad, V.; Shurey, S.; Ghatei, M.A.; Patel, A.G.; Bloom, S.R. Gut Hormone Profiles Following Bariatric Surgery Favor an Anorectic State, Facilitate Weight Loss, and Improve Metabolic Parameters. Ann. Surg. 2006, 243, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Lin, K.; Du, N.; Ng, D.M.; Lou, D.; Chen, P. Differences in the effects of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass on gut hormones: Systematic and meta-analysis. Surg. Obes. Relat. Dis. 2021, 17, 444–455. [Google Scholar] [CrossRef]
- Yehoshua, R.T.; Eidelman, L.A.; Stein, M.; Fichman, S.; Mazor, A.; Chen, J.; Bernstine, H.; Singer, P.; Dickman, R.; Shikora, S.A.; et al. Laparoscopic Sleeve Gastrectomy—Volume and Pressure Assessment. Obes. Surg. 2008, 18, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, C.; Quero, G.; Dallemagne, B.; Curcic, J.; Fox, M.; Perretta, S. Effects of Laparoscopic Sleeve Gastrectomy on Gastric Structure and Function Documented by Magnetic Resonance Imaging Are Strongly Associated with Post-operative Weight Loss and Quality of Life: A Prospective Study. Obes. Surg. 2020, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Garay, M.; Balagué, C.; Rodríguez-Otero, C.; Gonzalo, B.; Domenech, A.; Pernas, J.C.; Gich, I.J.; Miñambres, I.; Fernández-Ananín, S.; Targarona, E.M. Influence of antrum size on gastric emptying and weight-loss outcomes after laparoscopic sleeve gastrectomy (preliminary analysis of a randomized trial). Surg. Endosc. 2018, 32, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, T.R.; Jirapinyo, P.; Thompson, C.C. 1128 Effect of Sleeve Gastrectomy on Ghrelin, GLP-1, PYY, and GIP Gut Hormones: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, S633–S634. [Google Scholar] [CrossRef]
- Wittgrove, A.C.; Clark, G.W. Laparoscopic Gastric Bypass, Roux en-Y—500 Patients: Technique and Results, with 3-60 month follow-up. Obes. Surg. 2000, 10, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-C.; Pang, Y.-C.; Chen, J.-W.; Cao, J.-Y.; Sheng, Z.; Yuan, J.-H.; Wang, R.; Zhang, C.-S.; Wang, L.-X.; Dong, J. Systematic Review and Meta-analysis of the Change in Ghrelin Levels After Roux-en-Y Gastric Bypass. Obes. Surg. 2019, 29, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Akkary, E.; Sidani, S.; Boonsiri, J.; Yu, S.; Dziura, J.; Duffy, A.J.; Bell, R.L. The paradox of the pouch: Prompt emptying predicts improved weight loss after laparoscopic Roux-Y gastric bypass. Surg. Endosc. 2008, 23, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Deden, L.N.; Cooiman, M.I.; Aarts, E.O.; Janssen, I.M.; Gotthardt, M.; Hendrickx, B.W.; Berends, F.J. Gastric pouch emptying of solid food in patients with successful and unsuccessful weight loss after Roux-en-Y gastric bypass surgery. Surg. Obes. Relat. Dis. 2017, 13, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- E Cummings, D. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int. J. Obes. 2009, 33, S33–S40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nguyen, N.Q.; Debreceni, T.L.; Burgstad, C.M.; Wishart, J.M.; Bellon, M.; Rayner, C.K.; Wittert, G.A.; Horowitz, M. Effects of Posture and Meal Volume on Gastric Emptying, Intestinal Transit, Oral Glucose Tolerance, Blood Pressure and Gastrointestinal Symptoms After Roux-en-Y Gastric Bypass. Obes. Surg. 2014, 25, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; Kamocka, A.; Pérez-Pevida, B.; Purkayastha, S.; Moorthy, K.; Patel, A.; Chahal, H.; Frost, G.; Bassett, P.; Castagnetto-Gissey, L.; et al. The Effect of Standard Versus Longer Intestinal Bypass on GLP-1 Regulation and Glucose Metabolism in Patients With Type 2 Diabetes Undergoing Roux-en-Y Gastric Bypass: The Long-Limb Study. Diabetes Care 2020, dc200762. [Google Scholar] [CrossRef] [PubMed]
- Verdich, C.; Madsen, J.L.; Toubro, S.; Buemann, B.; Holst, J.; Astrup, A. Effect of obesity and major weight reduction on gastric emptying. Int. J. Obes. 2000, 24, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Kadouh, H.; Chedid, V.; Halawi, H.; Burton, D.D.; Clark, M.M.; Khemani, D.; Vella, A.; Acosta, A.; Camilleri, M. GLP-1 Analog Modulates Appetite, Taste Preference, Gut Hormones, and Regional Body Fat Stores in Adults with Obesity. J. Clin. Endocrinol. Metab. 2019, 105, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Rosenstock, J.; Hincelin-Méry, A.; Roy-Duval, C.; Delfolie, A.; Coester, H.-V.; Menge, B.A.; Forst, T.; Kapitza, C. Contrasting Effects of Lixisenatide and Liraglutide on Postprandial Glycemic Control, Gastric Emptying, and Safety Parameters in Patients With Type 2 Diabetes on Optimized Insulin Glargine With or Without Metformin: A Randomized, Open-Label Trial. Diabetes Care 2015, 38, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Senda, M.; Naito, Y.; Tamura, M.; Watanabe, D.; Shuto, Y.; Urita, Y. Reduction of postprandial glucose by lixisenatide vs sitagliptin treatment in J apanese patients with type 2 diabetes on background insulin glargine: A randomized phase IV study (NEXTAGE Study). Diabetes, Obes. Metab. 2017, 19, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.B.; Shojaee-Moradie, F.; E Sharaf, S.; Jackson, N.C.; Fielding, B.; Hovorka, R.; Mendis, J.; Russell-Jones, D.; Umpleby, A.M. Lixisenatide Reduces Chylomicron Triacylglycerol by Increased Clearance. J. Clin. Endocrinol. Metab. 2019, 104, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.K.; Watson, L.E.; Phillips, L.K.; Lange, K.; Bound, M.J.; Grivell, J.; Wu, T.; Jones, K.L.; Horowitz, M.; Ferrannini, E.; et al. Effects of Sustained Treatment With Lixisenatide on Gastric Emptying and Postprandial Glucose Metabolism in Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2020, 43, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Velchik, M.G.; Kramer, F.M.; Stunkard, A.J.; Alavi, A. Effect of the Garren-Edwards gastric bubble on gastric emptying. J. Nucl. Med. 1989, 30, 692–696. [Google Scholar]
- Barrichello, S.; Badurdeen, D.; Hedjoudje, A.; Neto, M.G.; Yance, R.; Veinert, A.; Fayad, L.; Simsek, C.; Grecco, E.; De Souza, T.F.; et al. The Effect of the Intra-gastric Balloon on Gastric Emptying and the DeMeester Score. Obes. Surg. 2019, 30, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, J.; Hedenström, H.; Karlsson, F.A.; Edén-Engström, B.; Sundbom, M. Gastric Emptying and Postprandial PYY Response After Biliopancreatic Diversion with Duodenal Switch. Obes. Surg. 2010, 21, 609–615. [Google Scholar] [CrossRef]
- De Jong, J.R.; Van Ramshorst, B.; Gooszen, H.G.; Smout, A.J.P.M.; Buul, M.M.C.T.-V. Weight Loss after Laparoscopic Adjustable Gastric Banding is not Caused by Altered Gastric Emptying. Obes. Surg. 2008, 19, 287–292. [Google Scholar] [CrossRef]
- Näslund, I.; Beckman, K.-W. Gastric Emptying Rate after Gastric Bypass and Gastroplasty. Scand. J. Gastroenterol. 1987, 22, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Melissas, J.; Koukouraki, S.; Askoxylakis, J.; Stathaki, M.; Daskalakis, M.; Perisinakis, K.; Karkavitsas, N. Sleeve gastrectomy—A restrictive procedure? Obes. Surg. 2007, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Bernstine, H.; Tzioni-Yehoshua, R.; Groshar, D.; Beglaibter, N.; Shikora, S.; Rosenthal, R.J.; Rubin, M. Gastric Emptying is not Affected by Sleeve Gastrectomy—Scintigraphic Evaluation of Gastric Emptying after Sleeve Gastrectomy without Removal of the Gastric Antrum. Obes. Surg. 2008, 19, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Braghetto, I.; DaVanzo, C.; Korn, O.; Csendes, A.; Valladares, H.; Herrera, E.; Gonzalez, P.; Papapietro, K. Scintigraphic Evaluation of Gastric Emptying in Obese Patients Submitted to Sleeve Gastrectomy Compared to Normal Subjects. Obes. Surg. 2009, 19, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Michalsky, D.; Dvorak, P.; Belacek, J.; Kasalicky, M. Radical Resection of the Pyloric Antrum and Its Effect on Gastric Emptying After Sleeve Gastrectomy. Obes. Surg. 2013, 23, 567–573. [Google Scholar] [CrossRef]
- Pilone, V.; Tramontano, S.; Di Micco, R.; Monda, A.; Hasani, A.; Izzo, G.; Vitiello, A.; Caprio, M.; Cuocolo, A.; Forestieri, P. Gastric emptying after sleeve gastrectomy: Statistical evidence of a controlled prospective study with gastric scintigraphy (180 visite). Minerva Chir. 2013, 68, 385–392. [Google Scholar] [PubMed]
- Melissas, J.; Leventi, A.; Klinaki, I.; Perisinakis, K.; Koukouraki, S.; de Bree, E.; Karkavitsas, N. Alterations of global gastrointestinal motility after sleeve gastrectomy: A prospective study. Ann. Surg. 2013, 258, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, A.A.; Sarhan, M.D.; Hegazy, T.; Mahmoud, M.M.; Ali, M.H. Comparative assessment of gastric emptying in obese patients before and after laparoscopic sleeve gastrectomy using radionuclide scintigraphy. Nucl. Med. Commun. 2015, 36, 854–862. [Google Scholar] [CrossRef]
- Burgerhart, J.S.; Van Rutte, P.W.J.; Edelbroek, M.A.L.; Wyndaele, D.N.J.; Smulders, J.F.; Van De Meeberg, P.C.; Siersema, P.D.; Smout, A.J.P.M. Association Between Postprandial Symptoms and Gastric Emptying After Sleeve Gastrectomy. Obes. Surg. 2014, 25, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, B.; Wahal, A.; Aggarwal, S.; Priyadarshini, P.; Bhattacharjee, H.; Khadgawat, R.; Yadav, R. Impact of Sleeve Gastrectomy on Type 2 Diabetes Mellitus, Gastric Emptying Time, Glucagon-Like Peptide 1 (GLP-1), Ghrelin and Leptin in Non-morbidly Obese Subjects with BMI 30–35.0 kg/m2: A Prospective Study. Obes. Surg. 2016, 26, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Vives, M.; Molina, A.; Danús, M.; Rebenaque, E.; Blanco, S.; París, M.; Sánchez, A.; Sabench, F.; Del Castillo, D. Analysis of Gastric Physiology After Laparoscopic Sleeve Gastrectomy (LSG) With or Without Antral Preservation in Relation to Metabolic Response: A Randomised Study. Obes. Surg. 2017, 27, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Sista, F.; Abruzzese, V.; Clementi, M.; Carandina, S.; Cecilia, M.; Amicucci, G. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: A prospective study. Surg. Obes. Relat. Dis. 2017, 13, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-J.; Cheng, M.-F.; Yang, W.-S.; Tsai, M.-S.; Lee, P.-C.; Chen, C.-N.; Lin, M.-T.; Tseng, P.-H. A Higher Preoperative Glycemic Profile Is Associated with Rapid Gastric Emptying After Sleeve Gastrectomy for Obese Subjects. Obes. Surg. 2018, 29, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Jin, L.; Wang, W.; Zeng, N.; Wang, L.; Zhao, K.; Xu, W.; Zhang, Z.; Yang, J. Alterations of Gastric Emptying Features Following Laparoscopic Sleeve Gastrectomy in Chinese Patients with Obesity: A Self-Controlled Observational Study. Obes. Surg. 2018, 29, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.A.; Mikhail, H.M.S.; Abdelsalam, A.; Abdallah, A.; Elshafey, H.E.; Abouelregal, T.E.; Omar, M.G.; ElKassar, H.; Ahmed, R.A.; Atallah, M.; et al. Acceleration of Gastric Emptying and Improvement of GERD Outcome After Laparoscopic Sleeve Gastrectomy in Non-diabetic Obese Patients. Obes. Surg. 2020, 30, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Johari, Y.; Wickremasinghe, A.; Kiswandono, P.; Yue, H.; Ooi, G.; Laurie, C.; Hebbard, G.; Beech, P.; Yap, K.; Brown, W.; et al. Mechanisms of Esophageal and Gastric Transit Following Sleeve Gastrectomy. Obes. Surg. 2021, 31, 725–737. [Google Scholar] [CrossRef]
- Svane, M.S.; Bojsen-Møller, K.N.; Martinussen, C.; Dirksen, C.; Madsen, J.L.; Reitelseder, S.; Holm, L.; Rehfeld, J.F.; Kristiansen, V.B.; van Hall, G.; et al. Postprandial Nutrient Handling and Gastrointestinal Hormone Secretion After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology 2019, 156, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, C.; Damgaard, M.; Bojsen-Møller, K.; Jørgensen, N.; Kielgast, U.; Jacobsen, S.; Naver, L.; Worm, D.; Holst, J.J.; Madsbad, S. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol. Motil. 2013, 25, 346-e255. [Google Scholar] [CrossRef]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Peters, T.; Beglinger, C.; Peterli, R. Incretin effects, gastric emptying and insulin responses to low oral glucose loads in patients after gastric bypass and lean and obese controls. Surg. Obes. Relat. Dis. 2016, 12, 1320–1327. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Method | Equipment Required | Principle |
|---|---|---|
| Scintigraphy | External gamma camera and isotope-labeled meal | Calculating volume content after ingestion of a isotope-labeled meal, with images obtained with a gamma camera at baseline 1, 2, and 4 h. |
| Ultrasonography | Ultrasound scanners | Measurement of changes in antral cross-sectional area or diameter over time. |
| Magnetic Resonance | MRI scanner | Measurement of gastric volume, secretion, emptying, and contractions derived from repetitive scans. Gastric meal volume is calculated by taking into account gastric secretion. |
| Isotope breath test | Breath collection vials and stable isotope-labeled meal | Measurement of breath excretion of 13CO2 after ingestion with a solid meal. After ingestion, it is absorbed in the proximal small intestine, metabolized by the liver, and excreted by the lungs, and results in a rise in expired 13CO2. |
| Drug Absorption | Plasma levels of paracetamol | Measurement of plasma concentrations, assuming that small intestine intestine absorption will reflect the gastric emptying rate. |
| Wireless pressure and pH capsule | Intraluminal capsule with miniaturized strain gauge and pH measurement. | The simultaneous intragastric measurement of pH and pressure is used to evaluate gastric emptying. |
| Barostat | Barostatically-controlled balloons. | A polyethylene balloon is inserted via the esophagus, situated in the gastric fundus in apposition with the wall, and distended until an intrabag volume of 30 mL is achieved or until respiratory variation is detected. The volume is calculated based on the changes in pressure and diameter. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuentes, L.; Camilleri, M.; Acosta, A. Gastric Sensory and Motor Functions and Energy Intake in Health and Obesity—Therapeutic Implications. Nutrients 2021, 13, 1158. https://doi.org/10.3390/nu13041158
Cifuentes L, Camilleri M, Acosta A. Gastric Sensory and Motor Functions and Energy Intake in Health and Obesity—Therapeutic Implications. Nutrients. 2021; 13(4):1158. https://doi.org/10.3390/nu13041158
Chicago/Turabian StyleCifuentes, Lizeth, Michael Camilleri, and Andres Acosta. 2021. "Gastric Sensory and Motor Functions and Energy Intake in Health and Obesity—Therapeutic Implications" Nutrients 13, no. 4: 1158. https://doi.org/10.3390/nu13041158
APA StyleCifuentes, L., Camilleri, M., & Acosta, A. (2021). Gastric Sensory and Motor Functions and Energy Intake in Health and Obesity—Therapeutic Implications. Nutrients, 13(4), 1158. https://doi.org/10.3390/nu13041158