Change in Exercise Performance and Markers of Acute Kidney Injury Following Heat Acclimation with Permissive Dehydration
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
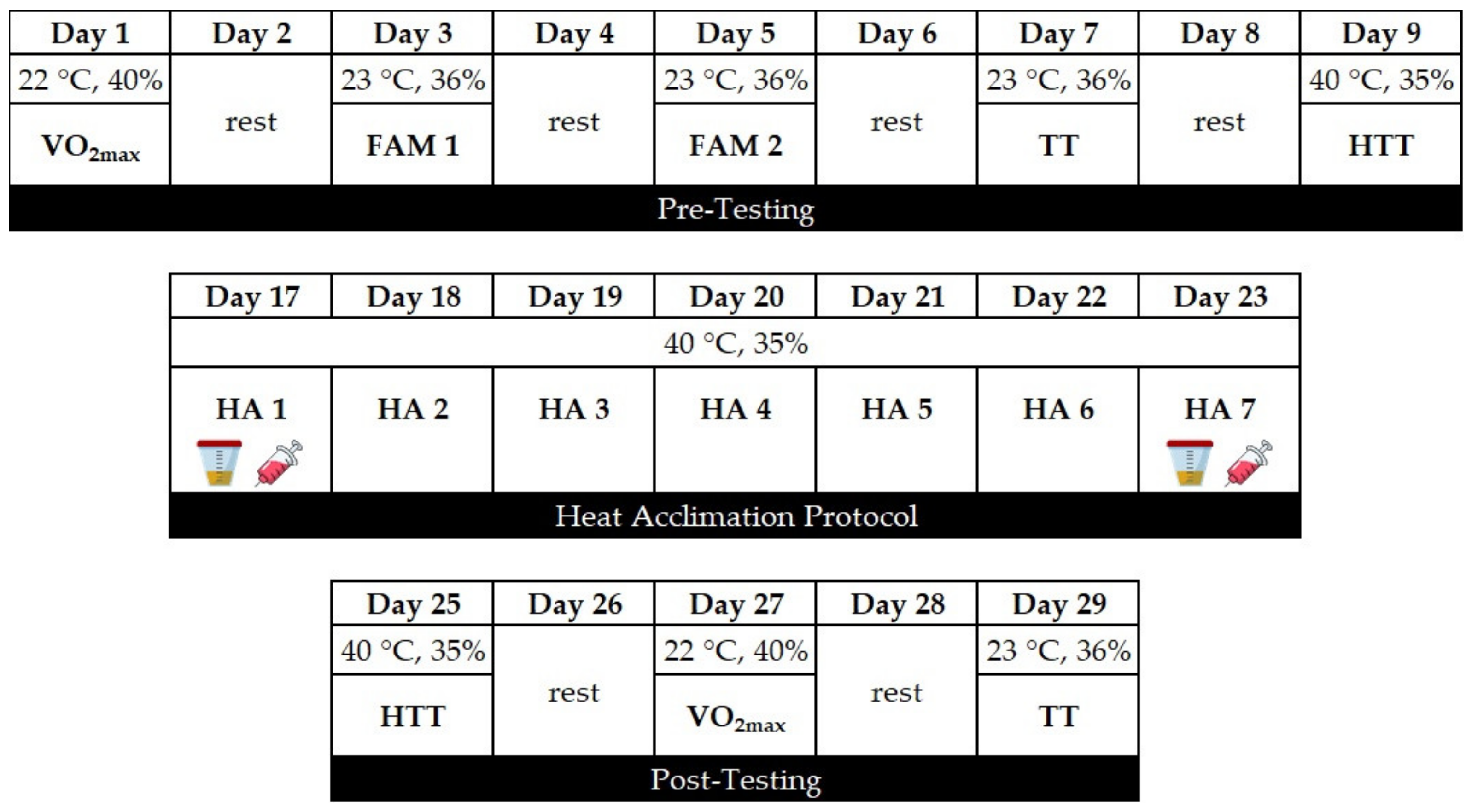
2.2. Experimental Design
2.3. Experimental Procedures
2.3.1. Maximal Oxygen Consumption
2.3.2. Cycling Time-Trial
2.3.3. Heat Tolerance Test
2.3.4. Heat Acclimation Protocol
2.3.5. Blood Sampling
2.3.6. Assessment of Hydration Level and Markers of Kidney Function
2.4. Statistical Analyses
3. Results
3.1. Heat Acclimation
3.2. Heat Tolerance
3.3. Changes in VO2max and Time-Trial Performance in Response to Heat Acclimation
3.4. Markers of Dehydration and Kidney Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wenger, C.B. Human heat adaptation to hot environments. In Medical Aspects of Harsh Environments; Pandolf, K.B., Burr, R.E., Eds.; Office of the Surgeon General, Department of the Army: Washington, DC, USA, 2001; Volume 1, pp. 51–86. [Google Scholar]
- Sawka, M.N.; Wenger, C.B.; Pandolf, K.B. Thermoregulatory responses to acute exercise-heat stress and heat acclimation. In Handbook of Physiology; Sec. 4, Environmental Physiology; Fregly, M.J., Blatteis, C.M., Eds.; Oxford University Press: New York, NY, USA, 1996; pp. 157–185. [Google Scholar]
- Nunneley, S.A.; Reardon, M.J. Prevention of heat illness. In Medical Aspects of Harsh Environments; Pandolf, K.B., Burr, R.E., Eds.; Office of the Surgeon General, Department of the Army: Washington, DC, USA, 2001; Volume 1, pp. 209–230. [Google Scholar]
- Minson, C.T.; Cotter, J.D. CrossTalk proposal: Heat acclimatization does improve performance in a cool condition. J. Physiol. 2015, 594, 241. [Google Scholar] [CrossRef]
- Nybo, L.; Lundby, C. Rebuttal by Lars Nybo and Carsten Lundby. J. Physiol. 2015, 594, 251. [Google Scholar] [CrossRef] [PubMed]
- Garrett, A.T.; Goosens, N.G.; Rehrer, N.J.; Patterson, M.J.; Harrison, J.; Sammut, I.; Cotter, J.D. Short-term heat acclimation is effective and may be enhanced rather than impaired by dehydration. Am. J. Hum. Biol. 2014, 26, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, S.; Halliwill, J.R.; Sawka, M.N.; Minson, C.T. Heat acclimation improves exercise performance. J. Appl. Physiol. 2010, 109, 1140–1147. [Google Scholar] [CrossRef]
- Moseley, P.L. Heat shock proteins and heat adaptation of the whole organism. J. Appl. Physiol. 1997, 83, 1314–1417. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.N.; Bayne, F.M.; Castelli, F.; Naughton, M.R.; Reeve, T.C.; Trangmar, S.J.; Mackenzie, R.W.A.; Tyler, C.J. Short-term isothermic heat acclimation elicits beneficial adaptations but medium-term elicits a more complete adaptation. Eur. J. Appl. Physiol. 2019, 120, 243–254. [Google Scholar] [CrossRef]
- Garrett, A.T.; Goosens, N.G.; Rehrer, N.J.; Patterson, M.J.; Cotter, J.D. Induction and decay of short-term heat acclimation. Eur. J. Appl. Physiol. 2009, 107, 659–670. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.P.; Hasday, J.D.; He, J.R.; Montain, S.J.; Cheuvront, S.N.; Sawka, M.N.; Singh, I.S. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, 185–191. [Google Scholar] [CrossRef]
- Cheung, S.S.; McLellan, T.M. Influence of hydration status and fluid replacement on heat tolerance while wearing NBC protective clothing. Eur. J. Appl. Occup. Physiol. 1998, 77, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.A.; Massey, H.C.; Tipton, M.J.; Young, J.S.; Corbett, J. Effect of permissive dehydration on induction and decay of heat acclimation, and temperate exercise performance. Front. Physiol. 2016, 7, 564. [Google Scholar] [CrossRef]
- Pandolf, K.B. Time course of heat acclimation and its decay. Int. J. Sports Med. 1998, 19, S157–S160. [Google Scholar] [CrossRef]
- Sawka, M.N.; Pandolf, K.B. Physical exercise in hot climates: Physiology, performance, and biomedical issues. In Medical Aspects of Harsh Environments; Pandolf, K.B., Burr, R.E., Eds.; Office of the Surgeon General, Department of the Army: Washington, DC, USA, 2001; Volume 1, pp. 87–133. [Google Scholar]
- Periard, J.D.; Racinais, S.; Sawka, M.N. Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand. J. Med. Sci. Sports 2015, 25, 20–38. [Google Scholar] [CrossRef]
- Yamada, P.M.; Amorim, F.T.; Moseley, P.; Robergs, R.; Schneider, S.M. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J. Appl. Physiol. 2007, 103, 1196–1204. [Google Scholar] [CrossRef]
- Poirier, M.P.; Gagnon, D.; Friesen, B.J.; Hardcastle, S.G.; Kenny, G.P. Whole-body heat exchange during heat acclimation and its decay. Med. Sci. Sports Exerc. 2015, 47, 390–400. [Google Scholar] [CrossRef]
- White, A.C.; Salgado, R.M.; Astorino, T.A.; Loeppky, J.A.; Schneider, S.M.; McCormick, J.J.; McLain, T.A.; Kravitz, L.; Mermier, C.M. The effect of ten days of heat acclimation on exercise performance at acute hypobaric hypoxia (4350 m). Temperature 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Neal, R.A.; Corbett, J.; Massey, H.C.; Tipton, M.J. Effect of short-term heat acclimation with permissive dehydration on thermoregulation and temperate exercise performance. Scand. J. Med. Sci. Sports 2016, 26, 875–884. [Google Scholar] [CrossRef]
- Akerman, A.P.; Tipton, M.; Minson, C.T.; Cotter, J.D. Heat stress and dehydration in adapting for performance—Good, bad, both, or neither. Temperature 2016, 3, 1–25. [Google Scholar] [CrossRef]
- Merry, T.L.; Ainslie, P.N.; Cotter, J.D. Effects of aerobic fitness on hypohydration-induced physiological strain and exercise impairment. Acta. Physiol. 2010, 198, 179–190. [Google Scholar] [CrossRef]
- Taylor, N.A.S.; Cotter, J. Heat adaptation: Guidelines for the optimization of human performance. Int. Sport Med. J. 2006, 7, 33–57. [Google Scholar]
- Garrett, A.T.; Creasy, R.; Rehrer, N.J.; Patterson, M.J.; Cotter, J.D. Effectiveness of short-term heat acclimation for highly trained athletes. Eur. J. Appl. Physiol. 2012, 112, 1827–1837. [Google Scholar] [CrossRef]
- Wesseling, C.; Crowe, J.; Hogstedt, C.; Jakobsson, K.; Lucas, R.; Wegman, D.H. Resolving the enigma of the mesoamerican nephropathy: A research workshop summary. First International Research Workshop on the Mesoamerican Nephropathy. Am. J. Kidney Dis. Mar. 2014, 63, 396–404. [Google Scholar] [CrossRef]
- Chapman, C.L.; Johnson, B.D.; Sackett, B.D.; Parker, M.D.; Schlader, Z.J. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, 189. [Google Scholar] [CrossRef] [PubMed]
- Divine, J.G.; Clark, J.F.; Colosimo, A.J.; Donaworth, M.; Hasselfeld, K.; Himmler, A.; Rauch, J.; Mangine, R. American football players in preseason training at risk of acute kidney injury without signs of rhabdomyolysis. Clin. J. Sport Med. 2018, 30, 556–561. [Google Scholar] [CrossRef]
- Pryor, R.R.; Pryor, L.J.; Vandermark, L.W.; Adams, E.L.; Brodeur, R.M.; Schlader, Z.J.; Armstrong, L.E.; Lee, E.C.; Maresh, C.M.; Casa, D.J. Acute kidney injury biomarker responses to short-term heat acclimation. Int. J. Environ. Res. Public Heath 2020, 17, 1325. [Google Scholar] [CrossRef]
- Schlader, Z.J.; Chapman, C.L.; Sarker, S.; Russo, L.; Rideout, T.C.; Parker, M.D.; Johnson, B.D.; Hostler, D. Firefighter work duration influences the extent of acute kidney injury. Med. Sci. Sport Exerc. 2017, 49, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Hvidberg, V.; Jacobsen, C.; Strong, R.K.; Cowland, J.B.; Moestrup, S.K.; Borregaard, N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005, 579, 773–779. [Google Scholar] [CrossRef]
- Jouffroy, R.; Lebreton, X.; Mansencal, N.; Anglicheau, D. Acute kidney injury during an ultra-endurance race. PLoS ONE 2019, 14, e222544. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.G.; Verma, G.; Pata, R.W.; Martin, T.G.; Perazella, M.A.; Parikh, C.R. Kidney injury and repair biomarkers in marathon runners. Am. J. Kidney Dis. 2017, 70, 252–261. [Google Scholar] [CrossRef]
- Lei, L.; Li, L.P.; Zeng, Z.; Mu, J.X.; Yang, X.; Zhou, C.; Wang, Z.L.; Zhang, H. Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci. Rep. 2018, 8, 7962. [Google Scholar] [CrossRef]
- Schlader, Z.; Hostler, D.; Parker, M.D.; Pryor, R.R.; Lohr, J.W.; Johnson, B.D.; Chapman, C.L. The potential for renal injury elicited by physical work in the heat. Nutrients 2019, 11, 2087. [Google Scholar] [CrossRef]
- Schrier, R.W.; Hano, J.; Keller, H.I.; Finkel, R.M.; Gilliland, P.F.; Cirksena, W.J.; Teschan, P.E. Renal, metabolic, and circulatory responses to heat and exercise: Studies in military recruits during summer training, with implications for acute renal failure. Ann. Int. Med. 1970, 73, 213–223. [Google Scholar] [CrossRef]
- Omassoli, J.; Hill, N.E.; Woods, D.R.; Delves, S.K.; Fallowfield, J.L.; Brett, S.J.; Wilson, D.; Corbett, R.W.; Allsopp, A.J.; Stacey, M.J. Variation in renal responses to exercise in the heat with progressive acclimatisation. J. Sci. Med. Sport 2019, 22, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sport Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Astorino, T.A.; White, A.C.; Dalleck, L.C. Supramaximal testing to confirm attainment of VO2max in sedentary men and women. Int. J. Sports Med. 2009, 30, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Dalleck, L.C.; Astorino, T.A.; Erickson, R.M.; McCarthy, C.M.; Beadell, A.A.; Botten, B.H. Suitability of verification testing to confirm attainment of VO2max in middle-aged and older adults. Res. Sports Med. 2012, 20, 118–128. [Google Scholar] [CrossRef]
- Scharhag-Rosenberger, F.; Carlsohn, A.; Cassel, M.; Mayer, F.; Scharhag, J. How to test maximal oxygen uptake: A study on timing and testing procedure of a supramaximal verification test. Appl. Physiol. Nutr. Metab. 2011, 36, 153–160. [Google Scholar] [CrossRef]
- Westgarth-Taylor, C.; Hawley, J.A.; Rickard, S.; Myburgh, K.H.; Noakes, T.D.; Dennis, S.C. Metabolic and performance adaptations to interval training in endurance-trained cyclists. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 298–304. [Google Scholar] [CrossRef]
- Astorino, T.A.; Cottrell, T.; Talhami Lozano, A.; Aburto-Pratt, K.; Duhon, J. Ergogenic effects of caffeine on simulated time-trial performance are independent of fitness level. J. Caffeine Res. 2011, 1, 179–185. [Google Scholar] [CrossRef]
- Dill, D.B.; Costill, D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974, 37, 247–248. [Google Scholar] [CrossRef]
- Racinais, S.; Periard, J.D.; Karlsen, A.; Nybo, L. Effect of heat acclimatization on cycling time trial performance and pacing. Med. Sci. Sports Exerc. 2015, 47, 601–606. [Google Scholar] [CrossRef]
- Costello, J.T.; Rendell, R.A.; Furber, M.; Massey, H.C.; Tipton, M.J.; Young, J.S.; Corbett, J. Effects of acute or chronic heat exposure, exercise and dehydration on plasma cortisol, IL-6 and CRP levels in trained males. Cytokine 2018, 110, 277–283. [Google Scholar] [CrossRef]
- McConell, G.K.; Burge, C.M.; Skinner, S.L.; Hargreaves, M. Influence of ingested fluid volume on physiological responses during prolonged exercise. Acta Physiol. Scand. 1997, 160, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Pethick, W.A.; Murray, H.J.; McFadyen, P.; Brodie, R.; Gaul, C.A.; Stellingwerff, T. Effects of hydration status during heat acclimation on plasma volume and performance. Scand. J. Med. Sci. Sports 2019, 29, 189–199. [Google Scholar] [CrossRef]
- Travers, G.; Nichols, D.; Riding, N.; Gonzalez-Alonso, J.; Periard, J.D. Heat acclimation with controlled heart rate: Influence of hydration status. Med. Sci. Sports Exerc. 2020, 52, 1815–1824. [Google Scholar] [CrossRef]
- Merry, T.L.; Ainslie, P.N.; Walker, R.; Cotter, J.D. Fitness alters regulatory but not behavioural responses to hypohydrated exercise. Physiol. Behav. 2008, 95, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Nybo, L.; Norgaard, S.J.; Jensen, M.V.; Bonne, T.; Racinais, S. Time course of natural heat acclimatization in well-trained cyclists during a 2-week training camp in the heat. Scand. J. Med. Sci. Sports 2015, 25, 240–249. [Google Scholar] [CrossRef]
- Keiser, S.; Fluck, D.; Huppin, F.; Stravs, A.; Hilty, M.P.; Lundby, C. Heat training increases exercise capacity in hot but not in temperate conditions: A mechanistic counter-balanced cross-over study. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Costill, D.L.; Fink, W.J. Changes in body water and electrolytes during heat acclimation: Effects of dietary sodium. Aviat. Space Environ. Med. 1987, 58, 143–148. [Google Scholar]
- Nielsen, B.; Strange, S.; Christensen, N.J.; Warberg, J.; Saltin, B. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflug. Arch. 1997, 434, 49–56. [Google Scholar] [CrossRef]
- Senay, L.C.; Mitchell, D.; Wyndham, C.H. Acclimatization in a hot, humid environment: Body fluid adjustments. J. Appl. Physiol. 1976, 40, 786–796. [Google Scholar] [CrossRef]
- Wyndham, C.H.; Benade, A.J.; Williams, C.G.; Strydom, N.B.; Goldin, A.; Heyns, A.J. Changes in central circulation and body fluid spaces during acclimatization to heat. J. Appl. Physiol. 1968, 25, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, S.; Minson, C.T. Heat acclimation improves cutaneous vascular function and sweating in trained cyclists. J. Appl. Physiol. 2010, 109, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Sawka, M.N.; Levine, L.; Cadarette, B.S.; Pandolf, K.B. Skeletal muscle metabolism during exercise is influenced by heat acclimation. J. Appl. Physiol. 1985, 59, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Brooks, G.A. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J. Appl. Physiol. 2012, 112, 354–361. [Google Scholar] [CrossRef]
- McCullough, P.A.; Chinnaiyan, K.M.; Gallagher, M.J.; Colar, J.M.; Geddes, T.; Gold, J.M.; Trivax, J.E. Changes in renal markers and acute kidney injury after marathon running. Nephrology 2011, 16, 194–199. [Google Scholar] [CrossRef]

| Group | Age (yr) | Body Height (cm) | Body Weight (kg) | VO2max (mL × kg−1 × min−1) | VO2max (L × min−1) |
|---|---|---|---|---|---|
| EUH | 25 ± 2 | 174.3 ± 3.8 | 77.9 ± 6.7 | 52.9 ± 7.1 | 4.2 ± 0.9 |
| DEH | 26 ± 7 | 172.4 ± 8.8 | 74.1 ± 11.2 | 50.3 ± 8.5 | 3.7 ± 0.3 |
| p-value | 0.813 | 0.612 | 0.453 | 0.554 | 0.213 |
| Parameter | EUH | DEH | ||
|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | |
| Resting HR (b × min−1) | 59 ± 9 | 54 ± 10 | 58 ± 7 | 53 ± 6 |
| Resting Tc (°C) | 37.2 ± 0.2 | 37.0 ± 0.2 | 37.0 ± 0.3 | 36.9 ± 0.3 |
| BW loss (%) | −0.6 ± 0.3 | −0.8 ± 0.4 | −2.2 ± 0.8 | −2.6 ± 0.5 |
| Sweat rate (L × h−1) | 1.78 ± 0.18 | 2.01 ± 0.28 | 1.59 ± 0.27 | 1.72 ± 0.28 |
| Average HR (b × min−1) | 150 ± 15 | 148 ± 18 | 150 ± 7 | 145 ± 5 |
| Tc final 60 (°C) | 38.7 ± 0.2 | 38.6 ± 0.1 | 38.7 ± 0.2 | 38.6 ± 0.1 |
| Time to 38.5 °C (min) | 30 ± 5.1 | 34 ± 5.4 | 29 ± 9.4 | 40 ± 12.8 |
| Hct (%) | 46.7 ± 0.9 | 43.3 ± 2.7 | 44.6 ± 3.1 | 42.6 ± 2.9 |
| Hb (g/dL) | 15.8 ± 0.6 | 14.5 ± 0.9 | 14.9 ± 0.8 | 14.2 ± 0.8 |
| PV expansion (%) | 16.5 ± 12.0 | 9.2 ± 4.0 | ||
| Parameter | EUH | DEH | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| HTTend HR (b × min−1) | 163 ± 13 | 152 ± 14 | 166 ± 9 | 143 ± 9 † |
| HTTend Tc (°C) | 38.7 ± 0.4 | 38.4 ± 0.3 | 38.8 ± 0.4 | 38.3 ± 0.3 |
| HTT sweat rate (L × h−1) | 1.38 ± 0.11 | 1.47 ± 0.22 | 1.06 ± 0.25 | 1.11 ± 0.42 |
| Parameter | EUH | DEH | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| VO2max (mL × kg−1 × min−1) | 52.9 ± 7.1 | 54.8 ± 6.2 | 50.3 ± 8.5 | 51.1 ± 8.2 |
| VO2max (L × min−1) | 4.2 ± 1.0 | 4.3 ± 0.9 | 3.7 ± 0.3 | 3.7 ± 0.3 |
| HRmax (b × min−1) | 186 ± 9 | 183 ± 6 | 184 ± 8 | 178 ± 8 |
| VO2max PPO (W) | 351 ± 24 | 372 ± 29 | 332 ± 25 | 348 ± 36 |
| TT time (s) | 1692.9 ± 57.8 | 1645.4 ± 53.7 | 1777.3 ± 63.7 | 1718.3 ± 51.2 |
| TT average PO (W) | 217 ± 21 | 233 ± 20 | 191 ± 16 | 208 ± 15 |
| TT end HR (b × min−1) | 185 ± 9 | 183 ± 10 | 180 ± 13 | 181 ± 9 |
| TT average RPE | 15 ± 1 | 16 ± 1 | 14 ± 1 | 15 ± 1 |
| Parameter | EUH | EUH | ||
| Day 1 | Day 7 | |||
| Pre | Post | Pre | Post | |
| USG (g/mL) | 1.007 ± 0.003 | 1.009 ± 0.004 | 1.005 ± 0.003 | 1.014 ± 0.007 |
| Plasma osmolality (mOsm/kg) | 300 ± 4 | 299 ± 2 | 298 ± 6 | 300 ± 3 † |
| Parameter | DEH | DEH | ||
| Day 1 | Day 7 | |||
| Pre | Post | Pre | Post | |
| USG (g/mL) | 1.006 ± 0.005 | 1.012 ± 0.008 | 1.006 ± 0.005 | 1.009 ± 0.007 |
| Plasma osmolality (mOsm/kg) | 301 ± 5 | 307 ± 6 | 301 ± 4 | 308 ± 3 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haroutounian, A.; Amorim, F.T.; Astorino, T.A.; Khodiguian, N.; Curtiss, K.M.; Matthews, A.R.D.; Estrada, M.J.; Fennel, Z.; McKenna, Z.; Nava, R.; et al. Change in Exercise Performance and Markers of Acute Kidney Injury Following Heat Acclimation with Permissive Dehydration. Nutrients 2021, 13, 841. https://doi.org/10.3390/nu13030841
Haroutounian A, Amorim FT, Astorino TA, Khodiguian N, Curtiss KM, Matthews ARD, Estrada MJ, Fennel Z, McKenna Z, Nava R, et al. Change in Exercise Performance and Markers of Acute Kidney Injury Following Heat Acclimation with Permissive Dehydration. Nutrients. 2021; 13(3):841. https://doi.org/10.3390/nu13030841
Chicago/Turabian StyleHaroutounian, Arpie, Fabiano T. Amorim, Todd A. Astorino, Nazareth Khodiguian, Katharine M. Curtiss, Aaron R. D. Matthews, Michael J. Estrada, Zachary Fennel, Zachary McKenna, Roberto Nava, and et al. 2021. "Change in Exercise Performance and Markers of Acute Kidney Injury Following Heat Acclimation with Permissive Dehydration" Nutrients 13, no. 3: 841. https://doi.org/10.3390/nu13030841
APA StyleHaroutounian, A., Amorim, F. T., Astorino, T. A., Khodiguian, N., Curtiss, K. M., Matthews, A. R. D., Estrada, M. J., Fennel, Z., McKenna, Z., Nava, R., & Sheard, A. C. (2021). Change in Exercise Performance and Markers of Acute Kidney Injury Following Heat Acclimation with Permissive Dehydration. Nutrients, 13(3), 841. https://doi.org/10.3390/nu13030841







