Free Fatty Acids Signature in Human Intestinal Disorders: Significant Association between Butyric Acid and Celiac Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Enrolment
2.2. FFAs Determination by GC–MS Analysis
2.3. Sample Preparation
2.4. Statistical Analysis
3. Results
3.1. Descriptive Analysis of Serum FFA Distributions
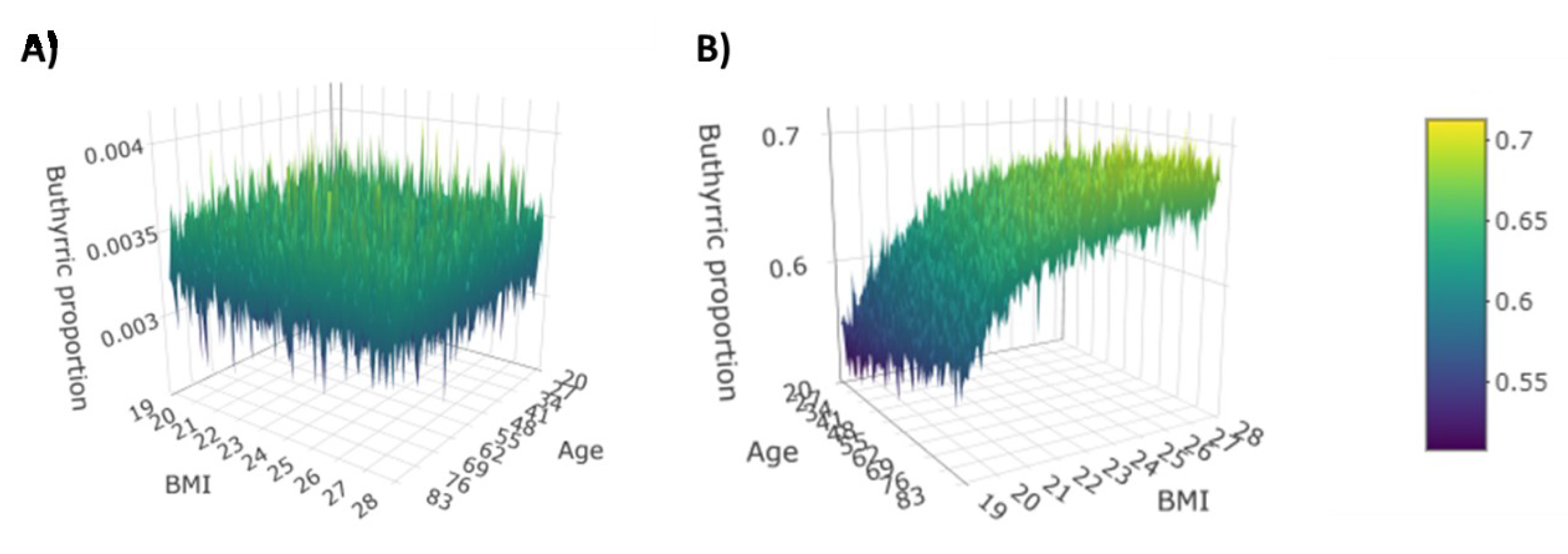
3.2. Dirichlet-Multinomial Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shores, D.R.; Binion, D.G.; Freeman, B.A.; Baker, P.R. New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 2192–2204. [Google Scholar] [CrossRef]
- Papackova, Z.; Cahova, M. Fatty acid signaling: The new function of intracellular lipases. Int. J. Mol. Sci. 2015, 16, 3831–3855. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Sieber, J.; Jehle, A.W. Free Fatty acids and their metabolism affect function and survival of podocytes. Front. Endocrinol. (Lausanne) 2014, 5, 186. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Goverse, G.; Molenaar, R.; Macia, L.; Tan, J.; Erkelens, M.N.; Konijn, T.; Knippenberg, M.; Cook, E.C.; Hanekamp, D.; Veldhoen, M.; et al. Diet-Derived Short Chain Fatty Acids Stimulate Intestinal Epithelial Cells to Induce Mucosal Tolerogenic Dendritic Cells. J. Immunol. 2017, 198, 2172–2181. [Google Scholar] [CrossRef]
- Kaur, H.; Golovko, S.; Golovko, M.Y.; Singh, S.; Darland, D.C.; Combs, C.K. Effects of Probiotic Supplementation on Short Chain Fatty Acids in the AppNL-G-F Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2020, 76, 1083–1102. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowicz, M.L.; Ma’ayan, A. GPR84: An immune response dial? Nat. Rev. Drug Discov. 2020, 19, 374. [Google Scholar] [CrossRef] [PubMed]
- Pinkosky, S.L.; Scott, J.W.; Desjardins, E.M.; Smith, B.K.; Day, E.A.; Ford, R.J.; Langendorf, C.G.; Ling, N.X.Y.; Nero, T.L.; Loh, K.; et al. Long-chain fatty acyl-CoA esters regulate metabolism via allosteric control of AMPK β1 isoforms. Nat. Metab. 2020, 2, 873–881. [Google Scholar] [CrossRef]
- Scoville, E.A.; Allaman, M.M.; Adams, D.W.; Motley, A.K.; Peyton, S.C.; Ferguson, S.L.; Horst, S.N.; Williams, C.S.; Beaulieu, D.B.; Schwartz, D.A.; et al. Serum Polyunsaturated Fatty Acids Correlate with Serum Cytokines and Clinical Disease Activity in Crohn’s Disease. Sci. Rep. 2019, 9, 2882. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragoulakis, V.; Sarandi, E.; Docea, A.O.; Papakonstaninou, E.; Tsilimidos, G.; Anamaterou, C.; Fragkiadaki, P.; Aschner, M.; Tsatsakis, A.; et al. Targeted Metabolomic Analysis of Serum Fatty Acids for the Prediction of Autoimmune Diseases. Front. Mol. BioSci. 2019, 6, 120. [Google Scholar] [CrossRef]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aljada, A.; Dandona, P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Rydén, M. Fatty Acids, Obesity and Insulin Resistance. Obes. Facts 2015, 8, 147–155. [Google Scholar] [CrossRef]
- Tian, Z.; Zhuang, X.; Luo, M.; Yin, W.; Xiong, L. The propionic acid and butyric acid in serum but not in feces are increased in patients with diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2020, 20, 73. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, Y.; Zhang, T.; He, J.; Luo, X.; Bian, X.; Wu, J.; Zou, C.; Wang, Y.; Fu, L. Identifying potential serum biomarkers of breast cancer through targeted free fatty acid profiles screening based on a GC-MS platform. Biomed. Chromatogr. 2020, 34, e4922. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Benson, J.; Ma, K.N.; Schaffer, D.; Potter, J.D. Trans-fatty acids and colon cancer. Nutr. Cancer 2001, 39, 170. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroenterol. 2019, 25, 5543–5558. [Google Scholar] [CrossRef]
- Pedone, M.; Stingo, F.C. Subject-Specific Bayesian Hierarchical Model for Compositional Data Analysis. In Book of Short Papers SIS 2020; Pearson Italia: Milano, Italy, 2020. [Google Scholar]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.C.; et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy 2019, 74, 1429–1444. [Google Scholar] [CrossRef]
- Djoussé, L.; Benkeser, D.; Arnold, A.; Kizer, J.R.; Zieman, S.J.; Lemaitre, R.N.; Tracy, R.P.; Gottdiener, J.S.; Mozaffarian, D.; Siscovick, D.S.; et al. Plasma free fatty acids and risk of heart failure: The Cardiovascular Health Study. Circ. Heart Fail. 2013, 6, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; He, J.; Lv, J.; Pan, R.; Lv, T. A high serum-free fatty acid level is associated with cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 705–710. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]
- Ukolov, A.I.; Orlova, T.I.; Savel’Eva, E.I.; Radilov, A.S. Chromatographic–mass spectrometric determination of free fatty acids in blood plasma and urine using extractive alkylation. J. Anal. Chem. 2015, 70, 1123–1130. [Google Scholar] [CrossRef]
- Neyraud, E.; Cabaret, S.; Brignot, H.; Chabanet, C.; Labouré, H.; Guichard, E.; Berdeaux, O. The basal free fatty acid concentration in human saliva is related to salivary lipolytic activity. Sci. Rep. 2017, 7, 5969. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.F.; Hermansson, M.; Liebisch, G.; Hodson, L.; Ejsing, C.S. Total Fatty Acid Analysis of Human Blood Samples in One Minute by High-Resolution Mass Spectrometry. Biomolecules 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Serafim, V.; Tiugan, D.-A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M.D. Development and Validation of a LC–MS/MS-Based Assay for Quantification of Free and Total Omega 3 and 6 Fatty Acids from Human Plasma. Molecules 2019, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Liu, O. Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acids. J. Pharm. Anal. 2015, 5, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, N.S.; Dias, D.A. A Robust GC-MS Method for the Quantitation of Fatty Acids in Biological Systems. Adv. Struct. Saf. Stud. 2013, 1055, 39–56. [Google Scholar] [CrossRef]
- Chiu, H.-H.; Kuo, C.-H. Gas chromatography-mass spectrometry-based analytical strategies for fatty acid analysis in biological samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef]
- Müller, M.; Hernández, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.E.; Van Eijk, H.; Canfora, E.E.; Blaak, E.E. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Yusuf, F.; Adewiah, S.; Fatchiyah, F. The Level Short Chain Fatty Acids and HSP 70 in Colorectal Cancer and Non-Colorectal Cancer. Acta Inform. Med. 2018, 26, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Dona, A.C.; Wijeyesekera, A.; Tournigand, C.; Baumgaertner, I.; Lebaleur, Y.; Sobhani, I.; Holmes, E. 1H NMR Spectroscopy of Fecal Extracts Enables Detection of Advanced Colorectal Neoplasia. J. Proteome Res. 2015, 14, 3871–3881. [Google Scholar] [CrossRef]
- Gomes, S.D.; Oliveira, C.S.; Azevedo-Silva, J.; Casanova, M.R.; Barreto, J.; Pereira, H.; Chaves, S.R.; Rodrigues, L.R.; Casal, M.; Côrte-Real, M.; et al. The Role of Diet Related Short-Chain Fatty Acids in Colorectal Cancer Metabolism and Survival: Prevention and Therapeutic Implications. Curr. Med. Chem. 2020, 27, 4087–4108. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Yu, E.; Wang, N.; Cai, Q.; Shuai, Q.; Yan, F.; Jiang, L.; Wang, H.; Liu, J.; et al. Changes in gut microbiota and plasma inflammatory factors across the stages of colorectal tumorigenesis: A case-control study. BMC Microbiol. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liberato, M.V.; Nascimento, A.S.; Ayers, S.D.; Lin, J.Z.; Cvoro, A.; Silveira, R.L.; Martínez, L.; Souza, P.C.T.; Saidemberg, D.; Deng, T.; et al. Medium Chain Fatty Acids Are Selective Peroxisome Proliferator Activated Receptor (PPAR) γ Activators and Pan-PPAR Partial Agonists. PLoS ONE 2012, 7, e36297. [Google Scholar] [CrossRef]
- Hara, T.; Kimura, I.; Inoue, D.; Ichimura, A.; Hirasawa, A. Free Fatty Acid Receptors and Their Role in Regulation of Energy Metabolism. Rev. Physiol. Biochem. Pharmacol. 2013, 164, 77–116. [Google Scholar] [CrossRef] [PubMed]
- Crotti, S.; Agnoletto, E.; Cancemi, G.; Di Marco, V.; Traldi, P.; Pucciarelli, S.; Nitti, D.; Agostini, M. Altered plasma levels of decanoic acid in colorectal cancer as a new diagnostic biomarker. Anal. Bioanal. Chem. 2016, 408, 6321–6328. [Google Scholar] [CrossRef] [PubMed]
- Iemoto, T.; Nishiumi, S.; Kobayashi, T.; Fujigaki, S.; Hamaguchi, T.; Kato, K.; Shoji, H.; Matsumura, Y.; Honda, K.; Yoshida, M. Serum level of octanoic acid predicts the efficacy of chemotherapy for colorectal cancer. Oncol. Lett. 2018, 17, 831–842. [Google Scholar] [CrossRef]
- Vockley, J. Long-chain fatty acid oxidation disorders and current management strategies. Am. J. Manag. Care 2020, 26, S147–S154. [Google Scholar] [PubMed]
- Cottet, V.; Vaysse, C.; Scherrer, M.-L.; Ortega-Deballon, P.; Lakkis, Z.; Delhorme, J.-B.; Deguelte-Lardière, S.; Combe, N.; Bonithon-Kopp, C. Fatty acid composition of adipose tissue and colorectal cancer: A case-control study. Am. J. Clin. Nutr. 2014, 101, 192–201. [Google Scholar] [CrossRef]
- Shen, S.; Yang, L.; Li, L.; Bai, Y.; Cai, C.; Liu, H. A plasma lipidomics strategy reveals perturbed lipid metabolic pathways and potential lipid biomarkers of human colorectal cancer. J. Chromatogr. B 2017, 41–48. [Google Scholar] [CrossRef]
- Fatima, S.; Hu, X.; Huang, C.; Zhang, W.; Cai, J.; Huang, M.; Gong, R.-H.; Chen, M.; Ho, A.H.M.; Su, T.; et al. High-fat diet feeding and palmitic acid increase CRC growth in β2AR-dependent manner. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Shimomoto, T.; Luo, Y.; Ohmori, H.; Chihara, Y.; Fujii, K.; Sasahira, T.; Denda, A.; Kuniyasu, H. Advanced glycation end products (AGE) induce the receptor for AGE in the colonic mucosa of azoxymethane-injected Fischer 344 rats fed with a high-linoleic acid and high-glucose diet. J. Gastroenterol. 2012, 47, 1073–1083. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Bjerregaard, J.H.; Skovbjerg, H.; Nyman, M. Fasting serum concentration of short-chain fatty acids in subjects with microscopic colitis and celiac disease: No difference compared with controls, but between genders. Scand. J. Gastroenterol. 2013, 48, 696–701. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.E.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef]
- Nistal, E.; Caminero, A.; Vivas, S.; Ruiz de Morales, J.M.; Sáenz de Miera, L.E.; Rodríguez-Aparicio, L.B.; Casqueiro, J. Differences in faecal bacteria populations and faecal bacteria metabolism in healthy adults and celiac disease patients. Biochimie 2012, 94, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Tjellström, B.; Högberg, L.; Stenhammar, L.; Fälth-Magnusson, K.; Magnusson, K.-E.; Norin, E.; Sundqvist, T.; Midtvedt, T. Faecal short-chain fatty acid pattern in childhood coeliac disease is normalised after more than one year’s gluten-free diet. Microb. Ecol. Heal. Dis. 2013, 24, 10. [Google Scholar] [CrossRef]
- De Preter, V.; Machiels, K.; Joossens, M.; Arijs, I.; Matthys, C.; Vermeire, S.; Rutgeerts, P.; Verbeke, K. Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut 2015, 64, 447–458. [Google Scholar] [CrossRef]
- Solakivi, T.; Kaukinen, K.; Kunnas, T.; Lehtimäki, T.; Mäki, M.; Nikkari, S.T. Serum fatty acid profile in celiac disease patients before and after a gluten-free diet. Scand. J. Gastroenterol. 2009, 44, 826–830. [Google Scholar] [CrossRef]
- Zeng, H.; Taussig, D.P.; Cheng, W.-H.; Johnson, L.K.; Hakkak, R. Butyrate Inhibits Cancerous HCT116 Colon Cell Proliferation but to a Lesser Extent in Noncancerous NCM460 Colon Cells. Nutrients. 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Coutzac, C.; Jouniaux, J.-M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Załęski, A.; Banaszkiewicz, A.; Walkowiak, J. Butyric acid in irritable bowel syndrome. Gastroenterol. Rev. 2013, 8, 350–353. [Google Scholar] [CrossRef]
- Cenit, M.C.; Olivares, M.; Codoñer-Franch, P.; Sanz, Y. Intestinal Microbiota and Celiac Disease: Cause, Consequence or Co-Evolution? Nutrition 2015, 7, 6900–6923. [Google Scholar] [CrossRef]
- Caminero, A.; Nistal, E.; Herrán, A.R.; Pérez-Andrés, J.; Ferrero, M.A.; Ayala, L.V.; Vivas, S.; De Morales, J.M.G.R.; Albillos, S.M.; Casqueiro, F.J. Differences in gluten metabolism among healthy volunteers, coeliac disease patients and first-degree relatives. Br. J. Nutr. 2015, 114, 1157–1167. [Google Scholar] [CrossRef]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef]
- Barengolts, E.; Green, S.J.; Chlipala, G.E.; Layden, B.T.; Eisenberg, Y.; Priyadarshini, M.; Dugas, L.R. Predictors of Obesity among Gut Microbiota Biomarkers in African American Men with and without Diabetes. Microorganisms 2019, 7, 320. [Google Scholar] [CrossRef]
- Irving, B.A.; Wood, G.C.; Bennotti, P.N.; Babu, E.; Deshpande, A.; Lent, M.R.; Petrick, A.; Gabrielsen, J.; Strodel, W.; Gerhard, G.S. Nutrient Transporter Expression in the Jejunum in Relation to Body Mass Index in Patients Undergoing Bariatric Surgery. Nutrients 2016, 8, 683. [Google Scholar] [CrossRef]
- Van Der Pals, M.; Myléus, A.; Norström, F.; Hammarroth, S.; Högberg, L.; Rosén, A.; Ivarsson, A.; Carlsson, A. Body mass index is not a reliable tool in predicting celiac disease in children. BMC Pediatr. 2014, 14, 165. [Google Scholar] [CrossRef]
- Cheng, J.; Brar, P.S.; Lee, A.R.; Green, P.H.R. Body mass index in celiac disease: Beneficial effect of a gluten-free diet. J. Clin. Gastroenterol. 2010, 44, 267–271. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Magkos, F.; Fabbrini, E.; Mohammed, B.; Klein, S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009, 17, 1872–1877. [Google Scholar] [CrossRef]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty Acids, Obesity, and Insulin Resistance: Time for a Reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef]
- Niccolai, E.; Boem, F.; Russo, E.; Amedei, A. The Gut⁻Brain Axis in the Neuropsychological Disease Model of Obesity: A Classical Movie Revised by the Emerging Director “Microbiome”. Nutrients 2019, 11, 156. [Google Scholar] [CrossRef]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romaní-Pérez, M.; Sanz, Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef]
- Freeman, H.J. Reproductive changes associated with celiac disease. World J. Gastroenterol. 2010, 16, 5810–5814. [Google Scholar] [CrossRef] [PubMed]
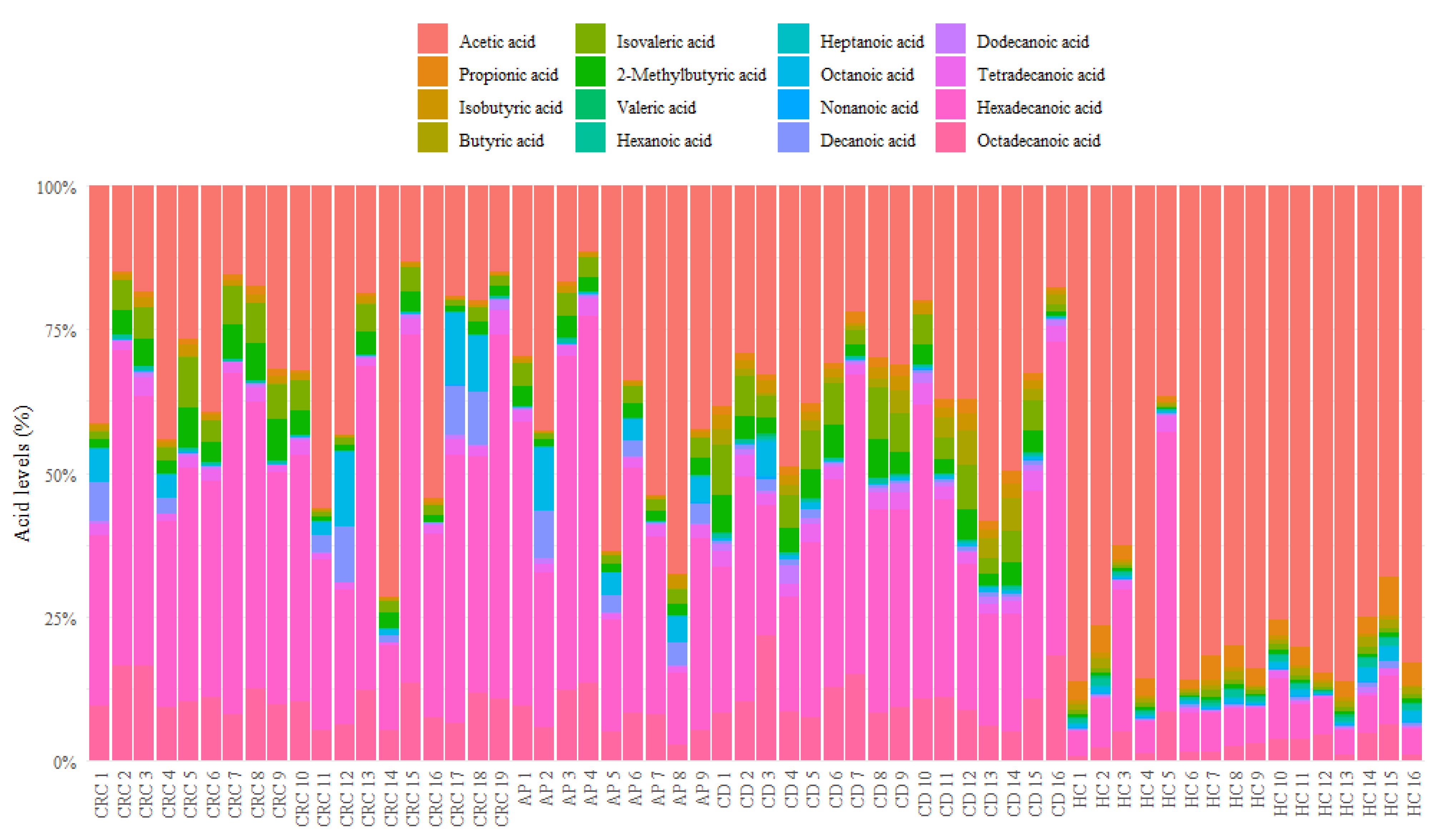
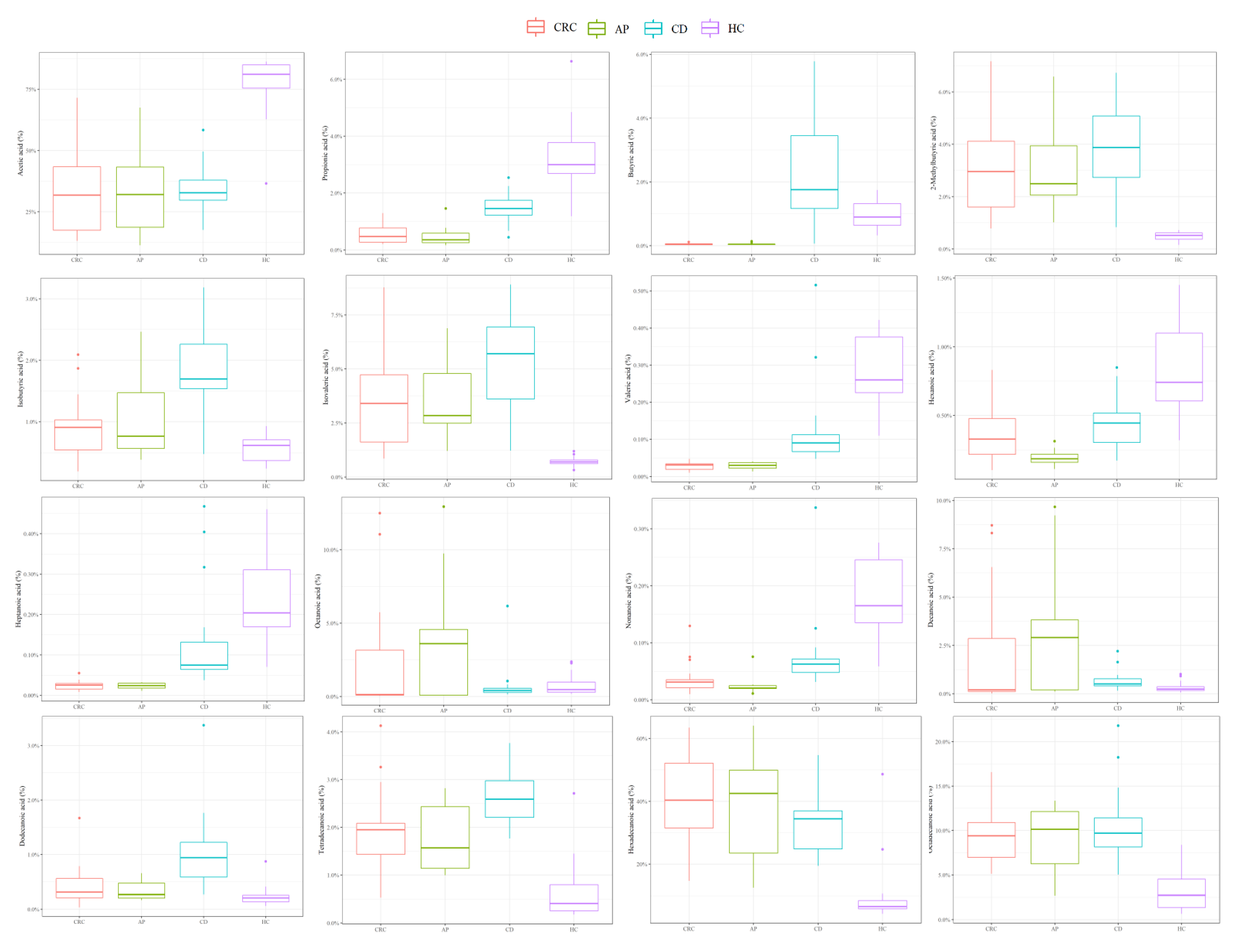

| ID Patients | State | Number | Male/Female Ratio, n | Age, Median (IQR) | BMI, Median (IQR) |
|---|---|---|---|---|---|
| HC | Healthy control | 16 | 1 (8/8) | 41.7 (31.7) | NA |
| CD | Celiac disease | 16 | 0.5 (6/10) | 35.5 (21) | 22.1 (3.7) |
| AP | Adenomatous polyposis | 9 | 1.2 (5/4) | 68 (28) | 24.9 (3.9) |
| CRC | Colorectal cancer | 19 | 8.5 (17/2) | 80 (13.5) | 24.5 (4.5) |
| FFA | CRC | AP | CD | HC |
|---|---|---|---|---|
| Acetic acid | 0.31768 (0.17476–0.43413) | 0.3204 (0.18718–0.43255) | 0.32748 (0.29762–0.37929) | 0.81064 (0.75446–0.84983) |
| Propionic acid | 0.00472 (0.00271–0.00774) | 0.00356 (0.00256–0.00586) | 0.01454 (0.01225–0.01744) | 0.02999 (0.02685–0.03777) |
| Isobutyric acid | 0.00909 (0.00546–0.01029) | 0.00763 (0.00569–0.01471) | 0.01695 (0.01537–0.0226) | 0.00615 (0.00372–0.00706) |
| Butyric acid | 0.00038 (0.00026–0.00058) | 0.00042 (0.00035–0.00048) | 0.01753 (0.01166–0.03449) | 0.00897 (0.00639–0.01317) |
| Isovaleric acid | 0.03399 (0.01612–0.04728) | 0.02838 (0.02483–0.04788) | 0.05703 (0.03606–0.06944) | 0.00701 (0.00621–0.00793) |
| 2–methylbutyric acid | 0.02959 (0.01603–0.04119) | 0.02493 (0.02058–0.0394) | 0.03875 (0.02733–0.05085) | 0.00517 (0.00375–0.00614) |
| Valeric acid | 0.00032 (2e–04–0.00035) | 0.00031 (0.00023–0.00038) | 0.0009 (0.00068–0.00113) | 0.0026 (0.00226–0.00376) |
| Hexanoic acid | 0.00328 (0.00219–0.00477) | 0.00185 (0.00161–0.00219) | 0.00444 (0.00303–0.00517) | 0.00741 (0.00606–0.01099) |
| Heptanoic acid | 0.00025 (0.00016–0.00029) | 0.00024 (0.00018–3e–04) | 0.00075 (0.00064–0.00132) | 0.00204 (0.0017–0.00311) |
| Octanoic acid | 0.00137 (0.00086–0.03154) | 0.03602 (0.00092–0.04563) | 0.00409 (0.00278–0.00555) | 0.00462 (0.00286–0.00977) |
| Nonanoic acid | 0.00031 (0.00021–0.00035) | 0.00021 (2e–04–0.00025) | 0.00063 (0.00048–0.00072) | 0.00165 (0.00135–0.00246) |
| Decanoic acid | 0.00205 (0.00117–0.02861) | 0.02912 (0.00203–0.03822) | 0.00509 (0.00409–0.00773) | 0.0024 (0.00165–0.0037) |
| Dodecanoic acid | 0.0031 (0.0021–0.00558) | 0.00266 (0.00202–0.00473) | 0.00943 (0.00584–0.01224) | 0.00206 (0.00136–0.00255) |
| Tetradecanoic acid | 0.0195 (0.01432–0.02084) | 0.01568 (0.0114–0.02433) | 0.02588 (0.02206–0.02976) | 0.00406 (0.00253–0.00795) |
| Hexadecanoic acid | 0.40353 (0.31485–0.52113) | 0.42495 (0.23516–0.49912) | 0.34432 (0.24853–0.36942) | 0.06569 (0.05869–0.08501) |
| Octadecanoic acid | 0.09405 (0.06981–0.10899) | 0.1013 (0.06261–0.12141) | 0.09689 (0.08155–0.11434) | 0.02715 (0.01355–0.04519) |
| FFA | HC vs. CD | HC vs. CRC | HC vs. AP | CD vs. CRC | CD vs. AP | CRC vs. AP |
|---|---|---|---|---|---|---|
| Acetic acid | 0.0000 | 0.0000 | 0.0000 | 0.7561 | 0.6368 | 0.9331 |
| Propionic acid | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.6291 |
| Isobutyric acid | 0.0000 | 0.0373 | 0.0741 | 0.0001 | 0.0096 | 0.6291 |
| Butyric acid | 0.0058 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.6993 |
| Isovaleric acid | 0.0000 | 0.0000 | 0.0000 | 0.0081 | 0.0272 | 0.7722 |
| 2 methylbutyric acid | 0.0000 | 0.0000 | 0.0000 | 0.1613 | 0.2071 | 0.8470 |
| Valeric acid | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.7355 |
| Hexanoic acid | 0.0019 | 0.0002 | 0.0000 | 0.2300 | 0.0003 | 0.0284 |
| Heptanoic acid | 0.4909 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.9615 |
| Octanoic acid | 0.0001 | 0.1515 | 0.0671 | 0.1613 | 0.8028 | 0.3829 |
| Nonanoic acid | 0.0058 | 0.0000 | 0.0000 | 0.0003 | 0.0003 | 0.1423 |
| Decanoic acid | 0.0000 | 0.9870 | 0.2071 | 0.3011 | 0.2976 | 0.1716 |
| Dodecanoic acid | 0.0000 | 0.0263 | 0.0842 | 0.0002 | 0.0003 | 0.7355 |
| Tetradecanoic acid | 0.0000 | 0.0000 | 0.0014 | 0.0065 | 0.0080 | 0.4679 |
| Hexadecanoic acid | 0.0000 | 0.0000 | 0.0001 | 0.0948 | 0.3876 | 0.9231 |
| Octadecanoic acid | 0.0000 | 0.0000 | 0.0003 | 0.4814 | 0.6771 | 0.9615 |
| Predicted | |||||
|---|---|---|---|---|---|
| CRC | AP | CD | HC | ||
| True status | CRC | 17 | 0 | 2 | 0 |
| AP | 8 | 0 | 1 | 0 | |
| CD | 2 | 0 | 14 | 0 | |
| HC | 1 | 0 | 0 | 15 | |
| Acid | Interaction | Gender | CRC | CD |
|---|---|---|---|---|
| Age | - | - | 0.0933 | |
| BMI | - | - | 0.1161 | |
| Butyric | Gender | - | - | −0.2278 |
| CRC | - | - | - | |
| CD | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldi, S.; Menicatti, M.; Nannini, G.; Niccolai, E.; Russo, E.; Ricci, F.; Pallecchi, M.; Romano, F.; Pedone, M.; Poli, G.; et al. Free Fatty Acids Signature in Human Intestinal Disorders: Significant Association between Butyric Acid and Celiac Disease. Nutrients 2021, 13, 742. https://doi.org/10.3390/nu13030742
Baldi S, Menicatti M, Nannini G, Niccolai E, Russo E, Ricci F, Pallecchi M, Romano F, Pedone M, Poli G, et al. Free Fatty Acids Signature in Human Intestinal Disorders: Significant Association between Butyric Acid and Celiac Disease. Nutrients. 2021; 13(3):742. https://doi.org/10.3390/nu13030742
Chicago/Turabian StyleBaldi, Simone, Marta Menicatti, Giulia Nannini, Elena Niccolai, Edda Russo, Federica Ricci, Marco Pallecchi, Francesca Romano, Matteo Pedone, Giovanni Poli, and et al. 2021. "Free Fatty Acids Signature in Human Intestinal Disorders: Significant Association between Butyric Acid and Celiac Disease" Nutrients 13, no. 3: 742. https://doi.org/10.3390/nu13030742
APA StyleBaldi, S., Menicatti, M., Nannini, G., Niccolai, E., Russo, E., Ricci, F., Pallecchi, M., Romano, F., Pedone, M., Poli, G., Renzi, D., Taddei, A., Calabrò, A. S., Stingo, F. C., Bartolucci, G., & Amedei, A. (2021). Free Fatty Acids Signature in Human Intestinal Disorders: Significant Association between Butyric Acid and Celiac Disease. Nutrients, 13(3), 742. https://doi.org/10.3390/nu13030742








