Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children—An Update and Novel Insights
Abstract
1. Introduction
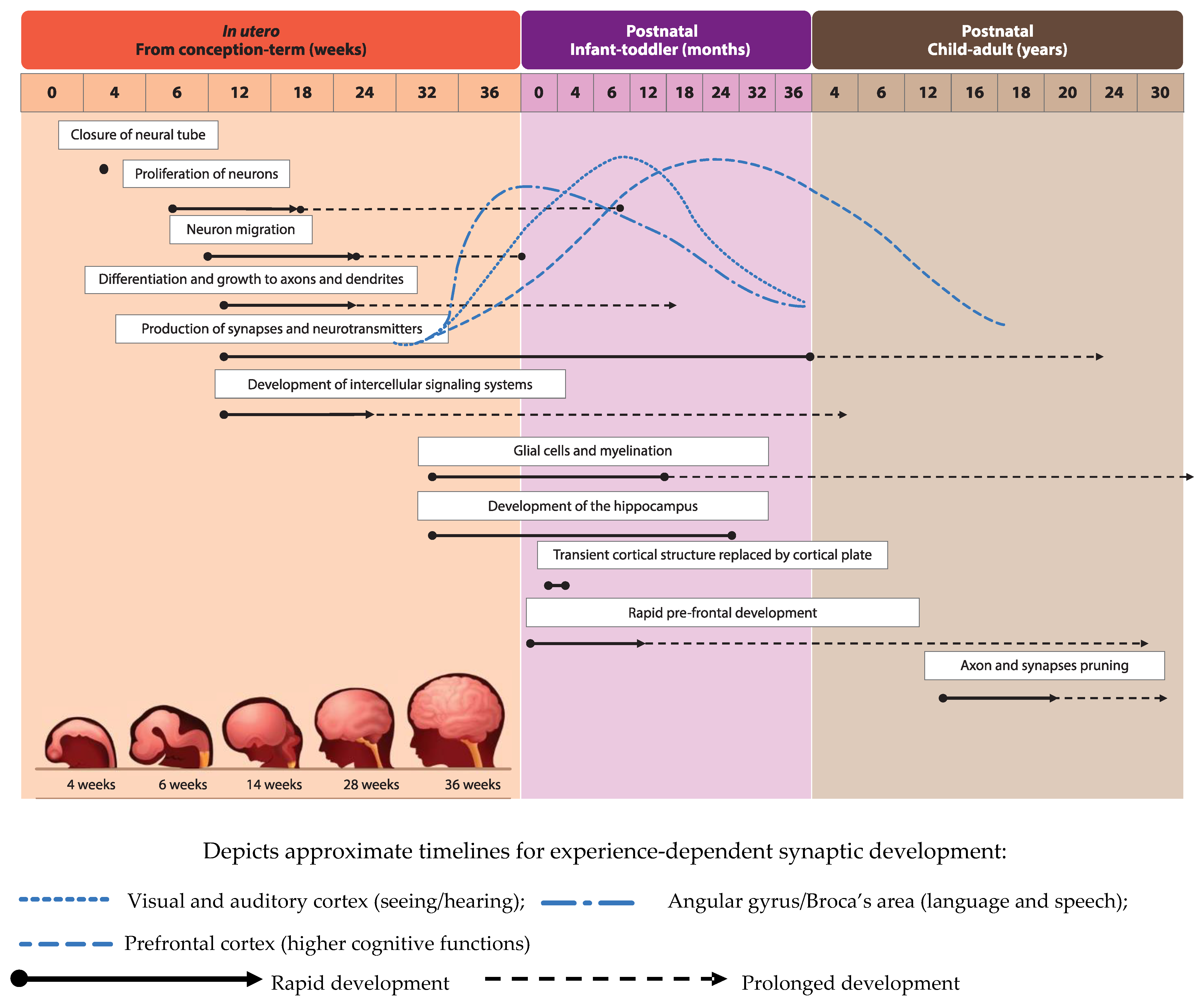
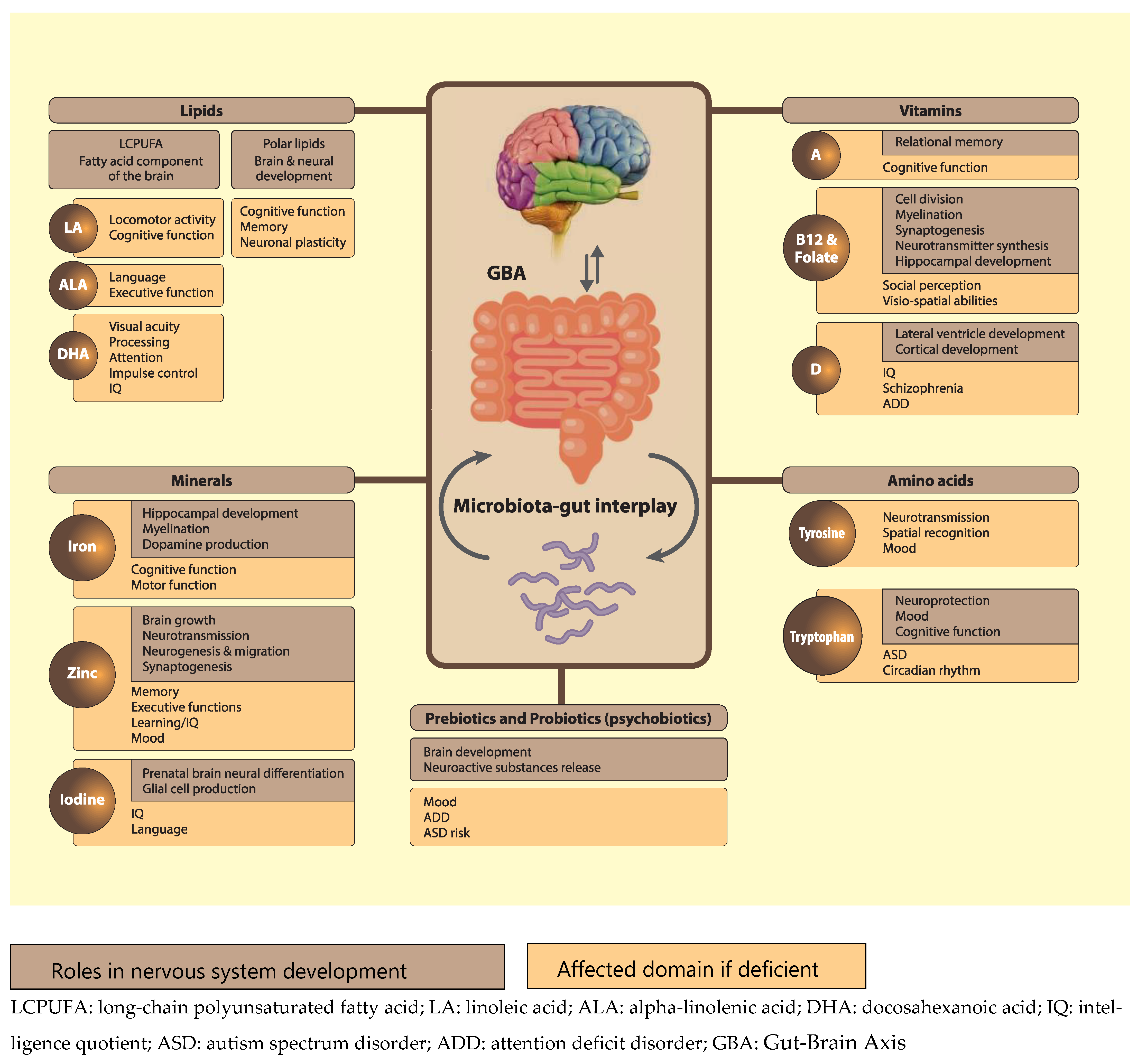
2. Nutrients that Play a Role in Neurodevelopment
2.1. Lipids
2.1.1. Long-Chain Polyunsaturated Fatty Acids
2.1.2. Polar Lipids
2.2. Minerals
2.2.1. Iron
2.2.2. Zinc
2.2.3. Iodine
2.3. Vitamins
2.3.1. Vitamin A
2.3.2. Vitamin B12
2.3.3. Vitamin D
2.4. Dietary Protein and Amino Acids
2.4.1. The Importance of Protein Quality
2.4.2. mTORC1: Linking Protein (in)Adequacy to Brain Development
2.4.3. Tryptophan
2.4.4. Tyrosine and Phenylalanine
2.4.5. Branched Chain Amino Acids
3. Nutrient Interactions through the Gut-Brain Axis (GBA)
3.1. What Is the Microbiome Gut-Brain Axis?
3.2. The Influence of the GBA on Cognitive, Emotional and Neural Development
3.3. The Impact of Pro- and Prebiotics through the GBA
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, M.M. Impact of nutrition on growth, brain, and cognition. Nestle Nutr. Inst. Workshop Ser. 2018, 89, 185–195. [Google Scholar]
- Hadders-Algra, M. Effect of long-chain polyunsaturated fatty acid supplementation on neurodevelopmental outcome in full-term infants. Nutrients 2010, 2, 790–804. [Google Scholar] [CrossRef]
- Grantham-McGregor, S.; Cheung, Y.B.; Cueto, S.; Glewwe, P.; Richter, L.; Strupp, B.; International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet 2007, 369, 60–70. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef]
- Cusick, S.E.; Georgieff, M.K. The role of nutrition in brain development: The golden opportunity of the “first 1000 days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Tamnes, C.K.; Herting, M.M.; Goddings, A.L.; Meuwese, R.; Blakemore, S.J.; Dahl, R.E.; Güroğlu, B.; Raznahan, A.; Sowell, E.R.; Crone, E.A.; et al. Development of the cerebral cortex across adolescence: A multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 2017, 37, 3402–3412. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, E.; Kim, Y.; Ha, E.H.; Chang, N. Association between maternal intake of n-6 to n-3 fatty acid ratio during pregnancy and infant neurodevelopment at 6 months of age: Results of the MOCEH cohort study. Nutr. J. 2017, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid quality in infant nutrition: Current knowledge and future opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef]
- Bernard, J.Y.; Armand, M.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B.; EDEN Mother-Child Cohort Study Group. The association between linoleic acid levels in colostrum and child cognition at 2 and 3 y in the EDEN cohort. Pediatr. Res. 2015, 77, 829–835. [Google Scholar] [CrossRef]
- Bernard, J.Y.; Armand, M.; Peyre, H.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B.; EDEN Mother-Child Cohort Study Group. Breastfeeding, polyunsaturated fatty acid levels in colostrum and child intelligence quotient at age 5–6 years. J. Pediatr. 2017, 183, 43–50.e3. [Google Scholar] [CrossRef]
- Dalmeijer, G.W.; Wijga, A.H.; Gehring, U.; Renders, C.M.; Koppelman, G.H.; Smit, H.A.; van Rossem, L. Fatty acid composition in breastfeeding and school performance in children aged 12 years. Eur. J. Nutr. 2016, 55, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Braarud, H.C.; Markhus, M.W.; Skotheim, S.; Stormark, K.M.; Frøyland, L.; Graff, I.E.; Kjellevold, M. Maternal DHA status during pregnancy has a positive impact on infant problem solving: A Norwegian prospective observation study. Nutrients 2018, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Sirois, S.; Wearden, A. Prenatal maternal docosahexaenoic acid intake and infant information processing at 4.5mo and 9mo: A longitudinal study. PLoS ONE 2019, 14, e0210984. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, U.; Gonzalez-Casanova, I.; Schnaas, L.; DiGirolamo, A.; Quezada, A.D.; Pallo, B.C.; Hao, W.; Neufeld, L.M.; Rivera, J.A.; Stein, A.D.; et al. Prenatal supplementation with DHA improves attention at 5 y of age: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 1075–1082. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P.; DOMInO, I.T. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef]
- Delgado-Noguera, M.F.; Calvache, J.A.; Bonfill Cosp, X.; Kotanidou, E.P.; Galli-Tsinopoulou, A. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 3, CD000376. [Google Scholar] [CrossRef]
- Colombo, J.; Jill Shaddy, D.; Kerling, E.H.; Gustafson, K.M.; Carlson, S.E. Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot. Essent. Fatty Acids 2017, 121, 52–56. [Google Scholar] [CrossRef]
- Drover, J.R.; Hoffman, D.R.; Castañeda, Y.S.; Morale, S.E.; Garfield, S.; Wheaton, D.H.; Birch, E.E. Cognitive function in 18-month-old term infants of the DIAMOND study: A randomized, controlled clinical trial with multiple dietary levels of docosahexaenoic acid. Early Hum. Dev. 2011, 87, 223–230. [Google Scholar] [CrossRef]
- Koletzko, B.; Uauy, R.; Palou, A.; Kok, F.; Hornstra, G.; Eilander, A.; Moretti, D.; Osendarp, S.; Zock, P.; Innis, S. Dietary intake of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in children—A workshop report. Br. J. Nutr. 2010, 103, 923–928. [Google Scholar] [CrossRef]
- Liao, K.; McCandliss, B.D.; Carlson, S.E.; Colombo, J.; Shaddy, D.J.; Kerling, E.H.; Lepping, R.J.; Sittiprapaporn, W.; Cheatham, C.L.; Gustafson, K.M. Event-related potential differences in children supplemented with long-chain polyunsaturated fatty acids during infancy. Dev. Sci. 2017, 20, e12455. [Google Scholar] [CrossRef] [PubMed]
- Devlin, A.M.; Chau, C.M.Y.; Dyer, R.; Matheson, J.; McCarthy, D.; Yurko-Mauro, K.; Innis, S.M.; Grunau, R.E. Developmental outcomes at 24 months of age in toddlers supplemented with arachidonic acid and docosahexaenoic acid: Results of a double blind randomized, controlled trial. Nutrients 2017, 9, 975. [Google Scholar] [CrossRef] [PubMed]
- Jumbe, T.; Comstock, S.S.; Harris, W.S.; Kinabo, J.; Pontifex, M.B.; Fenton, J.I. Whole-blood fatty acids are associated with executive function in Tanzanian children aged 4-6 years: A cross-sectional study. Br. J. Nutr. 2016, 116, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Adjepong, M.; Yakah, W.; Harris, W.S.; Annan, R.A.; Pontifex, M.B.; Fenton, J.I. Whole blood n-3 fatty acids are associated with executive function in 2-6-year-old Northern Ghanaian children. J. Nutr. Biochem. 2018, 57, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Øyen, J.; Kvestad, I.; Midtbø, L.K.; Graff, I.E.; Hysing, M.; Stormark, K.M.; Markhus, M.W.; Baste, V.; Frøyland, L.; Koletzko, B.; et al. Fatty fish intake and cognitive function: FINS-KIDS, a randomized controlled trial in preschool children. BMC Med. 2018, 16, 41. [Google Scholar] [CrossRef]
- Gallier, S.; Gragson, D.; Cabral, C.; Jiménez-Flores, R.; Everett, D.W. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. J. Agric. Food Chem. 2010, 58, 10503–10511. [Google Scholar] [CrossRef]
- Lopez, C.; Madec, M.-N.; Jimenez-Flores, R. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chem. 2010, 120, 22–33. [Google Scholar] [CrossRef]
- Zempleni, J.; Aguilar-Lozano, A.; Sadri, M.; Sukreet, S.; Manca, S.; Wu, D.; Zhou, F.; Mutai, E. Biological activities of extracellular vesicles and their cargos from bovine and human milk in humans and implications for infants. J. Nutr. 2017, 147, 3–10. [Google Scholar] [CrossRef]
- Hageman, J.H.J.; Danielsen, M.; Nieuwenhuizen, A.G.; Feitsma, A.L.; Dalsgaard, T.K. Comparison of bovine milk fat and vegetable fat for infant formula: Implications for infant health. Int. Dairy J. 2019, 92, 37–49. [Google Scholar] [CrossRef]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Zou, X.; Guo, Z.; Jin, Q.; Huang, J.; Cheong, L.; Xu, X.; Wang, X. Composition and microstructure of colostrum and mature bovine milk fat globule membrane. Food Chem. 2015, 185, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.; Xu, X.; Wang, X. Lipid composition analysis of milk fats from different mammalian species: Potential for use as human milk fat substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Dietary reference values for choline. EFSA J. 2016, 14, e04484. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar]
- Zheng, L.; Fleith, M.; Giuffrida, F.; O’Neill, B.V.; Schneider, N. Dietary polar lipids and cognitive development: A narrative review. Adv. Nutr. 2019, 10, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Anto, L.; Warykas, S.W.; Torres-Gonzalez, M.; Blesso, C.N. Milk polar lipids: Underappreciated lipids with emerging health benefits. Nutrients 2020, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.; van Dijk, G.J.; van der Beek, E.M. Milk lipid composition and structure; the relevance for infant brain development. OCL 2020, 27, 5. [Google Scholar] [CrossRef]
- Brink, L.R.; Lönnerdal, B. The role of milk fat globule membranes in behavior and cognitive function using a suckling rat pup supplementation model. J. Nutr. Biochem. 2018, 58, 131–137. [Google Scholar] [CrossRef]
- Norris, T.; Souza, R.; Xia, Y.; Zhang, T.; Rowan, A.; Gallier, S.; Zhang, H.; Qi, H.; Baker, P. Effect of supplementation of complex milk lipids in pregnancy on fetal growth: Results from the Complex Lipids in Mothers and Babies (CLIMB) randomized controlled trial. J. Matern. Fetal Neonatal Med. 2019, 1–10. [Google Scholar] [CrossRef]
- Veereman-Wauters, G.; Staelens, S.; Rombaut, R.; Dewettinck, K.; Deboutte, D.; Brummer, R.J.; Boone, M.; Le Ruyet, P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012, 28, 749–752. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, M.; Lönnerdal, B.; Hernell, O. Supplementation of infant formula with bovine milk fat globule membranes. Adv. Nutr. 2017, 8, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: A randomized, controlled trial. J. Pediatr. 2019, 215, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Rice, G.E.; Mitchell, M.D. The role of gangliosides in brain development and the potential benefits of perinatal supplementation. Nutr. Res. 2013, 33, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, Å. Role of sphingolipids in infant gut health and immunity. J. Pediatr. 2016, 173, S53–S59. [Google Scholar] [CrossRef]
- Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 2012, 3, 465S–472S. [Google Scholar] [CrossRef]
- Röhrig, C.H.; Choi, S.S.; Baldwin, N. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Crit. Rev. Food Sci. Nutr. 2017, 57, 1017–1038. [Google Scholar] [CrossRef]
- Larson, L.M.; Phiri, K.S.; Pasricha, S.R. Iron and cognitive development: What is the evidence? Ann. Nutr. Metab. 2017, 71 (Suppl. 3), 25–38. [Google Scholar] [CrossRef]
- Chmielewska, A.; Dziechciarz, P.; Gieruszczak-Białek, D.; Horvath, A.; Pieścik-Lech, M.; Ruszczyński, M.; Skórka, A.; Szajewska, H. Effects of prenatal and/or postnatal supplementation with iron, PUFA or folic acid on neurodevelopment: Update. Br. J. Nutr. 2019, 122, S10–S15. [Google Scholar] [CrossRef]
- González, H.F.; Visentin, S. Micronutrients and neurodevelopment: An update. Arch. Argent Pediatr. 2016, 114, 570–575. [Google Scholar]
- Szajewska, H.; Ruszczynski, M.; Chmielewska, A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2010, 91, 1684–1690. [Google Scholar] [CrossRef]
- Low, M.S.; Speedy, J.; Styles, C.E.; De-Regil, L.M.; Pasricha, S.R. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst. Rev. 2016, 4, CD009747. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Castillo, M.; Clark, K.M.; Smith, J.B. Iron-fortified vs. low-iron infant formula: Developmental outcome at 10 years. Arch. Pediatr. Adolesc. Med. 2012, 166, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics 2010, 2, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, L.E.; Putnick, D.L.; Zavaleta, N.; Lazarte, F.; Albornoz, C.; Chen, P.; Dipietro, J.A.; Bornstein, M.H. Maternal gestational zinc supplementation does not influence multiple aspects of child development at 54 mo of age in Peru. Am. J. Clin. Nutr. 2010, 92, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Zavaleta, N.; Kannass, K.N.; Lazarte, F.; Albornoz, C.; Kapa, L.L.; Caulfield, L.E. Zinc supplementation sustained normative neurodevelopment in a randomized, controlled trial of Peruvian infants aged 6–18 months. J. Nutr. 2014, 144, 1298–1305. [Google Scholar] [CrossRef]
- Gogia, S.; Sachdev, H.S. Zinc supplementation for mental and motor development in children. Cochrane Database Syst. Rev. 2012, 12, CD007991. [Google Scholar] [CrossRef]
- Nissensohn, M.; Sánchez-Villegas, A.; Fuentes Lugo, D.; Henríquez Sánchez, P.; Doreste Alonso, J.; Skinner, A.L.; Medina, M.W.; Lowe, N.M.; Hall Moran, V.; Serra-Majem, L. Effect of zinc intake on mental and motor development in infants: A meta-analysis. Int J. Vitam. Nutr. Res. 2013, 83, 203–215. [Google Scholar] [CrossRef]
- Bell, M.A.; Ross, A.P.; Goodman, G. Assessing infant cognitive development after prenatal iodine supplementation. Am. J. Clin. Nutr. 2016, 104 (Suppl. 3), 928S–934S. [Google Scholar] [CrossRef]
- Robinson, S.M.; Crozier, S.R.; Miles, E.A.; Gale, C.R.; Calder, P.C.; Cooper, C.; Inskip, H.M.; Godfrey, K.M. Preconception maternal iodine status is positively associated with iq but not with measures of executive function in childhood. J. Nutr. 2018, 148, 959–966. [Google Scholar] [CrossRef]
- Modesto, T.; Tiemeier, H.; Peeters, R.P.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; Ghassabian, A. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr. 2015, 169, 838–845. [Google Scholar] [CrossRef]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of iodine deficiency and excess in pregnant women: An overview of current knowns and unknowns. Am. J. Clin. Nutr. 2016, 104 (Suppl. 3), 918S–923S. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ding, G.; Lou, X.; Mo, Z.; Zhu, W.; Mao, G.; Zhou, J. A study on the influencing factors of urinary iodine concentration and the relationship between iodised salt concentration and urinary iodine concentration. Br. J. Nutr. 2015, 113, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.; Espada, M.; Julvez, J.; Llop, S.; Lopez-Espinosa, M.J.; Vioque, J.; Basterrechea, M.; Riaño, I.; González, L.; Alvarez-Pedrerol, M.; et al. Iodine intake from supplements and diet during pregnancy and child cognitive and motor development: The INMA Mother and Child Cohort Study. J. Epidemiol. Community Health 2018, 72, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, D.; Wu, W.; Li, H.; Cao, L.; Xu, J.; Yu, X.; Bian, X.; Yan, C.; Wang, W. Relationship between iodine concentration in maternal colostrum and neurobehavioral development of infants in Shanghai, China. J. Child Neurol. 2016, 31, 1108–1113. [Google Scholar] [CrossRef]
- Aboud, F.E.; Bougma, K.; Lemma, T.; Marquis, G.S. Evaluation of the effects of iodized salt on the mental development of preschool-aged children: A cluster randomized trial in northern Ethiopia. Matern. Child Nutr. 2017, 13, e12322. [Google Scholar] [CrossRef]
- Etchamendy, N.; Enderlin, V.; Marighetto, A.; Pallet, V.; Higueret, P.; Jaffard, R. Vitamin A deficiency and relational memory deficit in adult mice: Relationships with changes in brain retinoid signalling. Behav. Brain Res. 2003, 145, 37–49. [Google Scholar] [CrossRef]
- Lima, A.A.; Kvalsund, M.P.; Souza, P.P.; Figueiredo, Í.L.; Soares, A.M.; Mota, R.M.; Lima, N.L.; Pinkerton, R.C.; Patrick, P.P.; Guerrant, R.L.; et al. Zinc, vitamin A, and glutamine supplementation in Brazilian shantytown children at risk for diarrhea results in sex-specific improvements in verbal learning. Clinics (Sao Paulo) 2013, 68, 351–358. [Google Scholar] [CrossRef]
- Villamor, E.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr. Perinat. Epidemiol. 2012, 26, 328–335. [Google Scholar] [CrossRef]
- Lai, J.S.; Cai, S.; Feng, L.; Shek, L.P.; Yap, F.; Tan, K.H.; Chong, Y.S.; Godfrey, K.M.; Meaney, M.J.; Rifkin-Graboi, A.; et al. Associations of maternal zinc and magnesium with offspring learning abilities and cognitive development at 4 years in GUSTO. Nutr. Neurosci. 2019, 1–10. [Google Scholar] [CrossRef]
- Kvestad, I.; Hysing, M.; Shrestha, M.; Ulak, M.; Thorne-Lyman, A.L.; Henjum, S.; Ueland, P.M.; Midttun, Ø.; Fawzi, W.; Chandyo, R.K.; et al. Vitamin B-12 status in infancy is positively associated with development and cognitive functioning 5 y later in Nepalese children. Am. J. Clin. Nutr. 2017, 105, 1122–1131. [Google Scholar] [CrossRef]
- Strand, T.A.; Taneja, S.; Ueland, P.M.; Refsum, H.; Bahl, R.; Schneede, J.; Sommerfelt, H.; Bhandari, N. Cobalamin and folate status predicts mental development scores in North Indian children 12–18 mo of age. Am. J. Clin. Nutr. 2013, 97, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Strand, T.A.; Ulak, M.; Chandyo, R.K.; Kvestad, I.; Hysing, M.; Shrestha, M.; Basnet, S.; Ranjitkar, S.; Shrestha, L.; Shrestha, P.S. The effect of vitamin B12 supplementation in Nepalese infants on growth and development: Study protocol for a randomized controlled trial. Trials 2017, 18, 187. [Google Scholar] [CrossRef][Green Version]
- Rauh-Pfeiffer, A.; Handel, U.; Demmelmair, H.; Peissner, W.; Niesser, M.; Moretti, D.; Martens, V.; Wiseman, S.; Weichert, J.; Heene, M.; et al. Three-month B vitamin supplementation in pre-school children affects folate status and homocysteine, but not cognitive performance. Eur. J. Nutr. 2014, 53, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- O’Loan, J.; Eyles, D.W.; Kesby, J.; Ko, P.; McGrath, J.J.; Burne, T.H. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology 2007, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Eyles, D.; Mowry, B.; Yolken, R.; Buka, S. Low maternal vitamin D as a risk factor for schizophrenia: A pilot study using banked sera. Schizophr. Res. 2003, 63, 73–78. [Google Scholar] [CrossRef]
- Whitehouse, A.J.; Holt, B.J.; Serralha, M.; Holt, P.G.; Hart, P.H.; Kusel, M.M. Maternal vitamin D levels and the autism phenotype among offspring. J. Autism Dev. Disord. 2013, 43, 1495–1504. [Google Scholar] [CrossRef]
- Zhu, P.; Tong, S.L.; Hao, J.H.; Tao, R.X.; Huang, K.; Hu, W.B.; Zhou, Q.F.; Jiang, X.M.; Tao, F.B. Cord blood vitamin D and neurocognitive development are nonlinearly related in toddlers. J. Nutr. 2015, 145, 1232–1238. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef]
- Chavez, A.; Martinez, C.; Yaschine, T. Nutrition, behavioral development, and mother-child interaction in young rural children. Fed. Proc. 1975, 34, 1574–1582. [Google Scholar]
- Pollitt, E.; Gorman, K.S.; Engle, P.L.; Martorell, R.; Rivera, T.; Wachs, T.D.; Scrimshaw, N.S. Early supplemenatry feeding and cognition: Effects over two decades. Monogr. Soc. Res. Child Dev. 1993, 58, 1–99. [Google Scholar] [CrossRef]
- Li, H.; Barnhart, H.X.; Stein, A.D.; Martorell, R. Effects of early childhood supplementation on the educational achievement of women. Pediatrics 2003, 112, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Morgane, P.J.; Mokler, D.J.; Galler, J.R. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci. Biobehav. Rev. 2002, 26, 471–483. [Google Scholar] [CrossRef]
- Chertoff, M. Protein malnutrition and brain development. Brain Disord. Ther. 2015, 4, 168. [Google Scholar] [CrossRef]
- Laus, M.F.; Vales, L.D.; Costa, T.M.; Almeida, S.S. Early postnatal protein-calorie malnutrition and cognition: A review of human and animal studies. Int. J. Environ. Res. Public Health 2011, 8, 590–612. [Google Scholar] [CrossRef] [PubMed]
- Hemb, M.; Cammarota, M.; Nunes, M.L. Effects of early malnutrition, isolation and seizures on memory and spatial learning in the developing rat. Int. J. Dev. Neurosci. 2010, 28, 303–307. [Google Scholar] [CrossRef]
- Valadares, C.T.; Fukuda, M.T.; Françolin-Silva, A.L.; Hernandes, A.S.; Almeida, S.S. Effects of postnatal protein malnutrition on learning and memory procedures. Nutr. Neurosci. 2010, 13, 274–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Yang, Z. Perinatal food restriction impaired spatial learning and memory behavior and decreased the density of nitric oxide synthase neurons in the hippocampus of adult male rat offspring. Toxicol. Lett. 2010, 193, 167–172. [Google Scholar] [CrossRef]
- Harris, T.E.; Lawrence, J.C. TOR signaling. Sci. STKE 2003, 2003, re15. [Google Scholar] [CrossRef]
- Gal-Ben-Ari, S.; Kenney, J.W.; Ounalla-Saad, H.; Taha, E.; David, O.; Levitan, D.; Gildish, I.; Panja, D.; Pai, B.; Wibrand, K.; et al. Consolidation and translation regulation. Learn Mem. 2012, 19, 410–422. [Google Scholar] [CrossRef]
- Burket, J.A.; Benson, A.D.; Tang, A.H.; Deutsch, S.I. NMDA receptor activation regulates sociability by its effect on mTOR signaling activity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 60–65. [Google Scholar] [CrossRef]
- Ishizuka, Y.; Kakiya, N.; Nawa, H.; Takei, N. Leucine induces phosphorylation and activation of p70S6K in cortical neurons via the system L amino acid transporter. J. Neurochem. 2008, 106, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kang, B.N.; Tian, J.; Liu, Y.; Luo, H.R.; Hester, L.; Snyder, S.H. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J. Neurosci. 2007, 27, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, C.; Salinas, M.; Alcázar, A. Regulation of cap-dependent translation by insulin-like growth factor-1 in neuronal cells. Biochem. Biophys. Res. Commun. 2002, 291, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Huang, C.C.; Wu, M.Y.; Hsu, K.S. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J. Biol. Chem. 2005, 280, 18543–18550. [Google Scholar] [CrossRef] [PubMed]
- Avruch, J.; Hara, K.; Lin, Y.; Liu, M.; Long, X.; Ortiz-Vega, S.; Yonezawa, K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene 2006, 25, 6361–6372. [Google Scholar] [CrossRef] [PubMed]
- Chenal, J.; Pierre, K.; Pellerin, L. Insulin and IGF-1 enhance the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin pathway. Eur. J. Neurosci. 2008, 27, 53–65. [Google Scholar] [CrossRef]
- Lewin, G.R.; Barde, Y.A. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996, 19, 289–317. [Google Scholar] [CrossRef]
- Nawa, H.; Takei, N. BDNF as an anterophin; a novel neurotrophic relationship between brain neurons. Trends Neurosci. 2001, 24, 683–684. [Google Scholar] [CrossRef]
- Takei, N.; Kawamura, M.; Hara, K.; Yonezawa, K.; Nawa, H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: Comparison with the effects of insulin. J. Biol. Chem. 2001, 276, 42818–42825. [Google Scholar] [CrossRef]
- Takei, N.; Inamura, N.; Kawamura, M.; Namba, H.; Hara, K.; Yonezawa, K.; Nawa, H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J. Neurosci. 2004, 24, 9760–9769. [Google Scholar] [CrossRef]
- Hara, K.; Yonezawa, K.; Weng, Q.P.; Kozlowski, M.T.; Belham, C.; Avruch, J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998, 273, 14484–14494. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.E.; Wang, X.; Proud, C.G. Nutrients differentially regulate multiple translation factors and their control by insulin. Biochem. J. 1999, 344, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, Y.; Kakiya, N.; Witters, L.A.; Oshiro, N.; Shirao, T.; Nawa, H.; Takei, N. AMP-activated protein kinase counteracts brain-derived neurotrophic factor-induced mammalian target of rapamycin complex 1 signaling in neurons. J. Neurochem. 2013, 127, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Casadio, A.; Martin, K.C.; Giustetto, M.; Zhu, H.; Chen, M.; Bartsch, D.; Bailey, C.H.; Kandel, E.R. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 1999, 99, 221–237. [Google Scholar] [CrossRef]
- Carroll, M.; Warren, O.; Fan, X.; Sossin, W.S. 5-HT stimulates eEF2 dephosphorylation in a rapamycin-sensitive manner in Aplysia neurites. J. Neurochem. 2004, 90, 1464–1476. [Google Scholar] [CrossRef]
- Carroll, M.; Dyer, J.; Sossin, W.S. Serotonin increases phosphorylation of synaptic 4EBP through TOR, but eukaryotic initiation factor 4E levels do not limit somatic cap-dependent translation in aplysia neurons. Mol. Cell. Biol. 2006, 26, 8586–8598. [Google Scholar] [CrossRef]
- Garelick, M.G.; Kennedy, B.K. TOR on the brain. Exp. Gerontol. 2011, 46, 155–163. [Google Scholar] [CrossRef]
- Ryskalin, L.; Lazzeri, G.; Flaibani, M.; Biagioni, F.; Gambardella, S.; Frati, A.; Fornai, F. mTOR-dependent cell proliferation in the brain. Biomed. Res. Int. 2017, 2017, 7082696. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan metabolites along the microbiota-gut-brain axis: An interkingdom communication system influencing the gut in health and disease. Int. J. Tryptophan Res. 2020, 13, 1178646920928984. [Google Scholar] [CrossRef]
- Cowen, P.; Sherwood, A.C. The role of serotonin in cognitive function: Evidence from recent studies and implications for understanding depression. J. Psychopharmacol. 2013, 27, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, L.A.; O’Connell, N.C.; Hatch, T.F.; Picciano, M.F.; Birch, L.L. Tryptophan intake influences infants’ sleep latency. J. Nutr. 1992, 122, 1781–1791. [Google Scholar] [CrossRef]
- Yogman, M.W.; Zeisel, S.H. Diet and sleep patterns in newborn infants. N. Engl. J. Med. 1983, 309, 1147–1149. [Google Scholar] [CrossRef]
- Aparicio, S.; Garau, C.; Esteban, S.; Nicolau, M.C.; Rivero, M.; Rial, R.V. Chrononutrition: Use of dissociated day/night infant milk formulas to improve the development of the wake-sleep rhythms. Effects of tryptophan. Nutr. Neurosci. 2007, 10, 137–143. [Google Scholar] [CrossRef]
- Esteban, S.; Nicolaus, C.; Garmundi, A.; Rial, R.V.; Rodríguez, A.B.; Ortega, E.; Ibars, C.B. Effect of orally administered L-tryptophan on serotonin, melatonin, and the innate immune response in the rat. Mol. Cell. Biochem. 2004, 267, 39–46. [Google Scholar] [CrossRef]
- Harada, T.; Hirotani, M.; Maeda, M.; Nomura, H.; Takeuchi, H. Correlation between breakfast tryptophan content and morning-evening in Japanese infants and students aged 0–15 yrs. J. Physiol. Anthropol. 2007, 26, 201–207. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Mahanonda, R.; Sa-Ard-Iam, N.; Montreekachon, P.; Pimkhaokham, A.; Yongvanichit, K.; Fukuda, M.M.; Pichyangkul, S. IL-8 and IDO expression by human gingival fibroblasts via TLRs. J. Immunol. 2007, 178, 1151–1157. [Google Scholar] [CrossRef]
- Clarke, G.; McKernan, D.P.; Gaszner, G.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor activation in irritable bowel syndrome. Front. Pharmacol. 2012, 3, 90. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.E.; Myint, A.M.; Kubera, M.; Verkerk, R. The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar]
- Quera Salva, M.A.; Hartley, S.; Barbot, F.; Alvarez, J.C.; Lofaso, F.; Guilleminault, C. Circadian rhythms, melatonin and depression. Curr. Pharm. Des. 2011, 17, 1459–1470. [Google Scholar] [CrossRef]
- Suh, H.S.; Zhao, M.L.; Rivieccio, M.; Choi, S.; Connolly, E.; Zhao, Y.; Takikawa, O.; Brosnan, C.F.; Lee, S.C. Astrocyte indoleamine 2,3-dioxygenase is induced by the TLR3 ligand poly(I:C): Mechanism of induction and role in antiviral response. J. Virol. 2007, 81, 9838–9850. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Lutz, C.; Lanz, T.V.; Tritschler, I.; Köppel, A.; Tolosa, E.; Hoberg, M.; Anderl, J.; Aicher, W.K.; et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells 2009, 27, 909–919. [Google Scholar] [CrossRef]
- Asp, L.; Holtze, M.; Powell, S.B.; Karlsson, H.; Erhardt, S. Neonatal infection with neurotropic influenza A virus induces the kynurenine pathway in early life and disrupts sensorimotor gating in adult Tap1-/- mice. Int. J. Neuropsychopharmacol. 2010, 13, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Khalil, O.S.; Pisar, M.; McNair, K.; Kornisiuk, E.; Snitcofsky, M.; Gonzalez, N.; Jerusalinsky, D.; Darlington, L.G.; Stone, T.W. Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience 2013, 254, 241–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forrest, C.M.; Khalil, O.S.; Pisar, M.; Darlington, L.G.; Stone, T.W. Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 2013, 1504, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Pocivavsek, A. Elevated kynurenine pathway metabolism during neurodevelopment: Implications for brain and behavior. Neuropharmacology 2017, 112, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Pisar, M.; Forrest, C.M.; Khalil, O.S.; McNair, K.; Vincenten, M.C.; Qasem, S.; Darlington, L.G.; Stone, T.W. Modified neocortical and cerebellar protein expression and morphology in adult rats following prenatal inhibition of the kynurenine pathway. Brain Res. 2014, 1576, 1–17. [Google Scholar] [CrossRef]
- Kapalka, G. Nutritional and herbal therapies for children and adolescents. In A Handbook for Mental Health Clinicians; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- Peters, J.C.; Harper, A.E. Adaptation of rats to diets containing different levels of protein: Effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J. Nutr. 1985, 115, 382–398. [Google Scholar] [CrossRef]
- Harmer, C.J.; McTavish, S.F.; Clark, L.; Goodwin, G.M.; Cowen, P.J. Tyrosine depletion attenuates dopamine function in healthy volunteers. Psychopharmacol. Berl. 2001, 154, 105–111. [Google Scholar] [CrossRef]
- Leyton, M.; Young, S.N.; Pihl, R.O.; Etezadi, S.; Lauze, C.; Blier, P.; Baker, G.B.; Benkelfat, C. Effects on mood of acute phenylalanine/tyrosine depletion in healthy women. Neuropsychopharmacology 2000, 22, 52–63. [Google Scholar] [CrossRef]
- McLean, A.; Rubinsztein, J.S.; Robbins, T.W.; Sahakian, B.J. The effects of tyrosine depletion in normal healthy volunteers: Implications for unipolar depression. Psychopharmacology 2004, 171, 286–297. [Google Scholar] [CrossRef]
- Akimitsu, O.; Wada, K.; Noji, T.; Taniwaki, N.; Krejci, M.; Nakade, M.; Takeuchi, H.; Harada, T. The relationship between consumption of tyrosine and phenylalanine as precursors of catecholamine at breakfast and the circadian typology and mental health in Japanese infants aged 2 to 5 years. J. Physiol. Anthropol. 2013, 32, 13. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Branched-chain amino acids and brain function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.A.; Shoaf, S.E.; Hommer, D.; Rawlings, R.; Linnoila, M. Effects of acute tryptophan depletion on plasma and cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid in normal volunteers. J. Neurochem. 1999, 72, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, M.H.; Fernstrom, J.D. Acute tyrosine depletion reduces tyrosine hydroxylation rate in rat central nervous system. Life Sci. 1995, 57, PL97–PL102. [Google Scholar] [CrossRef]
- Human, M.P.C. A framework for human microbiome research. Nature 2012, 486, 215–221. [Google Scholar]
- Liang, S.; Wu, X.; Jin, F. Gut-brain psychology: Rethinking psychology from the microbiota-gut-brain axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.R.; Hauck, L.; Jeffrey, B.M.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L.E. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 2015, 300, 128–140. [Google Scholar] [CrossRef]
- De Weerth, C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci. Biobehav. Rev. 2017, 83, 458–471. [Google Scholar] [CrossRef]
- Diaz Heijtz, R. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin. Fetal Neonatal Med. 2016, 21, 410–417. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Blumberg, R.S. Correlation between early-life regulation of the immune system by microbiota and allergy development. J. Allergy Clin. Immunol. 2017, 139, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N. Microbiome, HPA axis and production of endocrine hormones in the gut. Adv. Exp. Med. Biol. 2014, 817, 177–194. [Google Scholar] [PubMed]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Cryan, J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Borojevic, R.; Verdu, E.F.; Huizinga, J.D.; Ratcliffe, E.M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol. Motil. 2014, 26, 98–107. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Grossman, M.I. Neural and hormonal regulation of gastrointestinal function: An overview. Annu. Rev. Physiol. 1979, 41, 27–33. [Google Scholar] [CrossRef]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef]
- Oldham, M.C.; Konopka, G.; Iwamoto, K.; Langfelder, P.; Kato, T.; Horvath, S.; Geschwind, D.H. Functional organization of the transcriptome in human brain. Nat. Neurosci. 2008, 11, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Caputo, V.; Sinibaldi, L.; Fiorentino, A.; Parisi, C.; Catalanotto, C.; Pasini, A.; Cogoni, C.; Pizzuti, A. Brain derived neurotrophic factor (BDNF) expression is regulated by microRNAs miR-26a and miR-26b allele-specific binding. PLoS ONE 2011, 6, e28656. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, F.; Hong, G.; Pang, M.; Xu, H.; Li, H.; Tian, F.; Fang, R.; Yao, Y.; Liu, J. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci. Lett. 2016, 618, 159–166. [Google Scholar] [CrossRef]
- Spiljar, M.; Merkler, D.; Trajkovski, M. The immune system bridges the gut microbiota with systemic energy homeostasis: Focus on tlrs, mucosal barrier, and scfas. Front. Immunol. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Guan, N.L.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Arentsen, T.; Qian, Y.; Gkotzis, S.; Femenia, T.; Wang, T.; Udekwu, K.; Forssberg, H.; Diaz Heijtz, R. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry 2017, 22, 257–266. [Google Scholar] [CrossRef]
- de Weerth, C.; Fuentes, S.; Puylaert, P.; de Vos, W.M. Intestinal microbiota of infants with colic: Development and specific signatures. Pediatrics 2013, 131, e550–e558. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Harty, S.; Johnson, K.V.; Moeller, A.H.; Archie, E.A.; Schell, L.D.; Carmody, R.N.; Clutton-Brock, T.H.; Dunbar, R.I.M.; Burnet, P.W.J. Microbial transmission in animal social networks and the social microbiome. Nat. Ecol. Evol. 2020, 4, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef]
- Burnet, P.W.; Cowen, P.J. Psychobiotics highlight the pathways to happiness. Biol. Psychiatry 2013, 74, 708–709. [Google Scholar] [CrossRef]
- Burnett, S.; Sebastian, C.; Cohen Kadosh, K.; Blakemore, S.J. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neurosci. Biobehav. Rev. 2011, 35, 1654–1664. [Google Scholar] [CrossRef]
- Johnson, M.H. Interactive specialization: A domain-general framework for human functional brain development. Dev. Cogn. Neurosci. 2011, 1, 7–21. [Google Scholar] [CrossRef]
- Johnson, M.H.; Grossmann, T.; Cohen Kadosh, K. Mapping functional brain development: Building a social brain through interactive specialization. Dev. Psychol. 2009, 45, 151–159. [Google Scholar] [CrossRef]
- Chen, J.J.; Zeng, B.H.; Li, W.W.; Zhou, C.J.; Fan, S.H.; Cheng, K.; Zeng, L.; Zheng, P.; Fang, L.; Wei, H.; et al. Effects of gut microbiota on the microRNA and mRNA expression in the hippocampus of mice. Behav. Brain Res. 2017, 322, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Ryan, F.J.; Hoban, A.E.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Microbes & neurodevelopment—Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav. Immun. 2015, 50, 209–220. [Google Scholar] [PubMed]
- Marungruang, N.; Arévalo Sureda, E.; Lefrançoise, A.; Weström, B.; Nyman, M.; Prykhodko, O.; Fåk Hållenius, F. Impact of dietary induced precocious gut maturation on cecal microbiota and its relation to the blood-brain barrier during the postnatal period in rats. Neurogastroenterol. Motil. 2018, 30, e13285. [Google Scholar] [CrossRef] [PubMed]
- Aatsinki, A.K.; Lahti, L.; Uusitupa, H.M.; Munukka, E.; Keskitalo, A.; Nolvi, S.; O’Mahony, S.; Pietilä, S.; Elo, L.L.; Eerola, E.; et al. Gut microbiota composition is associated with temperament traits in infants. Brain Behav. Immun. 2019, 80, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.M.; Galley, J.D.; Hade, E.M.; Schoppe-Sullivan, S.; Kamp Dush, C.; Bailey, M.T. Gut microbiome composition is associated with temperament during early childhood. Brain Behav. Immun. 2015, 45, 118–127. [Google Scholar] [CrossRef]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant gut microbiome associated with cognitive development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef]
- Oriá, R.B.; Murray-Kolb, L.E.; Scharf, R.J.; Pendergast, L.L.; Lang, D.R.; Kolling, G.L.; Guerrant, R.L. Early-life enteric infections: Relation between chronic systemic inflammation and poor cognition in children. Nutr. Rev. 2016, 74, 374–386. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Van Best, N.; Hornef, M.W.; Savelkoul, P.H.; Penders, J. On the origin of species: Factors shaping the establishment of infant’s gut microbiota. Birth Defects Res. C. Embryo Today 2015, 105, 240–251. [Google Scholar] [CrossRef]
- Grześkowiak, Ł.; Collado, M.C.; Mangani, C.; Maleta, K.; Laitinen, K.; Ashorn, P.; Isolauri, E.; Salminen, S. Distinct gut microbiota in southeastern African and northern European infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 812–816. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Janik, R.; Thomason, L.A.M.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 2016, 125, 988–995. [Google Scholar] [CrossRef]
- Kodish, I.; Rockhill, C.; Ryan, S.; Varley, C. Pharmacotherapy for anxiety disorders in children and adolescents. Pediatri. Clin. 2011, 58, 55–72. [Google Scholar] [CrossRef]
- Pärtty, A.; Kalliomäki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res. 2015, 77, 823–828. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401.e1. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3’Sialyllactose and 6’Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Szklany, K.; Wopereis, H.; de Waard, C.; van Wageningen, T.; An, R.; van Limpt, K.; Knol, J.; Garssen, J.; Knippels, L.M.J.; Belzer, C.; et al. Supplementation of dietary non-digestible oligosaccharides from birth onwards improve social and reduce anxiety-like behaviour in male BALB/c mice. Nutr. Neurosci. 2020, 23, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, A.; Dwipoerwantoro, P.G.; Kadim, M.; Alatas, S.; Conus, N.; Lestarina, L.; Bouisset, F.; Steenhout, P. Improved growth of toddlers fed a milk containing synbiotics. Asia Pac. J. Clin. Nutr. 2011, 20, 69–76. [Google Scholar]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef]
- Johnstone, N.; Milesi, C.; Burn, O.; van den Bogert, B.; Nauta, A.; Hart, K.; Sowden, P.; Burnet, P.W.J.; Kadosh, K.C. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy female volunteers are associated with reduced negative bias and the gut bacterial composition. medRxiv 2019, 19011403. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Shoaie, S.; Bergentall, M.; Ghaffari, P.; Zhang, C.; Larsson, E.; Bäckhed, F.; Nielsen, J. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol. Syst. Biol. 2015, 11, 834. [Google Scholar] [CrossRef]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.; Lane, H.Y.; Tsai, G.E. Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation. Curr. Pharm. Des. 2014, 20, 5180–5185. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.I.; Pepe, G.J.; Burket, J.A.; Winebarger, E.E.; Herndon, A.L.; Benson, A.D. D-cycloserine improves sociability and spontaneous stereotypic behaviors in 4-week old mice. Brain Res. 2012, 1439, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Cormack, B.E.; Harding, J.E.; Miller, S.P.; Bloomfield, F.H. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: A narrative review. Nutrients 2019, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen Kadosh, K.; Muhardi, L.; Parikh, P.; Basso, M.; Jan Mohamed, H.J.; Prawitasari, T.; Samuel, F.; Ma, G.; Geurts, J.M.W. Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children—An Update and Novel Insights. Nutrients 2021, 13, 199. https://doi.org/10.3390/nu13010199
Cohen Kadosh K, Muhardi L, Parikh P, Basso M, Jan Mohamed HJ, Prawitasari T, Samuel F, Ma G, Geurts JMW. Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children—An Update and Novel Insights. Nutrients. 2021; 13(1):199. https://doi.org/10.3390/nu13010199
Chicago/Turabian StyleCohen Kadosh, Kathrin, Leilani Muhardi, Panam Parikh, Melissa Basso, Hamid Jan Jan Mohamed, Titis Prawitasari, Folake Samuel, Guansheng Ma, and Jan M. W. Geurts. 2021. "Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children—An Update and Novel Insights" Nutrients 13, no. 1: 199. https://doi.org/10.3390/nu13010199
APA StyleCohen Kadosh, K., Muhardi, L., Parikh, P., Basso, M., Jan Mohamed, H. J., Prawitasari, T., Samuel, F., Ma, G., & Geurts, J. M. W. (2021). Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children—An Update and Novel Insights. Nutrients, 13(1), 199. https://doi.org/10.3390/nu13010199