Low Dietary Magnesium and Overweight/Obesity in a Mediterranean Population: A Detrimental Synergy for the Development of Hypertension. The SUN Project
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Ethics
2.3. Dietary and Exposure Assessment
2.4. Ascertainment of Incident Hypertension
2.5. Other Covariates
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Participants
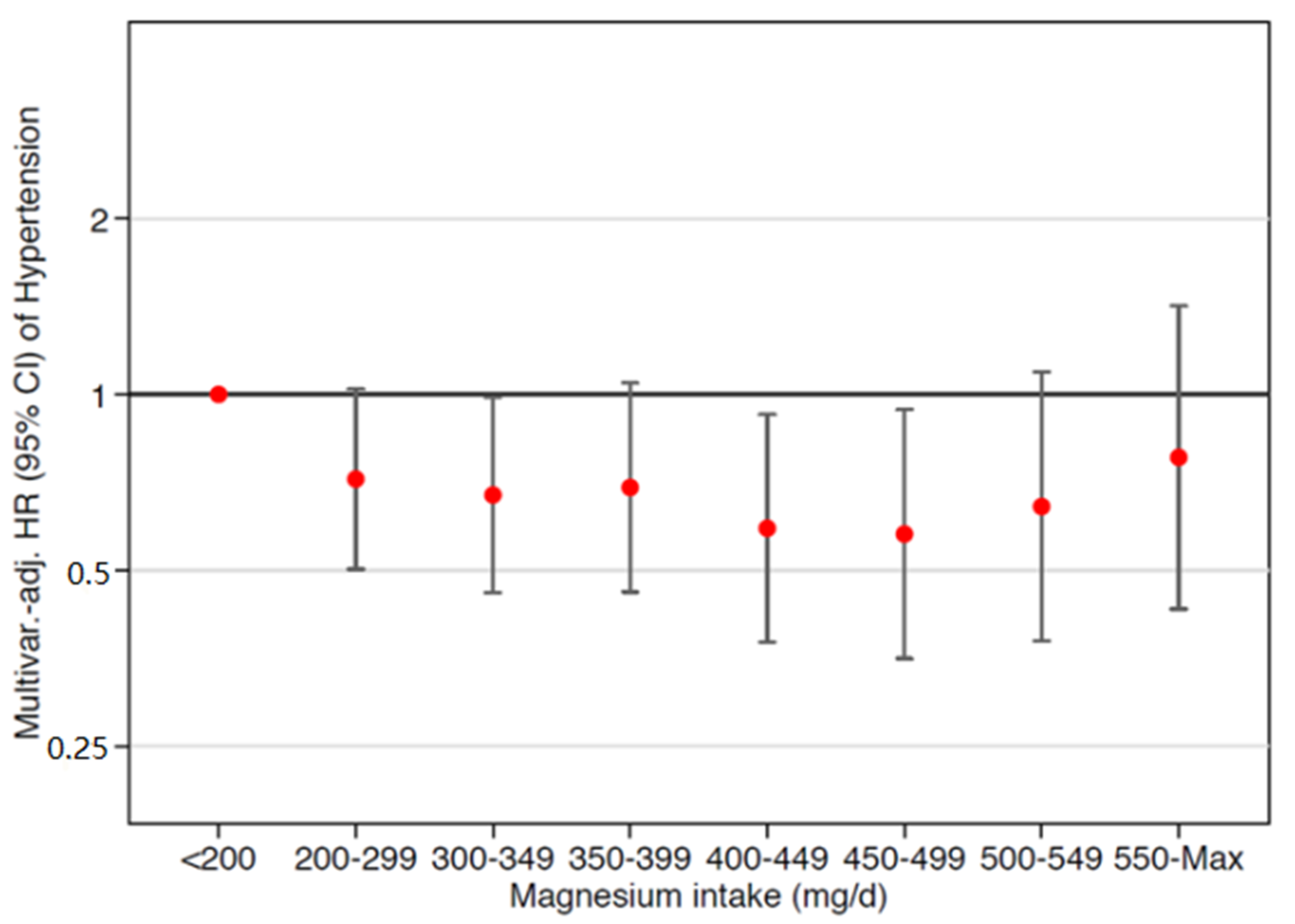
3.2. Magnesium Intake, Incident Hypertension, and Obesity
3.3. Magnesium Intake, Incident Hypertension, and Mediterranean Diet
3.4. Sensitivity Analyses
4. Discussion
5. Future Lines of Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Health Observatory (GHO) Data. Blood Pressure. 2018. Available online: http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence/en/ (accessed on 20 May 2020).
- Olsen, M.H.; Angell, S.Y.; Asma, S.; Boutouyrie, P.; Burger, D.; Chirinos, J.A.; Damasceno, A.; Delles, C.; Gimenez-Roqueplo, A.P.; Hering, D.; et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The Lancet Commission on hypertension. Lancet 2016, 388, 2665–2712. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Busardò, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Asp. Med. 2003, 24, 39–52. [Google Scholar] [CrossRef]
- Grober, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbagallo, M.; Dominguez, L.J.; Resnick, L.M. Magnesium metabolism in hypertension and type 2 diabetes mellitus. Am. J. Ther. 2007, 14, 375–385. [Google Scholar] [CrossRef]
- Villa-Bellosta, R. Impact of magnesium: Calcium ratio on calcification of the aortic wall. PLoS ONE 2017, 12, e0178872. [Google Scholar] [CrossRef] [Green Version]
- Resnick, L.M.; Laragh, J.H.; Sealey, J.E.; Alderman, M.H. Divalent cations in essential hypertension. Relations between serum ionized calcium, magnesium, and plasma renin activity. N. Engl. J. Med. 1983, 309, 888–891. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Pineo, A.; Belvedere, M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes. Res. 2010, 23, 131–137. [Google Scholar]
- Shechter, M.; Sharir, M.; Labrador, M.J.; Forrester, J.; Silver, B.; Bairey Merz, C.N. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 2000, 102, 2353–2358. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, T.Y.; van Dam, R.M.; Manson, J.E.; Hu, F.B. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am. J. Clin. Nutr. 2007, 85, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Halacheva, L. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soave, P.M.; Conti, G.; Costa, R.; Arcangeli, A. Magnesium and anaesthesia. Curr. Drug Targets 2009, 10, 734–743. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Magnesium; National Institutes of Health: Bethesda, MD, USA, 2018. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ (accessed on 20 May 2020).
- Ford, E.S.; Mokdad, A.H. Dietary magnesium intake in a national sample of US adults. J. Nutr. 2003, 133, 2879–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensink, G.B.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, D.E.; Mainous, A.G.; Geesey, M.E., 3rd; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef]
- Rosanoff, A.; Dai, Q.; Shapses, S.A. Essential Nutrient Interactions: Does Low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Adv. Nutr. 2016, 7, 25–43. [Google Scholar] [CrossRef] [Green Version]
- US Department of Health and Human Services. US Department of Agriculture (2015) 2015–2020 Dietary Guidelines for Americans, 8th ed.; US Department of Health and Human Services: Washington, DC, USA, 2015. Available online: http://www.health.gov/DietaryGuidelines (accessed on 20 May 2020).
- Barbagallo, M.; Belvedere, M.; Dominguez, L.J. Magnesium homeostasis and aging. Magnes. Res. 2009, 22, 235–246. [Google Scholar] [CrossRef] [Green Version]
- King, D.E.; Mainous, A.G.; Geesey, M.E., 3rd; Ellis, T. Magnesium intake and serum C-reactive protein levels in children. Magnes. Res. 2007, 20, 32–36. [Google Scholar]
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B.; et al. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2020, 59, 263–272. [Google Scholar] [CrossRef]
- Van Leer, E.M.; Seidell, J.C.; Kromhout, D. Dietary calcium, potassium, magnesium and blood pressure in the Netherlands. Int. J. Epidemiol. 1995, 24, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Folsom, A.R.; Melnick, S.L.; Eckfeldt, J.H.; Sharrett, A.R.; Nabulsi, A.A.; Hutchinson, R.G.; Metcalf, P.A. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: The ARIC study. Atherosclerosis Risk in Communities Study. J. Clin. Epidemiol. 1995, 48, 927–940. [Google Scholar] [CrossRef]
- Kesteloot, H.; Joossens, J.V. Relationship of dietary sodium, potassium, calcium, and magnesium with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension 1988, 12, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witteman, J.C.; Willett, W.C.; Stampfer, M.J.; Colditz, G.A.; Sacks, F.M.; Speizer, F.E.; Rosner, B.; Hennekens, C.H. A prospective study of nutritional factors and hypertension among US women. Circulation 1989, 80, 1320–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascherio, A.; Hennekens, C.; Willett, W.C.; Sacks, F.; Rosner, B.; Manson, J.; Witteman, J.; Stampfer, M.J. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension 1996, 27, 1065–1072. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Giovannucci, E.L.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Sacks, F.; Stampfer, M.J. A prospective study of nutritional factors and hypertension among US men. Circulation 1992, 86, 1475–1484. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Liu, K.; Daviglus, M.L.; Morris, S.J.; Loria, C.M.; Van Horn, L.; Jacobs, D.R., Jr.; Savage, P.J. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006, 113, 1675–1682. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Sesso, H.D.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Dietary magnesium intake and risk of incident hypertension among middle-aged and older US women in a 10-year follow-up study. Am. J. Cardiol. 2006, 98, 1616–1621. [Google Scholar] [CrossRef]
- Peacock, J.M.; Folsom, A.R.; Arnett, D.K.; Eckfeldt, J.H.; Szklo, M. Relationship of serum and dietary magnesium to incident hypertension: The Atherosclerosis Risk in Communities (ARIC) Study. Ann. Epidemiol. 1999, 9, 159–165. [Google Scholar] [CrossRef]
- Charlton, K.E.; Steyn, K.; Levitt, N.S.; Zulu, J.V.; Jonathan, D.; Veldman, F.J.; Nel, J.H. Diet and blood pressure in South Africa: Intake of foods containing sodium, potassium, calcium, and magnesium in three ethnic groups. Nutrition 2005, 21, 39–50. [Google Scholar] [CrossRef]
- Townsend, M.S.; Fulgoni, V.L.; Stern, J.S., 3rd; Adu-Afarwuah, S.; McCarron, D.A. Low mineral intake is associated with high systolic blood pressure in the Third and Fourth National Health and Nutrition Examination Surveys: Could we all be right? Am. J. Hypertens. 2005, 18 Pt 1, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T.; Xun, P.; Song, Y.; Rosanoff, A.; Shechter, M.; He, K. The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 921–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Jee, S.H.; Miller, E.R.; Guallar, E., 3rd; Singh, V.K.; Appel, L.J.; Klag, M.J. The effect of magnesium supplementation on blood pressure: A meta-analysis of randomized clinical trials. Am. J. Hypertens. 2002, 15, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e426–e483. [Google Scholar]
- Martinez-Gonzalez, M.A.; Sanchez-Villegas, A.; De Irala, J.; Marti, A.; Martinez, J.A. Mediterranean diet and stroke: Objectives and design of the SUN project. Seguimiento Universidad de Navarra. Nutr. Neurosci. 2002, 5, 65–73. [Google Scholar] [CrossRef]
- Segui-Gomez, M.; de la Fuente, C.; Vazquez, Z.; de Irala, J.; Martinez-Gonzalez, M.A. Cohort profile: The ‘Seguimiento Universidad de Navarra’ (SUN) study. Int. J. Epidemiol. 2006, 35, 1417–1422. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C. Issues in analysis and presentation of dietary data. In Nutritional Epidemiology, 3rd ed.; Willett, W.C., Ed.; Oxford University Press: New York, NY, USA, 2012; pp. 321–346. [Google Scholar]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef]
- Fernandez-Ballart, J.D.; Pinol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.A.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente-Arrillaga, C.; Ruiz, Z.V.; Bes-Rastrollo, M.; Sampson, L.; Martinez-Gonzalez, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Mataix Verdu, J. Tabla de Composición de Alimentos, 5th ed.; Universidad de Granada: Granada, Spain, 2009. [Google Scholar]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos, 16th ed.; Piramide: Madrid, Spain, 2013. [Google Scholar]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, A.; Beunza, J.J.; Delgado-Rodriguez, M.; Martinez-Gonzalez, M.A. Validation of self reported diagnosis of hypertension in a cohort of university graduates in Spain. BMC Public Health 2005, 5, 94. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Montero, A.; Beunza, J.J.; Bes-Rastrollo, M.; Barrio, M.T.; de la Fuente-Arrillaga, C.; Moreno-Galarraga, L.; Martínez-González, M.A. Validación de los componentes del síndrome metabólico autodeclarados en un estudio de cohortes. Gac. Sanit. 2011, 25, 303–307. [Google Scholar] [CrossRef] [Green Version]
- Barrio-Lopez, M.T.; Bes-Rastrollo, M.; Beunza, J.J.; Fernandez-Montero, A.; Garcia-Lopez, M.; Martinez-Gonzalez, M.A. Validation of metabolic syndrome using medical records in the SUN cohort. BMC Public Health 2011, 11, 867. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Lopez-Fontana, C.; Varo, J.J.; Sanchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32 (Suppl. 9), S498–S504. [Google Scholar] [CrossRef] [Green Version]
- Bes-Rastrollo, M.; Perez Valdivieso, J.R.; Sanchez-Villegas, A.; Alonso, A.; Martinez-Gonzalez, M.A. Validación del peso e índice de masa corporal auto-declarados de los participantes de una cohorte de graduados universitarios. Rev. Esp. Obes. 2005, 3, 183–189. [Google Scholar]
- Greenland, S. Analysis of Polytomous exposures and outcomes. In Modern Epidemiology, 3rd ed.; Rothman, K.J., Greenland, S., Lash, T., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Iqbal, S.; Klammer, N.; Ekmekcioglu, C. The Effect of Electrolytes on Blood Pressure: A Brief Summary of Meta-Analyses. Nutrients 2019, 11, 1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; You, J.; Zhao, N.; Xu, H. Magnesium Regulates Endothelial Barrier Functions through TRPM7, MagT1, and S1P1. Adv. Sci. 2019, 6, 1901166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linderman, G.C.; Lu, J.; Lu, Y.; Sun, X.; Xu, W.; Nasir, K.; Schulz, W.; Jiang, L.; Krumholz, H.M. Association of Body Mass Index with Blood Pressure among 1.7 Million Chinese Adults. JAMA Netw. Open 2018, 1, e181271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelber, R.P.; Gaziano, J.M.; Manson, J.E.; Buring, J.E.; Sesso, H.D. A prospective study of body mass index and the risk of developing hypertension in men. Am. J. Hypertens. 2007, 20, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuger, S.L.; Sui, X.; Church, T.S.; Meriwether, R.A.; Blair, S.N. Body mass index as a predictor of hypertension incidence among initially healthy normotensive women. Am. J. Hypertens. 2008, 21, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Ruderman, N.; Chisholm, D.; Pi-Sunyer, X.; Schneider, S. The metabolically obese, normal-weight individual revisited. Diabetes 1998, 47, 699–713. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Schneider, S.H.; Berchtold, P. The “metabolically-obese,” normal-weight individual. Am. J. Clin. Nutr. 1981, 34, 1617–1621. [Google Scholar] [CrossRef]
- Lee, S.H.; Han, K.; Yang, H.K.; Kim, H.-S.; Cho, J.-H.; Kwon, H.-S.; Park, Y.-M.; Cha, B.-Y.; Yoon, K.-H. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr. Diabetes 2015, 5, e149. [Google Scholar] [CrossRef] [Green Version]
- Yaghootkar, H.; Scott, R.A.; White, C.C.; Zhang, W.; Speliotes, E.; Munroe, P.B.; Ehret, G.B.; Bis, J.C.; Fox, C.S.; Walker, M. Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes 2014, 63, 4369–4377. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Romero, F.; Rodriguez-Moran, M. Serum magnesium in the metabolically-obese normal-weight and healthy-obese subjects. Eur. J. Intern. Med. 2013, 24, 639–643. [Google Scholar] [CrossRef]
- Rodriguez-Moran, M.; Guerrero-Romero, F. Oral magnesium supplementation improves the metabolic profile of metabolically obese, normal-weight individuals: A randomized double-blind placebo-controlled trial. Arch. Med. Res. 2014, 45, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Durazzo, M.; Guidi, S. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am. J. Clin. Nutr. 2006, 84, 1062–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, T.T.; Hu, F.B.; Pereira, M.A.; Liu, S.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Whole-grain intake and the risk of type 2 diabetes: A prospective study in men. Am. J. Clin. Nutr. 2002, 76, 535–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Wilson, P.W.F.; Jacques, P.F. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am. J. Clin. Nutr. 2002, 76, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzetto, L.; Costabile, G.; Della Pepa, G.; Ciciola, P.; Vetrani, C.; Vitale, M.; Rivellese, A.A.; Annuzzi, A. Dietary Fibre as a Unifying Remedy for the Whole Spectrum of Obesity-Associated Cardiovascular Risk. Nutrients 2018, 10, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melse-Boonstra, A. Bioavailability of Micronutrients from Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 2000, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Chaimani, A.; Schwedhelm, C.; Toledo, E.; Pünsch, M.; Hoffmann, G.; Boeing, H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: A systematic review and network meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2674–2687. [Google Scholar] [CrossRef]
- Sun, Q.; Geng, Z.; Willett, W.; Hu, F. Re: The use of food frequency questionnaires (FFQs) is both pseudo-scientific and illogical. BMJ 2016, 355, i5796. [Google Scholar]


| Dietary Magnesium Source | % of Magnesium Intake | Cumulative R2 |
|---|---|---|
| Vegetables | 27.0 | 0.36 |
| Fruit | 13.0 | 0.50 |
| Dairy | 15.0 | 0.63 |
| Nuts | 3.7 | 0.69 |
| Legumes | 6.9 | 0.73 |
| Fish and seafood | 7.3 | 0.85 |
| Magnesium Intake (mg/d) | |||
|---|---|---|---|
| <200 | 200–500 | >500 | |
| Mg intake (mg/d) | 155.1 ± 42.5 | 370.8 ± 72.8 | 588.7 ± 85.6 |
| N | 267 | 10782 | 3008 |
| Women, % | 63.7 | 66.6 | 68.7 |
| Age, y | 37.0 (11.3) | 34.8 (10.5) | 36.7 (11.2) |
| Married women, % | 44.6 | 45.8 | 47.3 |
| Married men, % | 55.4 | 54.2 | 52.7 |
| University education, y | 5.1 | 5.0 | 5.0 |
| BMI, kg/m2 | 23.5 (3.4) | 23.0 (3.2) | 22.9 (3.1) |
| Smoking | |||
| Current, % | 33.3 | 27.3 | 21.7 |
| Former smoker, % | 18.7 | 21.5 | 23.5 |
| Alcohol (g/d) | 3.32 (5.21) | 5.8 (8.48) | 6.38 (9.75) |
| Leisure-time physical activity, METs-h/week | 19.8 (21.4) | 25.4 (21.7) | 32.2 (28.6) |
| Television watching, h/d | 1.6 (1.1) | 1.6 (1.2) | 1.6 (1.2) |
| History of depression at baseline, % | 16.1 | 10.5 | 11.5 |
| Hypercholesterolemia at baseline, % | 16.9 | 13.0 | 14.1 |
| Total energy intake, kcal/d | 1110 (290) | 2216 (518) | 2947 (450) |
| Adoption of special diets, % | 8.3 | 6.8 | 8.1 |
| Between-meal snacking, % | 28.2 | 34.5 | 64.7 |
| Dietary consumption | |||
| Mediterranean diet score b | 2.49 ± 1.1 | 3.74 ± 1.7 | 5.22 ± 1.6 |
| Vegetables (g/d) | 146 (137) | 452 (229) | 820 (465) |
| Fruit (g/d) | 88 (109) | 282 (203) | 573 (420) |
| Legumes (g/d) | 8.5 (10.2) | 21 (13) | 31 (28) |
| Cereals (g/d) | 33 (38) | 96 (66) | 127 (80) |
| Whole bread (g/d) | 1.2 (4.7) | 1.2 (4.7) | 29 (49) |
| Nuts (g/d) | 1.6 (2.9) | 5.4 (7.6) | 14 (19) |
| Olive oil (g/d) | 8.8 (12.3) | 15 (13) | 18 (14) |
| Eggs (g/d) | 14 (15) | 23 (15) | 25 (16) |
| Fish and other seafood (g/d) | 49 (42) | 88 (50) | 129 (77) |
| Whole dairy products (g/d) | 106 (139) | 197 (190) | 224 (232) |
| Low-fat dairy products (g/d) | 82 (121) | 203 (218) | 324 (315) |
| Meat (g/d) | 100 (69) | 173 (75) | 194 (89) |
| Coffee (cups/d) | 3.3 (2.6) | 3.8 (2.4) | 3.9 (2.5) |
| Sugar-sweetened beverages (servings/d) c | 0.20 (0.5) | 0.21 (0.4) | 0.19 (0.4) |
| Dietary intake | |||
| Carbohydrates (% of energy) | 39 (13) | 43 (7.0) | 46 (7.2) |
| Protein (% of energy) | 18 (7.4) | 18 (3.2) | 18 (3.1) |
| Total fat (% of energy) | 41 (10) | 37 (6.3) | 34 (6.3) |
| MUFAs (% of energy) | 18 (6.4) | 16 (3.6) | 15 (3.4) |
| SFAs (% of energy) | 15 (5.4) | 13 (3) | 11 (3.1) |
| PUFAs (% of energy) | 5.5 (2.5) | 5.2 (1.5) | 5.0 (1.5) |
| Vitamin C (mg/d) | 85 (72) | 241 (113) | 435 (193) |
| Vitamin D (mcg/d) | 1.9 (1.7) | 3.4 (2.1) | 4.9 (3.2) |
| Iron from heme sources (mg/d) | 6.7 (2.3) | 15 (3.3) | 23 (4.4) |
| Folate (mcg/d) | 136 (71) | 356 (111) | 615 (197) |
| Na intake (mg/d) | 2129 ± 1459 | 3785 ± 2112 | 4666 ± 2828 |
| K intake (g/d) | 1720 ± 612 | 4245 ± 962 | 6823 ± 1442 |
| Ca intake (g/d) | 502 ± 245 | 1129 ± 368 | 1651 ± 505 |
| Dietary fiber (g/1000 kcal/d) | 8.3 (4.8) | 24 (7.4) | 43 (13) |
| Dietary Magnesium | Categories of Daily Magnesium Intake (mg/day) | |||||
|---|---|---|---|---|---|---|
| <200 | 200–300 | 300–400 | 400–500 | >500 | p-Trend | |
| n | 267 | 2051 | 4659 | 4072 | 3008 | |
| Incident hypertension (n) | 43 | 212 | 459 | 354 | 338 | |
| Person—years | 2698 | 19541 | 45294 | 39469 | 28359 | |
| Median (g/d) | 168.9 | 267.4 | 353.0 | 444.5 | 536.5 | |
| Crude incident hypertension rate (×10−3) | 1.59 | 1.08 | 1.10 | 0.90 | 1.12 | |
| Age- and sex-adjusted HR Model 1 | 1 (ref.) | 0.77 (0.56, 1.07) | 0.73 (0.53, 0.99) | 0.64 (0.46, 0.87) | 0.78 (0.57, 1.07) | 0.455 |
| Multivariate-adjusted HR b Model 2 | 1 (ref.) | 0.69 (0.49, 0.97) | 0.66 (0.47, 0.94) | 0.57 (0.39, 0.83) | 0.69 (0.45, 1.04) | 0.618 |
| Multivariate-adjusted HR c Model 3 | 1 (ref.) | 0.66 (0.47, 0.93) | 0.64 (0.45, 0.90) | 0.54 (0.37, 0.80) | 0.66 (0.43, 0.99) | 0.589 |
| Multivariate-adjusted HR d Model 4 | 1 (ref.) | 0.67 (0.47, 0.96) | 0.65 (0.45, 0.95) | 0.55 (0.36, 0.84) | 0.66 (0.39, 1.10) | 0.470 |
| n | Incident Hypertension n | HR (95% CI) b | |
|---|---|---|---|
| Main analysis | 14,057 | 1406 | 1.51 (1.08, 2.11) |
| Including only women | 9416 | 676 | 1.77 (1.10, 2.87) |
| Changing allowable energy limits (percentiles 1–99) c | 14,944 | 1473 | 1.20 (0.70, 2.05) |
| Censoring follow-up at 8 y | 9416 | 446 | 2.33 (1.25, 4.36) |
| Censoring follow-up at 6 y | 9416 | 365 | 2.42 (1.12, 5.23) |
| Excluding early incident hypertension (first 2 y) | 13,766 | 1210 | 1.54 (1.07, 2.21) |
| Adjusting for the Mediterranean diet score d | 14,057 | 1406 | 1.48 (1.06, 2.06) |
| Adjusting for fiber intake | 14,057 | 1406 | 1.48 (1.06, 2.06) |
| Adjusting for fiber intake below the median | 14,057 | 1406 | 1.76 (1.01, 3.09) |
| Adjusting for use of diuretics | 14,057 | 1406 | 1.48 (1.06, 2.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez, L.J.; Gea, A.; Ruiz-Estigarribia, L.; Sayón-Orea, C.; Fresán, U.; Barbagallo, M.; Ruiz-Canela, M.; Martínez-González, M.A. Low Dietary Magnesium and Overweight/Obesity in a Mediterranean Population: A Detrimental Synergy for the Development of Hypertension. The SUN Project. Nutrients 2021, 13, 125. https://doi.org/10.3390/nu13010125
Dominguez LJ, Gea A, Ruiz-Estigarribia L, Sayón-Orea C, Fresán U, Barbagallo M, Ruiz-Canela M, Martínez-González MA. Low Dietary Magnesium and Overweight/Obesity in a Mediterranean Population: A Detrimental Synergy for the Development of Hypertension. The SUN Project. Nutrients. 2021; 13(1):125. https://doi.org/10.3390/nu13010125
Chicago/Turabian StyleDominguez, Ligia J., Alfredo Gea, Liz Ruiz-Estigarribia, Carmen Sayón-Orea, Ujue Fresán, Mario Barbagallo, Miguel Ruiz-Canela, and Miguel A. Martínez-González. 2021. "Low Dietary Magnesium and Overweight/Obesity in a Mediterranean Population: A Detrimental Synergy for the Development of Hypertension. The SUN Project" Nutrients 13, no. 1: 125. https://doi.org/10.3390/nu13010125





