Mediterranean Diet Maintained Platelet Count within a Healthy Range and Decreased Thrombocytopenia-Related Mortality Risk: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Intervention
2.3. Outcomes
2.4. Covariates
2.5. Power Analyses
2.6. Statistical Analyses
3. Results
3.1. Study Population
3.2. MedDiet Interventions and Platelet Count
3.3. MedDiet Interventions and Platelet Count-Related Disorders
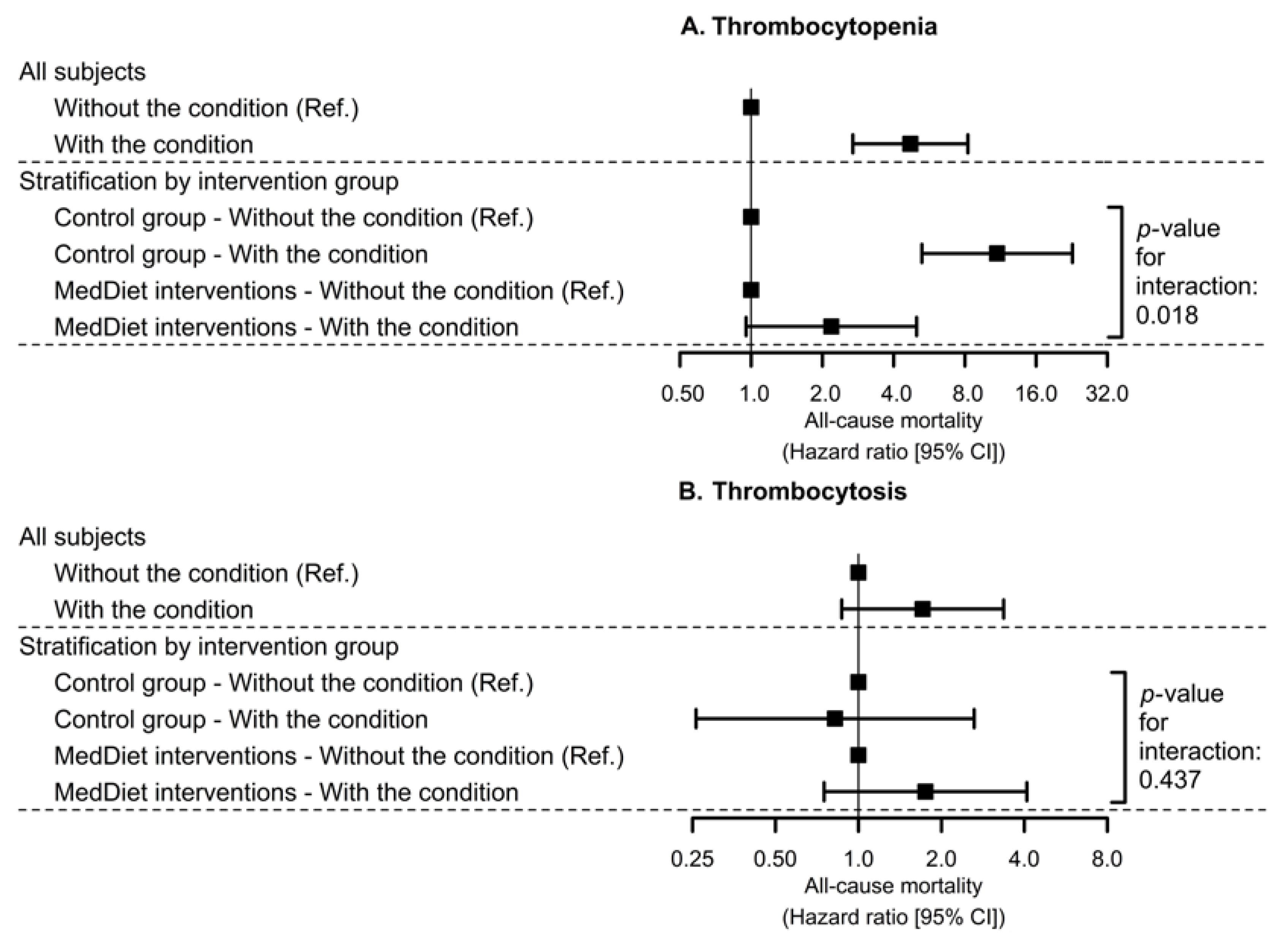
3.4. Interaction between Platelet Count-Related Disorders at Baseline and MedDiet on All-Cause Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; PREDIMED INVESTIGATORS. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, Á.; Castañer, O.; Tresserra-Rimbau, A.; Pintó, X.; Fitó, M.; Casas, R.; Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Lapetra, J.; et al. Mediterranean Diet and Atherothrombosis Biomarkers: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2020, 64, e2000350. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Pastori, D.; Farcomeni, A.; Nocella, C.; Bartimoccia, S.; Vicario, T.; Bucci, T.; Carnevale, R.; Violi, F. Mediterranean diet reduces thromboxane A2 production in atrial fibrillation patients. Clin. Nutr. 2015, 34, 899–903. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Sala-Vila, A.; Crespo, J.; Ros, E.; Estruch, R.; Badimon, L. The Mediterranean diet decreases prothrombotic microvesicle release in asymptomatic individuals at high cardiovascular risk. Clin. Nutr. 2020, 39, 3377–3384. [Google Scholar] [CrossRef]
- Zucoloto, A.Z.; Jenne, C.N. Platelet-Neutrophil Interplay: Insights Into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019, 6, 85. [Google Scholar] [CrossRef]
- Han, S.; Wu, P.; Duan, M.; Yang, F.; He, W.; Wu, N.; Hu, X.; Gan, D.; Wang, G.; Yang, M.; et al. The crosstalk between platelets and body fat: A reverse translational study. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; De Curtis, A.; Donati, M.B.; Cerletti, C.; De Gaetano, G.; Iacoviello, L. Age- and sex-based ranges of platelet count and cause-specific mortality risk in an adult general population: Prospective findings from the Moli-sani study. Platelets 2018, 29, 312–315. [Google Scholar] [CrossRef]
- Song, P.S.; Ahn, K.T.; Jeong, J.-O.; Jeon, K.-H.; Song, Y.B.; Gwon, H.-C.; Rha, S.-W.; Jeong, M.H.; Seong, I.-W. Association of baseline platelet count with all-cause mortality after acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2020. [Google Scholar] [CrossRef]
- Patti, G.; Di Martino, G.; Ricci, F.; Renda, G.; Gallina, S.; Hamrefors, V.; Melander, O.; Sutton, R.; Engström, G.; De Caterina, R.; et al. Platelet Indices and Risk of Death and Cardiovascular Events: Results from a Large Population-Based Cohort Study. Thromb. Haemost. 2019, 119, 1773–1784. [Google Scholar] [CrossRef]
- Lederer, A.K.; Maul-Pavicic, A.; Hannibal, L.; Hettich, M.; Steinborn, C.; Gründemann, C.; Zimmermann-Klemd, A.M.; Müller, A.; Sehnert, B.; Salzer, U.; et al. Vegan diet reduces neutrophils, monocytes and platelets related to branched-chain amino acids—A randomized, controlled trial. Clin. Nutr. 2020, 39, 3241–3250. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; De Curtis, A.; Costanzo, S.; Persichillo, M.; Donati, M.B.; Cerletti, C.; Iacoviello, L.; De Gaetano, G.; Moli-sani Project Investigators. Adherence to the Mediterranean diet is associated with lower platelet and leukocyte counts: Results from the Moli-sani study. Blood 2014, 123, 3037–3044. [Google Scholar] [CrossRef]
- Ambring, A.; Johansson, M.; Axelsen, M.; Gan, L.M.; Strandvik, B.; Friberg, P. Mediterranean-inspired diet lowers the ratio of serum phospholipid n-6 to n-3 fatty acids, the number of leukocytes and platelets, and vascular endothelial growth factor in healthy subjects. Am. J. Clin. Nutr. 2006, 83, 575–581. [Google Scholar] [CrossRef]
- Swain, F.; Bird, R. How I approach new onset thrombocytopenia. Platelets 2020, 31, 285–290. [Google Scholar] [CrossRef]
- Soltani, S.; Jayedi, A.; Shab-Bidar, S.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to the Mediterranean Diet in Relation to All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, 1029–1039. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ros, E.; Covas, M.I.; Fiol, M.; Warnberg, J.; Aros, F.; Ruiz-Gutierrez, V.; Lamuela-Raventos, R.M.; et al. Cohort Profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M.; Favaloro, E.J.; Lippi, G. Drug-Induced Thrombocytopenia: Mechanisms and Laboratory Diagnostics. Semin. Thromb. Hemost. 2020, 46, 264–274. [Google Scholar] [CrossRef]
- Babio, N.; Ibarrola-Jurado, N.; Bulló, M.; Martínez-González, M.Á.; Wärnberg, J.; Salaverría, I.; Ortega-Calvo, M.; Estruch, R.; Serra-Majem, L.; Covas, M.I.; et al. White Blood Cell Counts as Risk Markers of Developing Metabolic Syndrome and Its Components in the Predimed Study. PLoS ONE 2013, 8, e58354. [Google Scholar] [CrossRef] [PubMed]
- Biino, G.; Santimone, I.; Minelli, C.; Sorice, R.; Frongia, B.; Traglia, M.; Ulivi, S.; Di Castelnuovo, A.; Gögele, M.; Nutile, T.; et al. Age- And Sex-Related Variations in Platelet Count in Italy: A Proposal of Reference Ranges Based on 40987 Subjects’ Data. PLoS ONE 2013, 8, e54289. [Google Scholar] [CrossRef] [PubMed]
- Weiliang Qiu, A.; Chavarro, J.; Weiliang Qiu, M.; Qiu, W.; Chavarro, J.; Lazarus, R.; Rosner, B.; Ma, J. Package “PowerSurvEpi”: Power and Sample Size Calculation for Survival Analysis of Epidemiological Studies. 2018. Available online: https://cran.r-project.org/web/packages/powerSurvEpi/powerSurvEpi.pdf (accessed on 8 January 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Stringhini, S.; Zaninotto, P.; Kumari, M.; Kivimäki, M.; Batty, G.D. Lifecourse socioeconomic status and type 2 diabetes: The role of chronic inflammation in the English Longitudinal Study of Ageing. Sci. Rep. 2016, 6, 24780. [Google Scholar] [CrossRef]
- Therneau, T.M. Package “Survival”: Survival Analysis. 2018. Available online: https://cran.r-project.org/web/packages/survival/survival.pdf (accessed on 21 December 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: https://www.r-project.org/ (accessed on 21 December 2020).
- Vinholt, P.J.; Hvas, A.M.; Frederiksen, H.; Bathum, L.; Jørgensen, M.K.; Nybo, M. Platelet count is associated with cardiovascular disease, cancer and mortality: A population-based cohort study. Thromb. Res. 2016, 148, 136–142. [Google Scholar] [CrossRef]
- Gill, D.; Monori, G.; Georgakis, M.K.; Tzoulaki, I.; Laffan, M. Genetically Determined Platelet Count and Risk of Cardiovascular Disease: Mendelian Randomization Study. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wei, Y.; Zhang, R.; Dong, X.; Shen, S.; Zhao, Y.; Bai, J.; Albanes, D.; Caporaso, N.E.; Landi, M.T.; et al. Elevated platelet count appears to be causally associated with increased risk of lung cancer: A mendelian randomization analysis. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Chiva-Blanch, G.; Ros, E.; Martínez-González, M.-A.; Covas, M.-I.; Salas-Salvadó, J.; Fiol, M.; Arós, F.; et al. The Effects of the Mediterranean Diet on Biomarkers of Vascular Wall Inflammation and Plaque Vulnerability in Subjects with High Risk for Cardiovascular Disease. A Randomized Trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Corella, D.; Castañer, O.; Lamuela-Raventos, R.-M.; Salas-Salvadó, J.; Martínez-González, M.-A.; Ros, E.; Estruch, R. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvención con DIeta MEDiterránea (PREDIMED) Randomized Controlled Trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Thein, S.L. Platelets at the crossroads of thrombosis, inflammation and haemolysis. Br. J. Haematol. 2018, 180, 761–767. [Google Scholar] [CrossRef]
- Goliasch, G.; Forster, S.; El-Hamid, F.; Sulzgruber, P.; Meyer, N.; Siostrzonek, P.; Maurer, G.; Niessner, A. Platelet count predicts cardiovascular mortality in very elderly patients with myocardial infarction. Eur. J. Clin. Investig. 2013, 43, 332–340. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef]
- Puavilai, T.; Thadanipon, K.; Rattanasiri, S.; Ingsathit, A.; McEvoy, M.; Attia, J.; Thakkinstian, A. Treatment efficacy for adult persistent immune thrombocytopenia: A systematic review and network meta-analysis. Br. J. Haematol. 2020, 188, 450–459. [Google Scholar] [CrossRef] [PubMed]
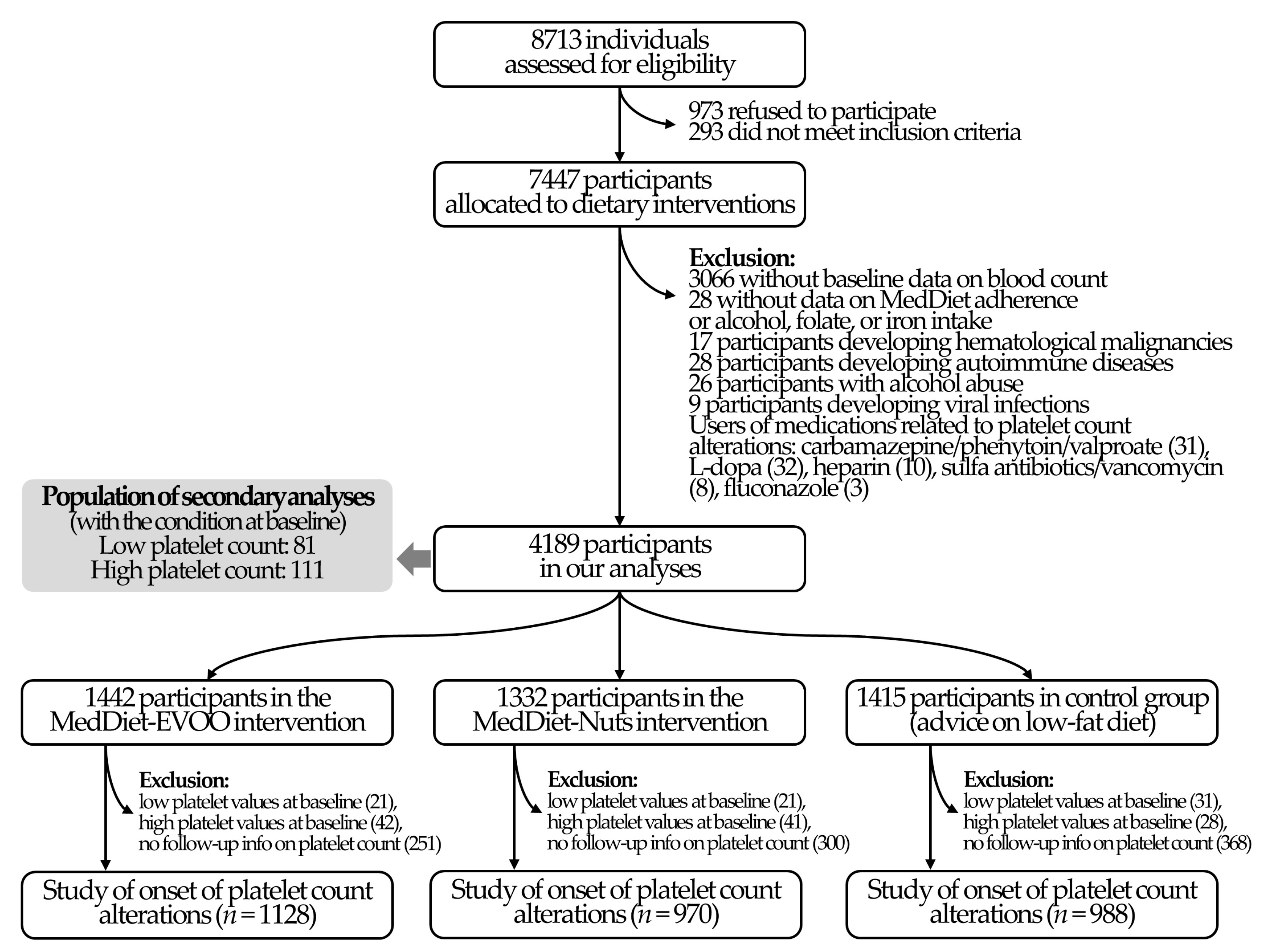
| All Participants | MedDiet-EVOO | MedDiet-Nuts | Control Diet | |
|---|---|---|---|---|
| (n = 4189) | (n = 1442) | (n = 1332) | (n = 1415) | |
| Age (years), mean ± SD | 67.0 ± 6.14 | 66.7 ± 6.06 | 66.9 ± 6.04 | 67.5 ± 6.30 |
| Female sex, n (%) | 2430 (58.0) | 859 (59.6) | 725 (54.4) | 846 (59.8) |
| Diabetes, n (%) | 1954 (46.6) | 686 (47.6) | 611 (45.9) | 657 (46.4) |
| Hypercholesterolemia, n (%) | 3100 (74.0) | 1069 (74.1) | 996 (74.8) | 1035 (73.1) |
| Hypertriglyceridemia, n (%) | 1264 (30.2) | 439 (30.4) | 405 (30.4) | 420 (29.7) |
| Hypertension, n (%) | 3522 (84.1) | 1201 (83.3) | 1121 (84.2) | 1200 (84.8) |
| Antiplatelet users, n (%) | 830 (19.8) | 255 (17.7) | 284 (21.3) | 291 (20.6) |
| Smoking habit: | ||||
| Never smokers, n (%) | 2589 (61.8) | 895 (62.1) | 798 (59.9) | 896 (63.3) |
| Current smokers, n (%) | 562 (13.4) | 195 (13.5) | 186 (14.0) | 181 (12.8) |
| Former smokers, n (%) | 1038 (24.8) | 352 (24.4) | 348 (26.1) | 338 (23.9) |
| Weight (according to body mass index): | ||||
| 18.5–24.9 kg/m2, n (%) | 281 (6.71) | 100 (6.93) | 101 (7.58) | 80 (5.65) |
| 25.0–29.9 kg/m2, n (%) | 1860 (44.4) | 645 (44.7) | 610 (45.8) | 605 (42.8) |
| ≥30.0 kg/m2, n (%) | 2048 (48.9) | 697 (48.3) | 621 (46.6) | 730 (51.6) |
| Leisure-time physical activity (metabolic equivalents of task-min/day), median (1st–3rd quartile) | 166 (56.1–312) | 175 (60.8–319) | 182 (63.3–326) | 150 (46.4–280) |
| Baseline | 1 Year | 2–3 Years | 4–5 Years | Time Effect | Time × Group Effect | |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | (109 Units/L·Year, [95% CI]) | (109 Units/L·Year, [95% CI], vs. Control Diet) | |
| All individuals | ||||||
| Control diet | 235 ± 60.4 | 236 ± 60.7 | 237 ± 64.1 | 238 ± 64.2 | +0.98 | Ref. |
| MedDiet combined | 235 ± 60.5 | 237 ± 61.9 | 236 ± 60.8 | 236 ± 65.6 | [0.12; 1.84] | −0.82 [−1.83; 0.19] |
| MedDiet-EVOO | 236 ± 60.1 | 236 ± 62.7 | 239 ± 61.9 | 239 ± 67.7 | −0.52 [−1.64; 0.60] | |
| MedDiet-Nuts | 235 ± 61.1 | 237 ± 61.1 | 234 ± 59.2 | 232 ± 62.4 | −1.21 [−2.39; −0.027] | |
| 1st quartile (<194·109 platelets/L at baseline) | ||||||
| Control diet | 166 ± 24.0 | 180 ± 40.3 | 176 ± 42.0 | 185 ± 42.6 | +3.67 | Ref. |
| MedDiet combined | 168 ± 23.8 | 180 ± 41.8 | 180 ± 36.6 | 183 ± 43.4 | [2.30; 5.04] | −0.024 [−1.65; 1.60] |
| MedDiet-EVOO | 167 ± 24.2 | 180 ± 37.9 | 181 ± 34.2 | 182 ± 41.6 | −0.056 [−1.87; 1.76] | |
| MedDiet-Nuts | 168 ± 23.5 | 180 ± 46.2 | 180 ± 39.4 | 184 ± 45.9 | 0.032 [−1.87; 1.93] | |
| 2nd quartile (194–229·109 platelets/L at baseline) | ||||||
| Control diet | 212 ± 9.71 | 217 ± 31.1 | 217 ± 34.0 | 221 ± 35.0 | +1.41 | Ref. |
| MedDiet combined | 212 ± 9.96 | 219 ± 36.1 | 216 ± 39.4 | 218 ± 38.8 | [0.12; 2.70] | −0.33 [−1.84; 1.18] |
| MedDiet-EVOO | 213 ± 9.65 | 218 ± 35.0 | 218 ± 43.6 | 218 ± 39.4 | −0.42 [−2.10; 1.26] | |
| MedDiet-Nuts | 211 ± 10.3 | 220 ± 37.3 | 214 ± 32.4 | 218 ± 38.2 | −0.22 [−1.99; 1.55] | |
| 3rd quartile (229–268·109 platelets/L at baseline) | ||||||
| Control diet | 248 ± 10.9 | 248 ± 33.3 | 245 ± 34.6 | 252 ± 40.3 | +0.86 | Ref. |
| MedDiet combined | 248 ± 11.0 | 247 ± 33.2 | 246 ± 34.6 | 252 ± 48.9 | [−0.56; 2.28] | −0.43 [−2.10; 1.24] |
| MedDiet-EVOO | 249 ± 11.1 | 247 ± 32.3 | 250 ± 35.3 | 256 ± 43.7 | 0.85 [−1.00; 2.70] | |
| MedDiet-Nuts | 248 ± 10.9 | 247 ± 34.1 | 241 ± 33.3 | 246 ± 54.7 | −2.17 [−4.13; −0.21] | |
| 4th quartile (≥268·109 platelets/L at baseline) | ||||||
| Control diet | 315 ± 44.5 | 300 ± 54.9 | 305 ± 56.6 | 318 ± 59.4 | −1.46 | Ref. |
| MedDiet combined | 316 ± 45.3 | 301 ± 60.7 | 297 ± 57.1 | 299 ± 67.7 | [−3.70; 0.78] | −3.20 [−5.81; −0.59] |
| MedDiet-EVOO | 316 ± 43.2 | 303 ± 65.5 | 299 ± 59.2 | 306 ± 70.9 | −2.48 [−5.36; 0.40] | |
| MedDiet-Nuts | 317 ± 47.6 | 299 ± 55.2 | 294 ± 54.3 | 288 ± 61.6 | −4.13 [−7.17; −1.09] | |
| Thrombocytopenia | Thrombocytosis | |||||
|---|---|---|---|---|---|---|
| Cases/Total | Model 1 | Model 2 | Cases/Total | Model 1 | Model 2 | |
| (Incidence Rate) | HR [95% CI] | HR [95% CI] | (Incidence Rate) | HR [95% CI] | HR [95% CI] | |
| Control diet | 22/988 | 18/988 | ||||
| (2.23%) | 1 (Ref.) | 1 (Ref.) | (1.82%) | 1 (Ref.) | 1 (Ref.) | |
| MedDiets combined | 19/2098 | 0.34 | 0.44 | 40/2098 | 0.96 | 1.29 |
| (0.91%) | [0.18; 0.64] | [0.23; 0.83] | (1.91%) | [0.55; 1.69] | [0.74; 2.26] | |
| MedDiet-EVOO | 10/1128 | 0.29 | 0.36 | 24/1128 | 1.04 | 1.57 |
| (0.89%) | [0.14; 0.62] | [0.16; 0.80] | (2.13%) | [0.56; 1.93] | [0.84; 2.97] | |
| MedDiet-Nuts | 9/970 | 0.40 | 0.56 | 16/970 | 0.86 | 1.04 |
| (0.93%) | [0.18; 0.89] | [0.26; 1.21] | (1.65%) | [0.44; 1.69] | [0.52; 2.06] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernáez, Á.; Lassale, C.; Castro-Barquero, S.; Ros, E.; Tresserra-Rimbau, A.; Castañer, O.; Pintó, X.; Vázquez-Ruiz, Z.; Sorlí, J.V.; Salas-Salvadó, J.; et al. Mediterranean Diet Maintained Platelet Count within a Healthy Range and Decreased Thrombocytopenia-Related Mortality Risk: A Randomized Controlled Trial. Nutrients 2021, 13, 559. https://doi.org/10.3390/nu13020559
Hernáez Á, Lassale C, Castro-Barquero S, Ros E, Tresserra-Rimbau A, Castañer O, Pintó X, Vázquez-Ruiz Z, Sorlí JV, Salas-Salvadó J, et al. Mediterranean Diet Maintained Platelet Count within a Healthy Range and Decreased Thrombocytopenia-Related Mortality Risk: A Randomized Controlled Trial. Nutrients. 2021; 13(2):559. https://doi.org/10.3390/nu13020559
Chicago/Turabian StyleHernáez, Álvaro, Camille Lassale, Sara Castro-Barquero, Emilio Ros, Anna Tresserra-Rimbau, Olga Castañer, Xavier Pintó, Zenaida Vázquez-Ruiz, José V. Sorlí, Jordi Salas-Salvadó, and et al. 2021. "Mediterranean Diet Maintained Platelet Count within a Healthy Range and Decreased Thrombocytopenia-Related Mortality Risk: A Randomized Controlled Trial" Nutrients 13, no. 2: 559. https://doi.org/10.3390/nu13020559
APA StyleHernáez, Á., Lassale, C., Castro-Barquero, S., Ros, E., Tresserra-Rimbau, A., Castañer, O., Pintó, X., Vázquez-Ruiz, Z., Sorlí, J. V., Salas-Salvadó, J., Lapetra, J., Gómez-Gracia, E., Alonso-Gómez, Á. M., Fiol, M., Serra-Majem, L., Sacanella, E., Razquin, C., Corella, D., Guasch-Ferré, M., ... Estruch, R. (2021). Mediterranean Diet Maintained Platelet Count within a Healthy Range and Decreased Thrombocytopenia-Related Mortality Risk: A Randomized Controlled Trial. Nutrients, 13(2), 559. https://doi.org/10.3390/nu13020559















