Serum 25-Hydroxyvitamin D Is Inversely Associated with Monocyte Percentage to HDL Cholesterol Ratio among Young Healthy Adults in Qatar
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Participants
2.2. Statistical Analysis
3. Results
3.1. Serum Vitamin D Concentrations in Young Healthy Adults in Qatar
3.2. Association between Serum 25(OH)D Concentrations and Inflammation Biomarkers and Serum Lipids
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikle, D. Vitamin D: Production, Metabolism, and Mechanisms of Action. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Badawi, A.; Arora, P.; Sadoun, E.; Al-Thani, A.A.; Thani, M.H. Prevalence of vitamin d insufficiency in qatar: A systematic review. J. Public Health Res. 2012, 1, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabhani, K.; Tsilidis, K.K.; Murphy, N.; Ward, H.A.; Elliott, P.; Riboli, E.; Gunter, M.; Tzoulaki, I. Prevalence of vitamin D deficiency and association with metabolic syndrome in a Qatari population. Nutr. Diabetes 2017, 7, e263. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszek-Matuszek, B.; Lenart-Lipińska, M.; Woźniakowska, E. Clinical implications of vitamin D deficiency. Prz. Menopauzalny 2015, 14, 75–81. [Google Scholar] [PubMed]
- Zughaier, S.M.; Lubberts, E.; Bener, A. Editorial: Immune-Modulatory Effects of Vitamin D. Front. Immunol. 2020, 11, 2385. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and Chronic Diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef]
- Ganji, V.; Zhang, X.; Shaikh, N.; Tangpricha, V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001–2006. Am. J. Clin. Nutr. 2011, 94, 225–233. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar]
- Zughaier, S.M.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Tangpricha, V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J. Clin. Transl. Endocrinol. 2014, 1, 19–25. [Google Scholar] [CrossRef]
- Kantari, C.; Pederzoli-Ribeil, M.; Witko-Sarsat, V. The role of neutrophils and monocytes in innate immunity. Contrib. Microbiol. 2008, 15, 118–146. [Google Scholar]
- Chapman Caroline, M.L.; Beilby John, P.; McQuillan Brendan, M.; Thompson Peter, L.; Hung, J. Monocyte Count, But Not C-Reactive Protein or Interleukin-6, Is an Independent Risk Marker for Subclinical Carotid Atherosclerosis. Stroke 2004, 35, 1619–1624. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Rubiano, L.A.; Angarita Ruidiaz, J.A.; Suarez Davila, H.F.; Suarez Rodriguez, A.; Rebolledo-Cobos, R.C.; Becerra, J.E. Relationship between Serum Vitamin D Levels and HDL Cholesterol in Postmenopausal Women from Colombian Caribbean. J. Nutr. Metab. 2018, 2018, 9638317. [Google Scholar] [CrossRef] [PubMed]
- AlQuaiz, A.M.; Kazi, A.; Youssef, R.M.; Alshehri, N.; Alduraywish, S.A. Association between standardized vitamin 25(OH)D and dyslipidemia: A community-based study in Riyadh, Saudi Arabia. Environ. Health Prev. Med. 2020, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Peng, M.; Chen, S.; Wu, S.; Zhang, W. Vitamin D deficiency is associated with dyslipidemia: A cross-sectional study in 3788 subjects. Curr. Med. Res. Opin. 2019, 35, 1059–1063. [Google Scholar] [CrossRef]
- Schmitt, E.B.; Nahas-Neto, J.; Bueloni-Dias, F.; Poloni, P.F.; Orsatti, C.L.; Petri Nahas, E.A. Vitamin D deficiency is associated with metabolic syndrome in postmenopausal women. Maturitas 2018, 107, 97–102. [Google Scholar] [CrossRef]
- Pan, G.T.; Guo, J.F.; Mei, S.L.; Zhang, M.X.; Hu, Z.Y.; Zhong, C.K.; Zeng, C.Y.; Liu, X.H.; Ma, Q.H.; Li, B.Y.; et al. Vitamin D Deficiency in Relation to the Risk of Metabolic Syndrome in Middle-Aged and Elderly Patients with Type 2 Diabetes Mellitus. J. Nutr. Sci. Vitam. (Tokyo) 2016, 62, 213–219. [Google Scholar] [CrossRef]
- Katipoglu, Z.; Mirza, E.; Oltulu, R.; Katipoglu, B. May Monocyte/HDL Cholesterol Ratio (MHR) and Neutrophil/Lymphocyte Ratio (NLR) Be an Indicator of Inflammation and Oxidative Stress in Patients with Keratoconus? Ocul. Immunol. Inflamm. 2020, 28, 632–636. [Google Scholar] [CrossRef]
- Osadnik, T.; Bujak, K.; Osadnik, K.; Czarnecka, H.; Pawlas, N.; Reguła, R.; Fronczek, M.; Lejawa, M.; Gawlita, M.; Gonera, M.; et al. Novel inflammatory biomarkers may reflect subclinical inflammation in young healthy adults with obesity. Endokrynol. Pol. 2019, 70, 135–142. [Google Scholar] [CrossRef]
- Çakýr, I.; Arifoðlu, H.B.; Ekici Günay, N.; Pangal, E.; Þahin, D.; Alýcý Sert, G.; Duru, N. Monocyte to High-Density Lipoprotein Ratio: A Novel Inflammation Marker Related to Diabetic Retinopathy. Ercýyes Med. J. 2020, 42, 190–194. [Google Scholar] [CrossRef]
- Yayla, K.G.; Canpolat, U.; Yayla, Ç.; Akboğa, M.K.; Akyel, A.; Akdi, A.; Çiçek, G.; Ozcan, F.; Turak, O.; Aydoğdu, S. A Novel Marker of Impaired Aortic Elasticity in Never Treated Hypertensive Patients: Monocyte/High-Density Lipoprotein Cholesterol Ratio. Acta Cardiol. Sin. 2017, 33, 41–49. [Google Scholar]
- Tani, S.; Matsumoto, M.; Anazawa, T.; Kawamata, H.; Furuya, S.; Takahashi, H.; Iida, K.; Washio, T.; Kumabe, N.; Kobori, M.; et al. Development of a model for prediction of coronary atherosclerotic regression: Evaluation of high-density lipoprotein cholesterol level and peripheral blood monocyte count. Heart Vessel. 2012, 27, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Karatas, A.; Turkmen, E.; Erdem, E.; Dugeroglu, H.; Kaya, Y. Monocyte to high-density lipoprotein cholesterol ratio in patients with diabetes mellitus and diabetic nephropathy. Biomark. Med. 2018, 12, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Demir, M.; Unal, S.; Yildiz, A.; Ozyazgan, B.; Demirtas, B.; Elalmis, O.U.; Ileri, M.; Guray, U. Monocyte-to-high density lipoprotein ratio (MHR) can predict the significance of angiographically intermediate coronary lesions. Int. J. Cardiovasc. Acad. 2017, 3, 16–20. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, Y.-J.; Park, B. Higher monocyte count with normal white blood cell count is positively associated with 10-year cardiovascular disease risk determined by Framingham risk score among community-dwelling Korean individuals. Medicine 2019, 98 (Suppl. 61), e15340. [Google Scholar] [CrossRef]
- Yakar, H.I.; Kanbay, A.; Ceylan, E. Could Monocyte /HDL cholesterol ratio predict cardiovascular events in patients with Chronic Obstructive Pulmonary Disease? Eur. Respir. J. 2017, 50, PA1007. [Google Scholar]
- Wei, X.B.; Chen, F.; Huang, J.L.; He, P.C.; Wei, Y.X.; Tan, N.; Chen, J.Y.; Yu, D.Q.; Liu, Y.H. Novel Risk Biomarker for Infective Endocarditis Patients With Normal Left Ventricular Ejection Fraction- Monocyte to High-Density Lipoprotein Cholesterol Ratio. Circ. J. 2017, 82, 283–288. [Google Scholar] [CrossRef]
- Oh, S.W.; Yi, H.J.; Lee, D.H.; Sung, J.H. Prognostic Significance of Various Inflammation-Based Scores in Patients with Mechanical Thrombectomy for Acute Ischemic Stroke. World Neurosurg. 2020, 141, e710–e717. [Google Scholar] [CrossRef]
- Al Thani, A.; Fthenou, E.; Paparrodopoulos, S.; Al Marri, A.; Shi, Z.; Qafoud, F.; Afifi, N. Qatar Biobank Cohort Study: Study Design and First Results. Am. J. Epidemiol. 2019, 188, 1420–1433. [Google Scholar] [CrossRef]
- Al Kuwari, H.; Al Thani, A.; Al Marri, A.; Al Kaabi, A.; Abderrahim, H.; Afifi, N.; Qafoud, F.; Chan, Q.; Tzoulaki, I.; Downey, P.; et al. The Qatar Biobank: Background and methods. BMC Public Health 2015, 15, 1208. [Google Scholar] [CrossRef]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; HB, D.V. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Ganjali, S.; Gotto Jr, A.M.; Ruscica, M.; Atkin, S.L.; Butler, A.E.; Banach, M.; Sahebkar, A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell. Physiol. 2018, 233, 9237–9246. [Google Scholar] [CrossRef] [PubMed]
- Bolayir, A.; Gokce, S.F.; Cigdem, B.; Bolayir, H.A.; Yildiz, O.K.; Bolayir, E.; Topaktas, S.A. Monocyte/high-density lipoprotein ratio predicts the mortality in ischemic stroke patients. Neurol. Neurochir. Pol. 2018, 52, 150–155. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Zhong, C.; Zheng, D.; Xu, J.; Zhang, X.; Liu, H.; Zhang, Y.; Shi, J.; Huang, Z.; Cao, Y.; et al. Monocyte to HDL cholesterol ratio is associated with discharge and 3-month outcome in patients with acute intracerebral hemorrhage. J. Neurol. Sci. 2017, 372, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Usta, A.; Avci, E.; Bulbul, C.B.; Kadi, H.; Adali, E. The monocyte counts to HDL cholesterol ratio in obese and lean patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2018, 16, 34. [Google Scholar] [CrossRef]
- Li, N.; Ren, L.; Wang, J.H.; Yan, Y.R.; Lin, Y.N.; Li, Q.Y. Relationship between monocyte to HDL cholesterol ratio and concomitant cardiovascular disease in Chinese Han patients with obstructive sleep apnea. Cardiovasc. Diagn. 2019, 9, 362–370. [Google Scholar] [CrossRef]
- Sercelik, A.; Besnili, A.F. Increased monocyte to high-density lipoprotein cholesterol ratio is associated with TIMI risk score in patients with ST-segment elevation myocardial infarction. Rev. Port. Cardiol. (Engl. Ed.) 2018, 37, 217–223. [Google Scholar] [CrossRef]
- Karlmark, K.R.; Tacke, F.; Dunay, I.R. Monocytes in health and disease—Minireview. Eur. J. Microbiol. Immunol. 2012, 2, 97–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.M.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D Inhibits Monocyte/Macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1. J. Immunol. 2012, 188, 2127. [Google Scholar] [CrossRef]
- Wöbke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front Physiol 2014, 5, 244. [Google Scholar] [CrossRef]
- Riek, A.E.; Oh, J.; Sprague, J.E.; Timpson, A.; de las Fuentes, L.; Bernal-Mizrachi, L.; Schechtman, K.B.; Bernal-Mizrachi, C. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J. Biol. Chem. 2012, 287, 38482–38494. [Google Scholar] [CrossRef]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zugel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Buonomo, A.R.; Zappulo, E.; Scotto, R.; Pinchera, B.; Perruolo, G.; Formisano, P.; Borgia, G.; Gentile, I. Vitamin D deficiency is a risk factor for infections in patients affected by HCV-related liver cirrhosis. Int. J. Infect. Dis. 2017, 63, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ingham, T.R.; Jones, B.; Camargo, C.A.; Kirman, J.; Dowell, A.C.; Crane, J.; Stanley, T.V.; Grimwood, K.; The Whiti Te Ra Study, G. Association of vitamin D deficiency with severity of acute respiratory infection: A case-control study in New Zealand children. Eur. Respir. J. 2014, 44 (Suppl. 58), 439. [Google Scholar]
- Mohamed, W.A.; Al-Shehri, M.A. Cord blood 25-hydroxyvitamin D levels and the risk of acute lower respiratory tract infection in early childhood. J. Trop. Pediatr. 2013, 59, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Wadhera, V.; Leach, S.T.; Woodhead, H.J.; Lemberg, D.A.; Mendoza-Cruz, A.C.; Day, A.S. Vitamin D deficiency in children with inflammatory bowel disease. Dig. Dis. Sci. 2011, 56, 830–836. [Google Scholar] [CrossRef]
- Kostoglou-Athanassiou, I.; Athanassiou, P.; Lyraki, A.; Raftakis, I.; Antoniadis, C. Vitamin D and rheumatoid arthritis. Adv. Endocrinol. Metab. 2012, 3, 181–187. [Google Scholar] [CrossRef]
- Terrier, B.; Derian, N.; Schoindre, Y.; Chaara, W.; Geri, G.; Zahr, N.; Mariampillai, K.; Rosenzwajg, M.; Carpentier, W.; Musset, L.; et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res. 2012, 14, R221. [Google Scholar] [CrossRef]
- Li, M.; Chen, P.; Li, J.; Chu, R.; Xie, D.; Wang, H. Review: The impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 2327–2336. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Agrawal, V.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. The Possible Role of Vitamin D in Suppressing Cytokine Storm and Associated Mortality in COVID-19 Patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Alkhatatbeh, M.J.; Amara, N.A.; Abdul-Razzak, K.K. Association of 25-hydroxyvitamin D with HDL-cholesterol and other cardiovascular risk biomarkers in subjects with non-cardiac chest pain. Lipids Health Dis. 2019, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, H.; Xiao, H.; Tang, H.; Xiang, Z.; Wang, X.; Zou, H. Comparison of the Value of Neutrophil to High-Density Lipoprotein Cholesterol Ratio and Lymphocyte to High-Density Lipoprotein Cholesterol Ratio for Predicting Metabolic Syndrome Among a Population in the Southern Coast of China. Diabetes Metab. Syndr. Obes. 2020, 13, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, C.; Shao, X.; Ning, J.; Gao, P.; Xiao, H.; Chen, Y.; Zou, Z.; Hong, G.; Li, X.; et al. Lymphocyte To High-Density Lipoprotein Ratio As A New Indicator Of Inflammation And Metabolic Syndrome. Diabetes Metab. Syndr. Obes. 2019, 12, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
| Vitamin D Deficiency (serum 25(OH)D, <12 ng/mL) | Vitamin D Insufficiency (serum 25(OH)D, 12-˂20 ng/mL) | Vitamin D Sufficiency (serum 25(OH)D, ≥20ng/mL) | p-Value 2 | |
|---|---|---|---|---|
| N | 488 | 261 | 125 | |
| Gender 3 | ||||
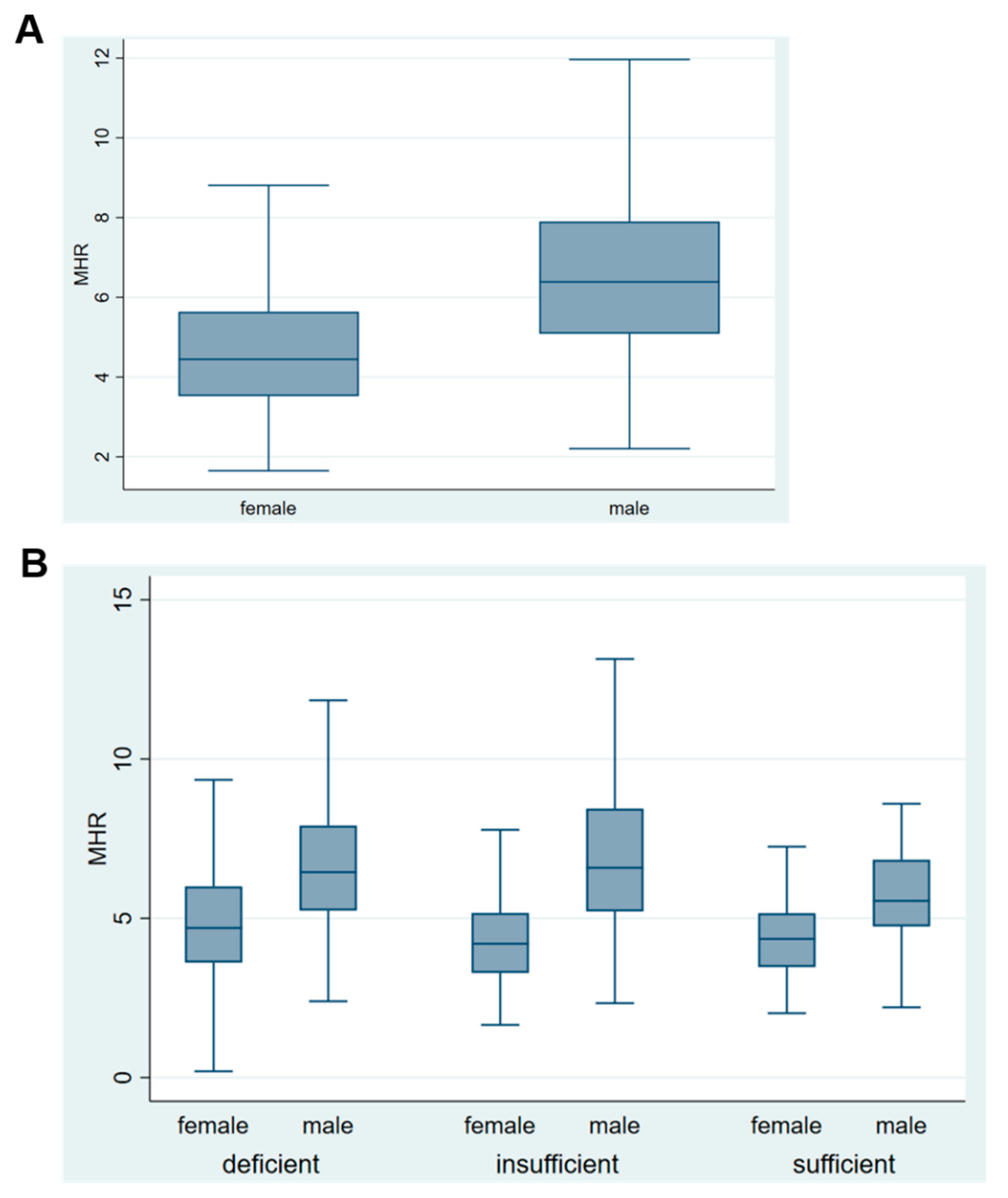
| Women | 274 (56%) | 131 (51%) | 56 (46%) | ns |
| Men | 214 (44%) | 130 (50%) | 55 (44%) | |
| Age (years) | 28.8 (6) | 30.3 (5.9) | 29.8 (5.8) | 0.002 |
| Body mass index, kg/m2 | 28.3 (6.8) | 27.6 (5.3) | 26.4 (5.5) | 0.008 |
| White blood cells, cells/109 L | 6.8 (2.0) | 6.8 (2.0) | 6.7 (1.8) | ns |
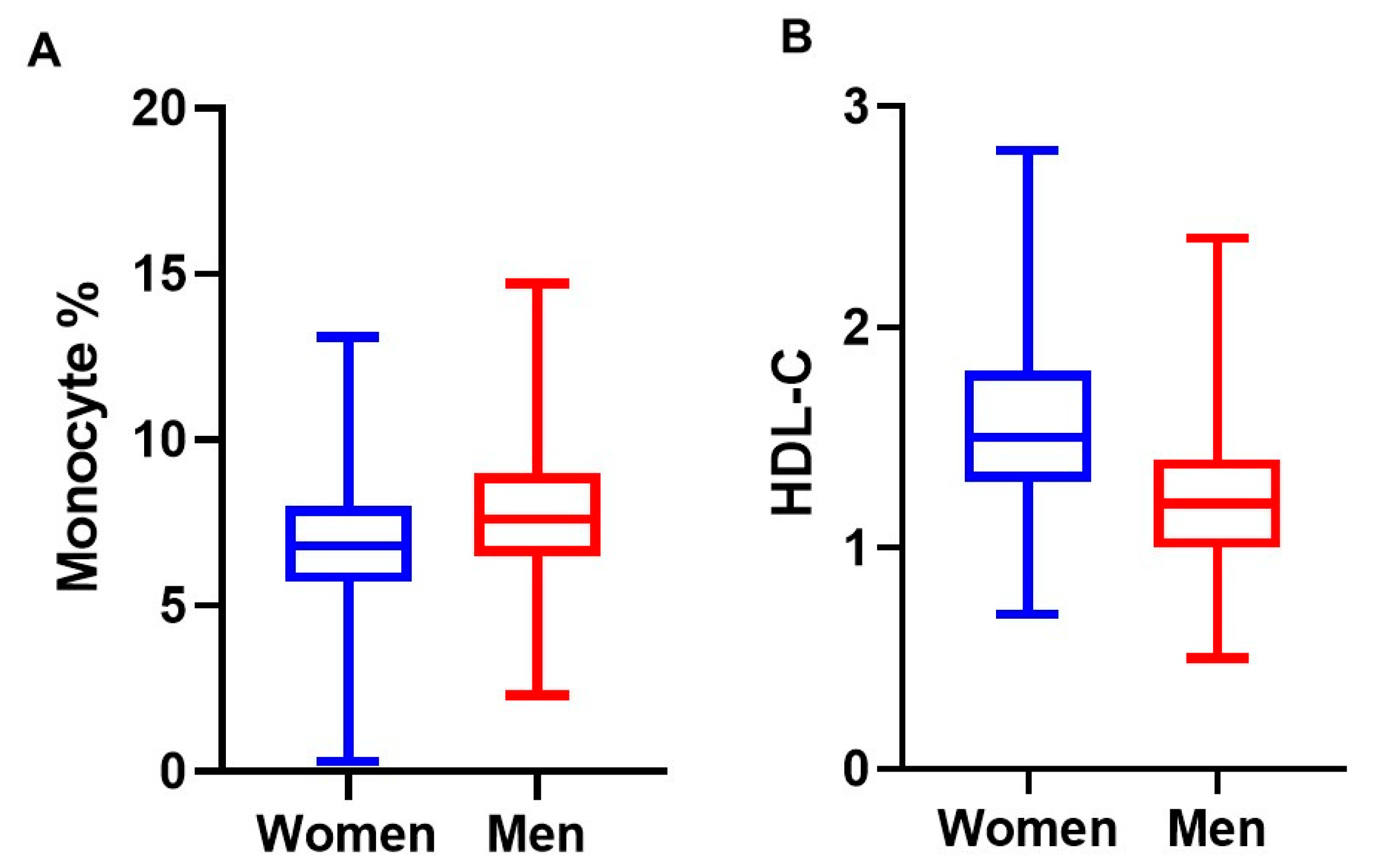
| Monocyte, % | 7.5 (1.9) | 7.3 (1.9) | 6.9 (1.6) | 0.014 |
| Lymphocyte, % | 35.6 (8.6) | 35.1 (9.4) | 36 (9.1) | ns |
| Neurtophil, % | 53 (9.8) | 54.1 (10.4) | 53.5 (10.1) | ns |
| Monocyte % to HDL ratio | 5.8 (2.3) | 5.7 (2.5) | 5.1 (1.8) | 0.011 |
| Lymphocyte % to HDL ratio | 27.3 (10.1) | 26.9 (11.1) | 26 (8.4) | ns |
| Neutrophil % to HDL ratio | 41 (14) | 42 (16.2) | 39 (12.2) | ns |
| Total cholesterol, mmol/L | 4.8 (0.8) | 4.8 (0.7) | 4.6 (0.8) | ns |
| HDL-cholesterol, mmol/L | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.3) | ns |
| LDL- cholesterol, mmol/L | 2.8 (0.8) | 2.8 (0.7) | 2.7 (0.7) | ns |
| Triacylglycerol, mmol/L | 1.2 (0.7) | 1.1 (0.7) | 1.0 (0.5) | ns |
| Glucose, mmol/L | 5.0 (0.7) | 5.0 (0.9) | 4.9 (0.7) | ns |
| Vitamin D Deficiency (˂12 ng/mL) 2 | Vitamin D Insufficiency (12-˂20 ng/mL) 2 | Continuous Association 3 | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | |
| White blood cell, cells/109 L (n = 702) | 0.01 (−0.11, 0.14) | ns | 0.02 (−0.11, 0.15) | ns | 0.09 (−0.22, 0.41) | ns |
| Monocyte % (n = 702) | 0.2 (0.06, 0.34) | 0.006 | 0.09 (−0.05, 0.24) | ns | −0.54 (−0.19, −0.2) | 0.002 |
| Lymphocyte % (n = 702) | −0.01 (−0.03, 0.02) | ns | −0.02 (−0.04, 0.01) | ns | 0.01 (−0.06, 0.08) | ns |
| Neurtophil % (n = 702) | −0.01 (−0.03, 0.02) | ns | 0.01 (−0.01, 0.04) | ns | 0.02 (−0.03, 0.08) | ns |
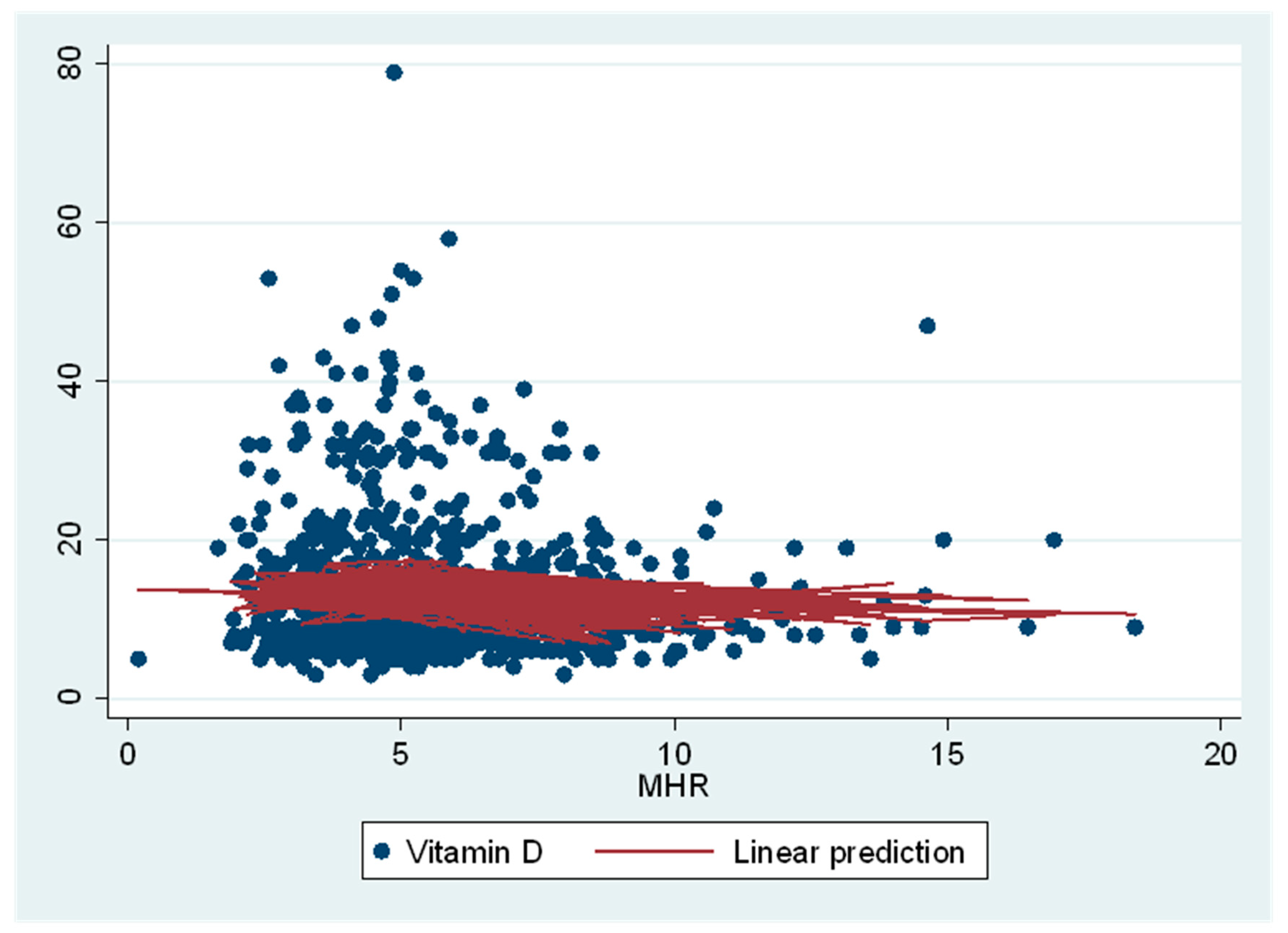
| Monocyte % to HDL ratio (n = 701) | 0.19 (0.06, 0.32) | 0.005 | 0.15 (0.02, 0.29) | 0.03 | −0.38 (−0.66, −0.1) | 0.008 |
| Lymphocyte % to HDL ratio (n = 701) | 0.01 (−0.02, 0.04) | ns | 0.01 (−0.02, 0.04) | ns | −0.02 (−0.08, 0.04) | ns |
| Neutrophil % to HDL ratio (n = 701) | 0.02 (−0.004, 0.04) | ns | 0.01 (−0.01, 0.02) | ns | 0.001 (−0.04, 0.04) | ns |
| Total cholesterol, mmol/L (n = 706) | 04 (0.08, 0.72) | 0.014 | 0.25 (−0.09, 0.58) | ns | −1.03, −1.81, −0.26) | 0.009 |
| HDL-cholesterol, mmol/L (n = 706) | −0.2 (−0.94, 0.54) | ns | −0.22 (−0.1, 0.58) | ns | 0.54 (−1.42, 2.4) | ns |
| LDL- cholesterol, mmol/L (n = 703) | 0.4 (0.05, 0.74) | 0.03 | 0.27 (−0.1, 0.63) | ns | −0.98 (−1.84, −0.12) | 0.026 |
| Triacylglycerol, mmol/L (n = 706) | 0.49 (0.02, 0.93) | 0.04 | 0.31 (−0.16, 0.78) | ns | −1.06 (−2.0, −0.11) | 0.028 |
| Glucose, mmol/L (n = 706) | 0.2 ( 0.18, 0.58) | ns | 0.1 (−0.26,0.55) | ns | −0.34 (−1.2, 0.49) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, H.; Islam, N.; Ganji, V.; Zughaier, S.M. Serum 25-Hydroxyvitamin D Is Inversely Associated with Monocyte Percentage to HDL Cholesterol Ratio among Young Healthy Adults in Qatar. Nutrients 2021, 13, 127. https://doi.org/10.3390/nu13010127
Mousa H, Islam N, Ganji V, Zughaier SM. Serum 25-Hydroxyvitamin D Is Inversely Associated with Monocyte Percentage to HDL Cholesterol Ratio among Young Healthy Adults in Qatar. Nutrients. 2021; 13(1):127. https://doi.org/10.3390/nu13010127
Chicago/Turabian StyleMousa, Hanaa, Nazmul Islam, Vijay Ganji, and Susu M. Zughaier. 2021. "Serum 25-Hydroxyvitamin D Is Inversely Associated with Monocyte Percentage to HDL Cholesterol Ratio among Young Healthy Adults in Qatar" Nutrients 13, no. 1: 127. https://doi.org/10.3390/nu13010127
APA StyleMousa, H., Islam, N., Ganji, V., & Zughaier, S. M. (2021). Serum 25-Hydroxyvitamin D Is Inversely Associated with Monocyte Percentage to HDL Cholesterol Ratio among Young Healthy Adults in Qatar. Nutrients, 13(1), 127. https://doi.org/10.3390/nu13010127






