The Health Effects of Vitamin D and Probiotic Co-Supplementation: A Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Design
2.2. Criteria for Study Inclusion
2.3. Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Data Synthesis
3. Results
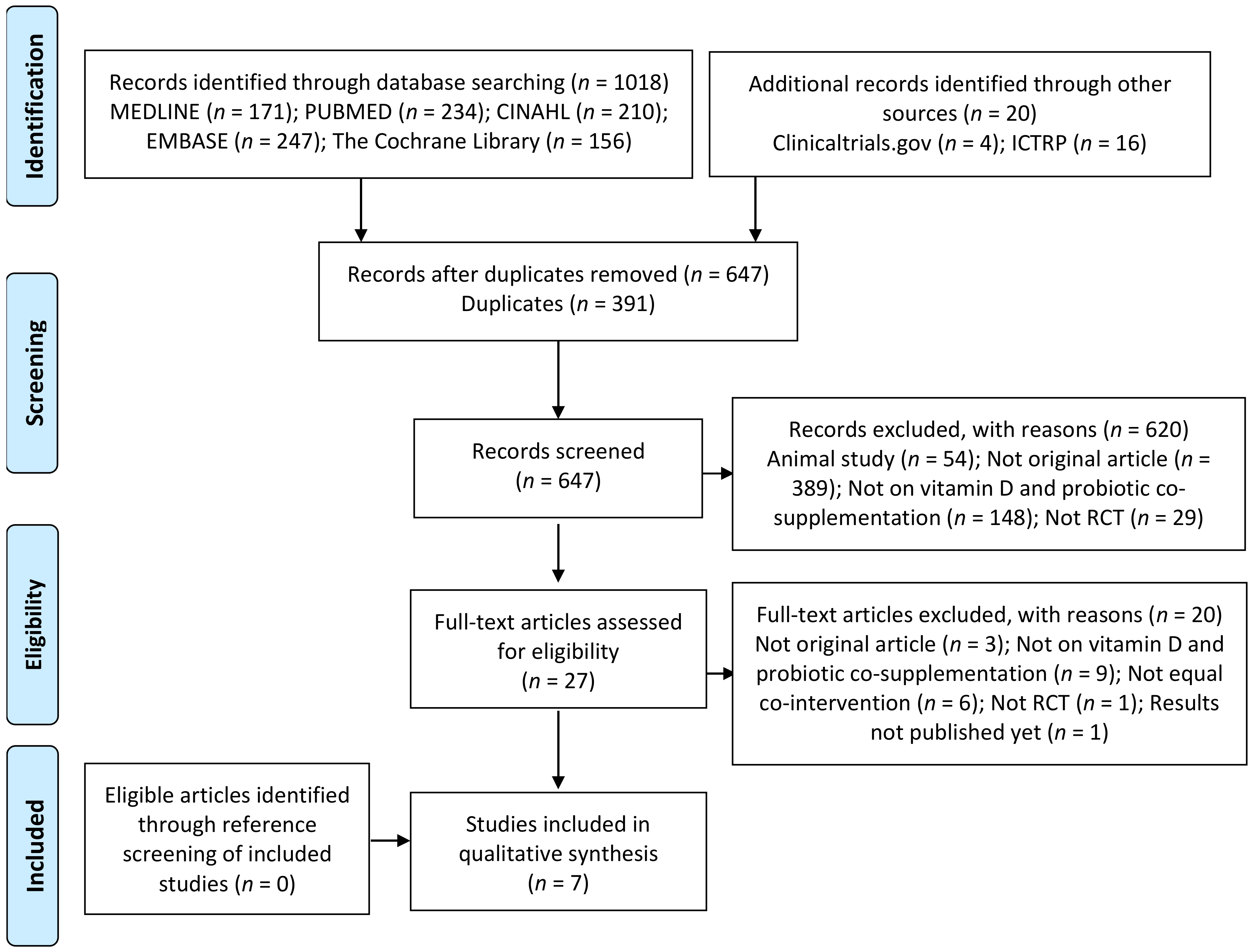
3.1. Search Results
3.2. Characteristics of Included Studies
| First Author, Year, Country | Study Design | Duration | Study Population | Intervention | Control | Co-Intervention | Compliance/Drop-out |
|---|---|---|---|---|---|---|---|
| Ghaderi, 2019, Iran [27] | Randomized, double-blind, placebo-controlled trial | 12 weeks | n = 60, aged 25–65, 93.33% men, diagnosed with schizophrenia using DSM-IV-TR criteria with disease duration ≥2 years, PANSS score ≥55, treated with chlorpromazine (300–1000 mg/day, except clozapine) and anticholinergic agents (Trihexyphenidyl, 4–8 mg/day) during the last 6 months | Vitamin D3 and probiotic supplement:
| Placebo similar shape and packaging | None | Compliance: >90%Drop out: I: 13.33% C: 13.33% (Intention-to-treat analysis) |
| Jafarnejad, 2017, Iran [31] | Randomized, double-blind, placebo-controlled clinical trial | 6 weeks | n = 50, age 50–72 years, women with mild bone loss (osteopenia) diagnosed based on the World Health Organization criteria (T-score between −1.0 and −2.5) | Probiotic supplement: Lactobacillus casei 1.3 × 1010 CFU, Bifidobacterium longum 5 × 1010 CFU, Lactobacillus acidophilus 1.5 × 1010 CFU, Lactobacillus rhamnosus 3.5 × 109 CFU, Lactobacillus bulgaricus 2.5 × 108 CFU, Bifidobacterium breve 1 × 1010 CFU, and Streptococcus thermophilus 1.5 × 108 CFU/500 mg | Placebo similar in shape, size, odor, color and packaging | Vitamin D (200 IU daily) and Calcium (500 mg daily) | Compliance 100% Drop out: I: 20% C: 16% |
| Jamilian, 2018, Iran [29] | Randomized, double-blind, placebo-controlled clinical trial | 6 weeks | n = 87, women with GDM diagnosed by a “one-step” 2-h 75-g oral glucose tolerance test based on the ADA guidelines | Vitamin D and probiotic supplement:
| C1: 8 × 109 CFU/day of probiotic supplements C2: Placebo Similar in appearance, color, shape, size, odor, taste and packaging | Vitamin D3: 1000 IU and Vitamin B9: 400 mg, daily from the beginning of pregnancy, and Ferrous sulfate: 60 mg, daily from the secondtrimester | Compliance: 100% Drop out: I: 0% C1: 6.66% C2: 10% |
| Ostadmohammadi, 2019, Iran [28] | Randomized, double-blind, placebo-controlled clinical trial | 12 weeks | n = 60, aged 18–40 years, women with PCOS, diagnosed based on the Rotterdam criteria with BMI: 17–34 kg/m2 and insulin resistance: 1.4–4 | Vitamin D and probiotic supplement:
| Placebo similar in appearance, color, shape, size, odor, taste and packaging | None | Compliance 100%; No drop out |
| Raygan, 2018, Iran [30] | Randomized, double-blind, placebo-controlled clinical trial | 12 weeks | n = 60, age 45–85 years, 50% men, with T2DM diagnosed based on the criteria of the ADA and with CHD diagnosed as per the AHA with 2- and 3-vessel CHD | Vitamin D3 and probiotic supplement:
| Placebo similar in appearance, color, shape, size, odor, taste and packaging | None | Compliance > 90% Drop out: I: 13.33% C: 13.33% (Intention-to-treat analysis) |
| Savino, 2015, Italy [25] | Single-blind, randomized controlled, parallel-group trial | 12 weeks | n = 105, newborns aged less than 10 days of life, 48.5% boys, with gestational age between 37 and 42 weeks, birth weight from 2500 to 4300 g, and normal physical examination | Vitamin D and probiotic supplement:
| Vitamin D (400 IU daily) | None | No infants lost to follow- ups |
| Tazzyman, 2015, United Kingdom [26] | Double-blind, randomized, three-arm parallel design trial | 12 weeks | n = 51, 7.8% men, with previous clinical diagnosis of IBS and met the Rome III criteria and stratified according to vitamin D status at baseline (deficient: 25(OH)D <20 ng/mL; repleted: 25(OH)D >20 ng/mL) | Vitamin D3 and probiotic supplement:
| C1: Double placebo C2: Placebo and Vitamin D3 (400 IU daily) Similar in form, containing identical buffers | None | Compliance: 98% Drop out: 0% |
3.3. Assessment of Risk of Bias
3.4. Results of Included Studies
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Ogbu, D.; Xia, E.; Sun, J. Gut instincts: Vitamin D/vitamin D receptor and microbiome in neurodevelopment disorders. Open Biol. 2020, 10, 200063. [Google Scholar] [CrossRef]
- Sun, J. Dietary Vitamin D, Vitamin D Receptor, and Microbiome. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Butel, M.-J.; Waligora-Dupriet, A.-J.; Wydau-Dematteis, S. The developing gut microbiota and its consequences for health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Bakke, D.; Chatterjee, I.; Agrawal, A.; Dai, Y.; Sun, J. Regulation of Microbiota by Vitamin D Receptor: A Nuclear Weapon in Metabolic Diseases. Nucl. Recept. Res. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Hawrelak, J.A.; Myers, S.P. The Causes of Intestinal Dysbiosis: A Review. Altern. Med. Rev. 2004, 9, 180–197. [Google Scholar]
- Battistini, C.; Nassani, N.; Saad, S.M.; Sun, J. Probiotics, Vitamin D, and Vitamin D Receptor in Health and Disease. In Lactic Acid Bacteria; Cavalcanti de Albuquerque, M.A., de Moreno de LeBlanc, A., LeBlanc, J.G., Bedani, R., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 93–105. ISBN 978-0-429-42259-1. [Google Scholar]
- Shang, M.; Sun, J. Vitamin D/VDR, Probiotics, and Gastrointestinal Diseases. Curr. Med. Chem. 2017, 24, 876–887. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Trehan, N.; Afonso, L.; Levine, D.L.; Levy, P.D. Vitamin D Deficiency, Supplementation, and Cardiovascular Health. Crit. Pathw. Cardiol. 2017, 16, 109–118. [Google Scholar] [CrossRef]
- AlAnouti, F.; Abboud, M.; Papandreou, D.; Mahboub, N.; Haidar, S.; Rizk, R. Effects of Vitamin D Supplementation on Lipid Profile in Adults with the Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 3352. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M. Vitamin D Supplementation and Blood Pressure in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1163. [Google Scholar] [CrossRef] [PubMed]
- Spedding, S. Vitamin D and Depression: A Systematic Review and Meta-Analysis Comparing Studies with and without Biological Flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A. Vitamin D and human health. Int. J. Mol. Sci. 2019, 20, 145. [Google Scholar] [CrossRef]
- Batacchi, Z.; Robinson-Cohen, C.; Hoofnagle, A.N.; Isakova, T.; Kestenbaum, B.; Martin, K.J.; Wolf, M.S.; De Boer, I.H. Effects of vitamin D2 supplementation on vitamin D3 metabolism in health and CKD. Clin. J. Am. Soc. Nephrol. 2017, 7, 1498–1506. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. BioMed Res. Int. 2019, 1–20. [Google Scholar] [CrossRef]
- Isolauri, E. Probiotics in the Development and Treatment of Allergic Disease. Gastroenterol. Clin. 2012, 41, 747–762. [Google Scholar] [CrossRef]
- Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Jones, M.L.; Labbé, A.; Rodes, L.; Kahouli, I.; Prakash, S. Effect of orally administered L. fermentum NCIMB 5221 on markers of metabolic syndrome: An in vivo analysis using ZDF rats. Appl. Microbiol. Biotechnol. 2014, 98, 115–126. [Google Scholar] [CrossRef]
- Varankovich, N.V.; Nickerson, M.T.; Korber, D.R. Probiotic-based strategies for therapeutic and prophylactic use against multiple gastrointestinal diseases. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Mohammadi-Sartang, M.; Bellissimo, N.; Mazloomi, S.M.; Fararouie, M.; Bedeltavana, A.; Famouri, M.; Mazloom, Z. The effect of daily fortified yogurt consumption on weight loss in adults with metabolic syndrome: A 10-week randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 565–574. [Google Scholar] [CrossRef]
- Shang, M.; Sun, J. Vitamin D, VDR, and probiotics in health and disease: A mini review. CAB Rev. 2017, 24, 876–887. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: New York, NY, USA, 2019. [Google Scholar]
- Savino, F.; Ceratto, S.; Poggi, E.; Cartosio, M.E.; Cordero di Montezemolo, L.; Giannattasio, A. Preventive effects of oral probiotic on infantile colic: A prospective, randomised, blinded, controlled trial using Lactobacillus reuteri DSM 17938. Benef. Microbes 2015, 6, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Tazzyman, S.; Richards, N.; Trueman, A.R.; Evans, A.L.; Grant, V.A.; Garaiova, I.; Plummer, S.F.; Williams, E.A.; Corfe, B.M. Vitamin D associates with improved quality of life in participants with irritable bowel syndrome: Outcomes from a pilot trial. BMJ Open Gastroenterol. 2015, 2, e000052. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, A.; Banafshe, H.R.; Mirhosseini, N.; Moradi, M.; Karimi, M.-A.; Mehrzad, F.; Bahmani, F.; Asemi, Z. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry 2019, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V. Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J. Ovarian. Res. 2019, 12. [Google Scholar] [CrossRef]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef]
- Raygan, F. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog. Neuropsychopharmacol. 2018, 8, 50–55. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Djafarian, K.; Fazeli, M.R.; Yekaninejad, M.S.; Rostamian, A.; Keshavarz, S.A. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 497–506. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Schippa, S.; Conte, M.P. Dysbiotic Events in Gut Microbiota: Impact on Human Health. Nutrients 2014, 6, 5786–5805. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, C.; Verlinden, L.; De Hertogh, G.; Kato, S.; Gysemans, C.; Mathieu, C.; Carmeliet, G.; Verstuyf, A. Impact on Experimental Colitis of Vitamin D Receptor Deletion in Intestinal Epithelial or Myeloid Cells. Endocrinology 2017, 158, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Barragan, M.; Good, M.; Kolls, J. Regulation of Dendritic Cell Function by Vitamin D. Nutrients 2015, 7, 8127–8151. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Y.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef]
- Yoon, S.S.; Sun, J. Probiotics, Nuclear Receptor Signaling, and Anti-Inflammatory Pathways. Gastroenterol. Res. Pract. 2011, 2011, 1–16. [Google Scholar] [CrossRef]
- Mencarelli, A.; Cipriani, S.; Renga, B.; Bruno, A.; D’Amore, C.; Distrutti, E.; Fiorucci, S. VSL#3 Resets Insulin Signaling and Protects against NASH and Atherosclerosis in a Model of Genetic Dyslipidemia and Intestinal Inflammation. PLoS ONE 2012, 7, e45425. [Google Scholar] [CrossRef]
- Fernandes de Abreu, D.A.; Eyles, D.; Féron, F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009, 34, S265–S277. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R. Vitamin D and the occurrence of depression: Causal association or circumstantial evidence? Nutr. Rev. 2009, 67, 481–492. [Google Scholar] [CrossRef]
- Humble, M.B. Vitamin D, light and mental health. J. Photochem. Photobiol. B 2010, 101, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Schmidt, C. Mental health: Thinking from the gut. Nature 2015, 25, S12–S15. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, j4008. [Google Scholar] [CrossRef]
| First Author, YEAR | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Ghaderi, 2019 [27] |  |  |  |  |  |  |  |
| Jafarnejad, 2017 [31] |  |  |  |  |  |  |  |
| Jamilian, 2018 [29] |  |  |  |  |  |  |  |
| Ostadmohammadi, 2019 [28] |  |  |  |  |  |  |  |
| Raygan, 2018 [30] |  |  |  |  |  |  |  |
| Savino, 2015 [25] |  |  |  |  |  |  |  |
| Tazzyman, 2015 [26] |  |  |  |  |  |  |  |
 Low risk of bias
Low risk of bias  Unclear risk of bias
Unclear risk of bias  High risk of bias.
High risk of bias.| First Author, Year, Country | Outcome Measures | Results | Conclusion |
|---|---|---|---|
| Ghaderi, 2019, Iran 1 [27] | BMI: weight in kg divided by height in meters squared (height and weight measured withoutshoes and in light clothing by a trained staff) Serum 25-hydroxyvitamin D: ELISA kit Severity of psychiatric symptoms: PANSS Domains of cognitive function: BPRS scores TAC: method of ferric reduction antioxidant power developed by Benzie and Strain GSH: Beutler method MDA: Thiobarbituric acid reactive substances spectrophotometric Test Serum hs-CRP: ELISA kit NO: Griess Method Serum insulin: ELISA kit HOMA-IR and QUICKI: calculated using standard formula FPG and lipid profiles: Enzymatic kits | At baseline and end line: No significant difference between-groups, in height, age, weight, BMI and METs At baseline: Significant difference between-groups for positive PANSS score, BPRS, GSH and plasma NO At end line: In the I group compared with the C group: Significant greater decrease in MDA (−0.3 ± 0.9 vs. +0.2 ± 0.4 μmol/L), serum hs-CRP (−2.3 ± 3.0 vs. −0.3 ± 0.8 mg/L), FPG (−7.0 ± 9.9 vs. −0.2 ± 9.9 mg/dL), serum insulin (−2.7 ± 2.3 vs. +0.4 ± 2.0 μIU/mL), HOMA-IR (−0.8 ± 0.7 vs. + 0.1 ± 0.7), TG (−7.8 ± 25.2 vs. +10.1 ± 30.8 mg/dL), TC (−4.9 ± 15.0 vs. +5.9 ± 19.5 mg/dL), and TC/HDL-C (−0.1 ±0.6 vs. +0.3 ± 0.8) Significant greater increase in 25-hydroxyvitamin D (+9.1 ± 4.1 vs. +0.2 ± 0.4 ng/mL), general PANSS score (−3.1 ± 4.7 vs. +0.3 ± 3.9), total PANSS score (−7.4 ± 8.7 vs. −1.9 ± 7.5), plasma TAC (+51.1 ± 129.7 vs. −20.7 ± 53.3 mmol/L), QUICKI (+0.02 ± 0.01 vs. +0.0003 ± 0.01) No significant difference in the change of BPRS score and other metabolic profilesIn the analysis adjusting for baseline values of biochemical parameters, age and BMI, and controlling for potential confounders: The difference in changes in TC/HDL between the two groups became non-significant The difference in changes in negative PANSS score, BPRS and plasma GSH became statistically significant Other metabolic profiles did not change statically | Probiotic and vitamin D co-supplementation for 12 weeks to patients with chronic schizophrenia had beneficial effects on the general and total PANSS scores, as well as their metabolic profiles, compared with placebo |
| Jafarnejad, 2017, Iran [31] | Nutrient intake: 3-day dietary recall (2 weekdays and one weekend day), through monthly interview throughout the study period; nutrient analysis: by Nutritionist IV software modified for Iranian foods Physical activity: daily physical activity questionnaires validated by Kelishady et al. and calculated as metabolic equivalents/day Body weight: measured wearing light clothes without shoes using digital scales with 100-g precision Height: measured using a stadiometer with 0.5-cm precision in a normal standing position without shoes. BMI: weight in kilograms divided by height in meters squared BMD: dual energy X-ray absorptiometry Bone and pro-inflammatory biomarkers (TNF-α and IL-1b), Total serum levels of BALP, Osteocalcin, CTX, Vitamin D, RANKL, Osteoprotegrin, Serum TNF-α and IL-1b, Serum PTH, Urinary deoxypyridinoline: ELISA kits Serum calcium, phosphorus, magnesium, albumin, creatinine, alkaline phosphatase, and urinary amounts of calcium, phosphorus, magnesium, and creatinine: Pars Azmoon kits | At baseline: No significant differences between-groups At end line: Significant between-group differences in BALP (U/L) (I: 19.65 ± 1.66 at baseline and 16.53 ± 0.90 at end line vs. C: 17.81 ± 1.35 at baseline and 18.63 ± 1.29 at end line); CTX (ng/mL) (I: 0.41 ± 0.02 at baseline and 0.35 ± 0.02 at end line vs. C: 0.45 ± 0.02 at baseline and 0.42 ± 0.02 at end line); TNF-α (pg/mL) (I: 4.24 ± 0.5 at baseline and 3.73 ± 0.43 at end line vs. 3.83 ± 0.47 at baseline and 4.32 ± 0.5 at end line); PTH (pg/mL) (I: 31.92 ± 1.39 at baseline and 29.05 ± 1.53 at end line vs. C: 30.65 ± 1.44 at baseline and 32.81 ± 1.72 at end line) No significant between-group difference in Spinal BMD, Total hip BMD, RANKL, osteoprotegrin, RANKL/ osteoprotegrin ratio, deoxypyridinoline, osteocalcin, IL-1, Vitamin D, serum calcium, 24-h urinary Calcium, Serum phosphorus, 24-h urinary phosphorus, Serum magnesium, 24-h urinary magnesium, Serum creatinine, 24-h urinary creatinine, ALP, Albumin | Supplementation with probiotics, vitamin D and calcium for 6 weeks to postmenopausal osteopenic women showed a possible role in suppressing bone resorption and bone turnover, but did not affect bone density and other serum indicators compared with placebo, vitamin D and calcium |
| Jamilian, 2018, Iran 2,3 [29] | BMI: weight in kg divided by height in meters squared (height and weight measured withoutshoes and in light clothing by a trained staff) Polyhydramnios: sonographic estimation method at post-intervention and defined as an AFI in excess of 25 cm Preterm delivery: defined as delivery occurred at <37 weeks of pregnancy Newborn’s macrosomia: defined as birth weight of >4000 g. 2.5 Serum 25-hydroxyvitamin D: ELISA kit Serum insulin: ELISA kit HOMA-IR and QUICKI: calculated according to the standard formula FPG, serum TG, VLDL-C, TC, LDL-C and HDL-C: enzymatic kits Serum hs-CRP: ELISA kit Plasma NO: Griess method TAC: method of ferric reducing antioxidant power developed by Benzie and Strain GSH: Beutler method MDA: Thiobarbituric acid reactive substances spectrophotometric Test Newborns’ hyperbilirubinemia: when the total serum bilirubin levels were at ≥15 mg/dL (257 mmol/L) among infants 25–48 h old, 18 mg/dL (308 mmol/L) in infants 49–72 h old, and 20 mg/dL (342 mmol/L) in infants >72 h old | At baseline and end line: No significant difference between-groups, in age, height, weight, BMI, METs and intakes of macro- and micronutrients At end line: In the I group compared with the C1 group Significant greater decrease in TG (β −15.82 mg/dL), VLDL-C (β −3.16 mg/dL) and hs-CRP (β −0.32 mg/L) Significant greater increase in serum 25-hydroxyvitamin D (β 16.16 ng/mL), TAC (β 63.26 mmol/L) and GSH (β 53.61 mmol/L)Lower incidence of hyperbilirubinemiain newborns (10.0% vs. 13.8%) Lower incidence of newborns’ hospitalization (10.0% vs. 10.3%) No significant changes in other pregnancy outcomes In the I group compared with C2 group: Significant greater decrease in FPG (β −10.99 mg/dL), serum insulin (β −1.95 mIU/mL), HOMA-IR (β −0.76; 95%), TG (β −37.56 mg/dL), VLDL-C (β −7.51 mg/dL), HDL/TC B: −0.52), hs-CRP (β −1.80 mg/L) and MDA (β −0.43 mmol/L) Significant greater increase in 25-hydroxyvitamin D (β 18.21 ng/mL), QUICKI (β 0.01) HDL-C (β 4.09 mg/dL) and TAC (β 97.77 mmol/L) No significant changes in other metabolic parameters Lower incidence of hyperbilirubinemia in newborns (10.0% vs. 35.7%) Lower incidence of newborns’ hospitalization (10.0% vs. 32.1%) No significant changes in other pregnancy outcomes In the C1 group compared with the C2 group Significant greater decrease in FPG (β −8.60 mg/dL), Insulin (β −1.34 μIU/mL), HOMA-IR (β −0.54), TG (β −21.73 mg/dL), VLDL-C (β −4.34 mg/dL) and hs-CRP (β −1.36 mg/L), and MDA (β −0.50 μmol/L) Significant greater increase in serum 25-hydroxyvitamin D (β 2.05 ng/mL) | High dose of vitamin D and probiotic co-supplementation for 6 weeks to women with GDM had beneficial effects on metabolic status and newborns’ outcomes compared with placebo and low dose of vitamin D or probiotic supplementation and a low dose of vitamin D |
| Ostadmohammadi, 2019, Iran 2,3 [28] | Hirsutism: mFG scoring system Mental health: BDI, GHQ-28 and DASS Quality of sleep: PSQI Serum 25-hydroxyvitamin D: ELISA kit Serum total testosterone and SHBG: ELISA kits hs-CRP: ELISA kit Plasma NO: Griess method TAC: Benzie and Strain method GSH: Beutler method MDA: Thiobarbituric acid reactive substances spectrophotometric Test | At baseline: No significant difference between-groups for mean age, height and dietary macro- and micro-nutrient intakes. At end line: In the I group compared with the C group: Significant greater decrease in BDI (β −0.58), GHQ (β − 0.93), DASS (β − 0.90), total testosterone (β − 0.19 ng/mL), hirsutism (β − 0.95), hs-CRP (β − 0.67 mg/L) and MDA (β − 0.25 μmol/L) Significant greater increase in TAC (β 82.81 mmol/L) and GSH (β 40.42 μmol/L) No significant effect on serum SHBG and plasma NO levels, acne, alopecia and PSQI | Vitamin D and probiotic co-supplementation for 12 weeks to women with PCOS had beneficial effects on mental health parameters, but did not affect serum SHBG, plasma NO levels, acne, alopecia and PSQI, compared with placebo |
| Raygan, 2018, Iran 1 [30] | Serum 25-hydroxyvitamin D: ELISA FPG and lipid profiles: Enzymatic kit Insulin: ELISA kit HOMA-IR and QUICKI: standard formula Hs-CRP: ELISA kit Plasma TAC: Benzie and Strain method GSH: Beutler and Gelbart method MDA: spectrophotometric test NO: Griess method SBP and DBP: sphygmomanometer (Not detailed) Mental health: BDI, BAI, GHQ-28 | At baseline and end line: No significant differences between-groups in mean age, height, weight, BMI and METs and macro and micronutrient intakes At end line: In the I group compared with the C group: Significant greater decrease in BDI (−2.8 ± 3.8 vs. −0.9 ± 2.1), BAI (−2.1 ± 2.3 vs. −0.8 ± 1.4) and GHQ scores (−3.9 ± 4.1 vs. −1.1 ± 3.4), Insulin (μIU/mL) (−2.8 ± 3.8 vs. +0.2 ± 4.9), HOMA-IR (−1.0 ± 1.6 vs. −0.1 ± 1.5), and hs-CRP (ng/mL) (−950.0 ± 1811.2 vs. +260.5 ± 2298.2) Significant greater increase in 25-hydroxyvitamin D (ng/mL) (+11.8 ± 5.9 vs. +0.1 ± 1.4), QUICKI (+0.03 ± 0.04 vs. −0.001 ± 0.01), serum HDL-cholesterol (mg/dL) (+2.3 ± 3.5 vs. −0.5 ± 3.8), plasma NO (μmol/L) (+1.7 ± 4.0 vs. −1.4 ± 6.7) and plasma TAC (mmol/L) (+12.6 ± 41.6 vs. −116.9 ± 324.2) No significant different changes in FPG, Triglycerides, VLDL-Cholesterol, LDL-Cholesterol, GSH, MDA, SBP and DBP | Vitamin D and probiotic co-supplementation for 12 weeks to diabetic people with CHD had beneficial effects on mental health, glycemic control, HDL-cholesterol levels, hs-CRP, NO and TAC, but did not affect other metabolic profiles and blood pressures, compared with placebo |
| Savino, 2015, Italy [25] | Administration of pain-relieving agents (cimetropium bromide at least three times per week or simethicone at least five times per week): daily reporting by parents % of infants switching from exclusive breastfeeding to partial or exclusive formula feeding: not detailed Number of phone-calls and visits due to infantile colic: noted by the pediatrician. | In the I group compared with the C group:
| Vitamin D and probiotic co-supplementation for 12 weeks to newborns was associated with a reduction of pediatric consultations for infantile colic, use of pain-relieving agents and of infant formula, compared with vitamin D supplementation |
| Tazzyman, 2015, United Kingdom [26] | Serum 25(OH)D: Cobas e411 automated immunoassay Dietary intake: Food frequency questionnaire analyzed using FETA open source software IBS symptom: questionnaire assessing abdominal pain (pain severity and number of days with pain), bloating, bowel habits (minimum and maximum bowel movement per day and satisfaction with bowel habit) and quality of life | At baseline: No significant differences between-groups At end line: In the I and C2 groups compared with the C1 group: - Significantly higher 25OHD (ng/mL) (37.2 ±9.3 and 37.1 ± 11.7 vs. 25.3 ± 8.0) No significant between-group differences for any symptom tested, and total symptom severity (same results obtained for participants who were 25(OH)D-deficient at baseline) | Vitamin D and probiotic co-supplementation had no significant effect on the symptoms of IBS, compared with vitamin D alone, or placebo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abboud, M.; Rizk, R.; AlAnouti, F.; Papandreou, D.; Haidar, S.; Mahboub, N. The Health Effects of Vitamin D and Probiotic Co-Supplementation: A Systematic Review of Randomized Controlled Trials. Nutrients 2021, 13, 111. https://doi.org/10.3390/nu13010111
Abboud M, Rizk R, AlAnouti F, Papandreou D, Haidar S, Mahboub N. The Health Effects of Vitamin D and Probiotic Co-Supplementation: A Systematic Review of Randomized Controlled Trials. Nutrients. 2021; 13(1):111. https://doi.org/10.3390/nu13010111
Chicago/Turabian StyleAbboud, Myriam, Rana Rizk, Fatme AlAnouti, Dimitrios Papandreou, Suzan Haidar, and Nadine Mahboub. 2021. "The Health Effects of Vitamin D and Probiotic Co-Supplementation: A Systematic Review of Randomized Controlled Trials" Nutrients 13, no. 1: 111. https://doi.org/10.3390/nu13010111
APA StyleAbboud, M., Rizk, R., AlAnouti, F., Papandreou, D., Haidar, S., & Mahboub, N. (2021). The Health Effects of Vitamin D and Probiotic Co-Supplementation: A Systematic Review of Randomized Controlled Trials. Nutrients, 13(1), 111. https://doi.org/10.3390/nu13010111








