Metabolic Bone Disease of Prematurity: Risk Factors and Associated Short-Term Outcomes
Abstract
1. Introduction
2. Materials and Methods
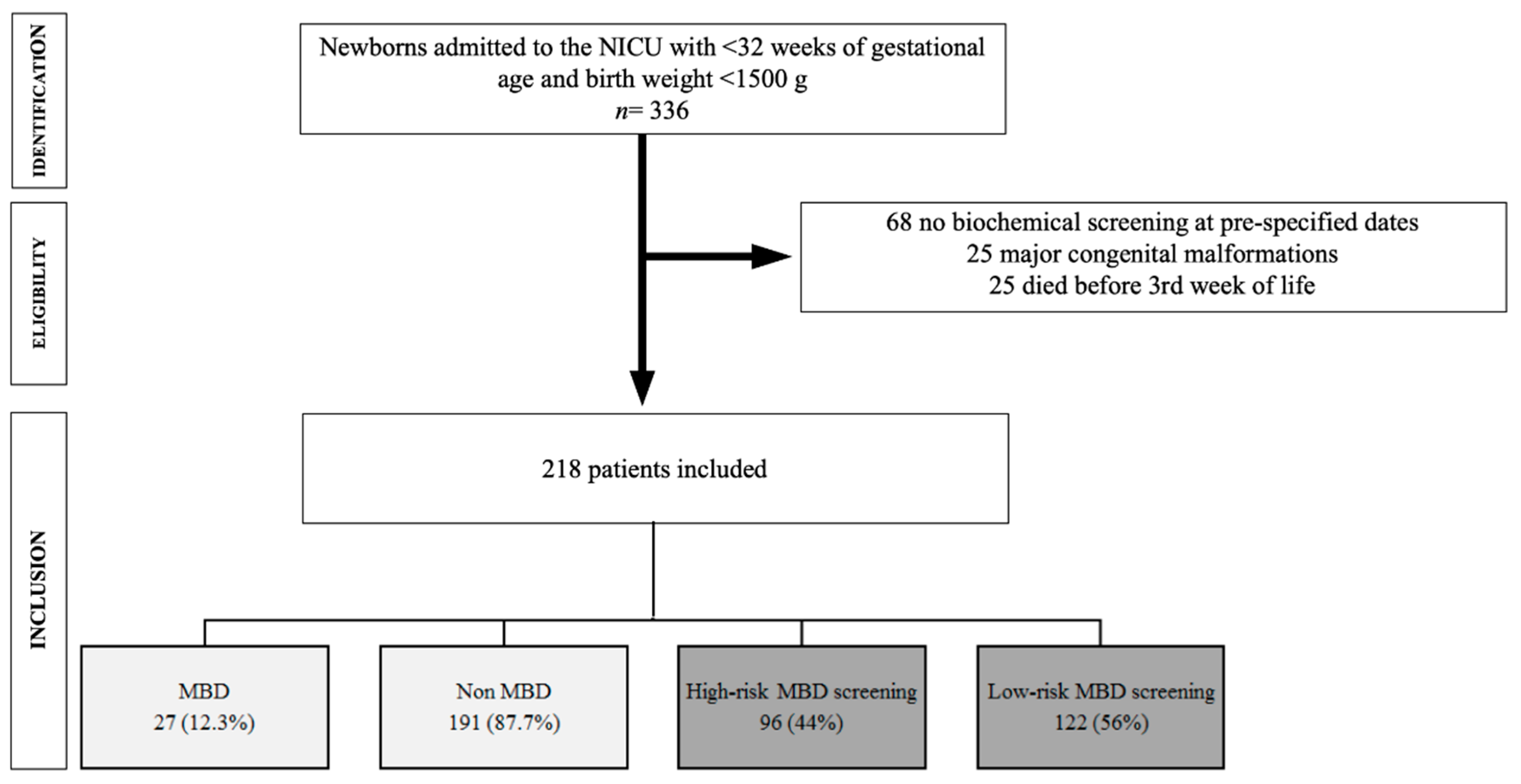
2.1. Study Design and Population
2.2. Nutrition Protocol
2.3. MBD Screening
2.4. Anthropometric and Analytical Measurements
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Univariate Analysis of Study Population
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chinoy, A.; Mughal, M.Z.; Padidela, R. Metabolic bone disease of prematurity: Causes, recognition, prevention, treatment and long-term consequences. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F560–F566. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Khasawneh, W.; McNelis, K.; Dykstra, C.; Amstadt, R.; Super, D.M.; Groh-Wargo, S.; Kumar, D. Metabolic Bone Disease. J. Parenter. Enter. Nutr. 2014, 38, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Rustico, S.E.; Calabria, A.C.; Garber, S.J. Metabolic bone disease of prematurity. J. Clin. Transl. Endocrinol. 2014, 1, 85–91. [Google Scholar] [CrossRef][Green Version]
- Rigo, J.; De Curtis, M.; Pieltain, C.; Picaud, J.C.; Salle, B.L.; Senterre, J. Bone mineral metabolism in the micropremie. Clin. Perinatol. 2000, 27, 147–170. [Google Scholar] [CrossRef]
- Rigo, J.; Pieltain, C.; Salle, B.; Senterre, J. Enteral calcium, phosphate and vitamin D requirements and bone mineralization in preterm infants. Acta Paediatr. Int. J. Paediatr. 2007, 96, 969–974. [Google Scholar] [CrossRef]
- Harrison, C.M.; Johnson, K.; McKechnie, E. Osteopenia of prematurity: A national survey and review of practice. Acta Paediatr. Int. J. Paediatr. 2008, 97, 407–413. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; Cole, T.J.; Bishop, N.J.; Lucas, A. Neonatal factors predicting childhood height in preterm infants: Evidence for a persisting effect of early metabolic bone disease? J. Pediatr. 2000, 137, 668–673. [Google Scholar] [CrossRef]
- Zozaya, C.; Díaz, C.; Saenz de Pipaón, M. How Should We Define Postnatal Growth Restriction in Preterm Infants? Neonatology 2018, 114, 177–180. [Google Scholar] [CrossRef]
- Cassady, S.J.; Lasso-Pirot, A.; Deepak, J. Phenotypes of Bronchopulmonary Dysplasia in Adults. Chest 2020. [Google Scholar] [CrossRef]
- Abrams, S.A.; Bhatia, J.J.S.; Corkins, M.R.; De Ferranti, S.D.; Golden, N.H.; Silverstein, J.; Grummer-Strawn, L.; Hubbard, V.; Marchand, V.; Silverman, B.M.; et al. Calcium and vitamin D requirements of enterally fed preterm infants. Pediatrics 2013, 131. [Google Scholar] [CrossRef]
- Montaner Ramón, A. Risk factors of bone mineral metabolic disorders. Semin. Fetal Neonatal Med. 2020, 25, 101068. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.K.; Doan, J.; Teoh, Y.S.; Stewart, A.; Forrest, P.; Simm, P.J. Outcomes of standardised approach to metabolic bone disease of prematurity. J. Paediatr. Child Health 2018, 54, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Faerk, J.; Peitersen, B.; Petersen, S.; Michaelsen, K.F. Bone mineralisation in premature infants cannot be predicted from serum alkaline phosphatase or serum phosphate. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Rayannavar, A.; Calabria, A.C. Screening for Metabolic Bone Disease of prematurity. Semin. Fetal Neonatal Med. 2020, 25, 101086. [Google Scholar] [CrossRef]
- Faienza, M.F.; D’Amato, E.; Natale, M.P.; Grano, M.; Chiarito, M.; Brunetti, G.; D’Amato, G. Metabolic bone disease of prematurity: Diagnosis and management. Front. Pediatr. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Kelly, A.; Kovatch, K.J.; Garber, S.J. Metabolic bone disease screening practices among U.S. Neonatologists. Clin. Pediatr. 2014, 53, 1077–1083. [Google Scholar] [CrossRef]
- Körnmann, M.N.; Christmann, V.; Gradussen, C.J.W.; Rodwell, L.; Gotthardt, M.; Van Goudoever, J.B.; Van Heijst, A.F.J. Growth and Bone Mineralization of Very Preterm Infants at Term Corrected Age in Relation to Different Nutritional Intakes in the Early Postnatal Period. Nutrients 2017, 9, 1318. [Google Scholar] [CrossRef]
- Chen, W.; Yang, C.; Chen, H.; Zhang, B. Risk factors analysis and prevention of metabolic bone disease of prematurity. Medicine 2018, 97, 1–5. [Google Scholar] [CrossRef]
- Visser, F.; Sprij, A.J.; Brus, F. The validity of biochemical markers in metabolic bone disease in preterm infants: A systematic review. Acta Paediatr. Int. J. Paediatr. 2012, 101, 562–568. [Google Scholar] [CrossRef]
- Nehra, D.; Carlson, S.J.; Fallon, E.M.; Kalish, B.; Potemkin, A.K.; Gura, K.M.; Simpser, E.; Compher, C.; Puder, M.; American Society for Parenteral and Enteral Nutrition. A.S.P.E.N. clinical guidelines: Nutrition support of neonatal patients at risk for metabolic bone disease. JPEN J. Parenter. Enteral Nutr. 2013, 37, 570–598. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Perinatal brain injury: From pathogenesis to neuroprotection. Ment. Retard. Dev. Disabil. Res. Rev. 2001, 7, 56–64. [Google Scholar] [CrossRef]
- Bancalari, E.; Claure, N. Definitions and diagnostic criteria for bronchopulmonary dysplasia. Semin. Perinatol. 2006, 30, 164–170. [Google Scholar] [CrossRef]
- Castro Conde, J.R.; Echániz Urcelay, I.; Botet Mussons, F.; Pallás Alonso, C.R.; Narbona, E.; Sánchez Luna, M.; Comisión de Estándares de la Sociedad Española de Neonatología. Retinopathy of prematurity. Prevention, screening and treatment guidelines. An. Pediatr. 2009, 71, 514–523. [Google Scholar] [CrossRef]
- Patel, A.L.; Engstrom, J.L.; Meier, P.P.; Jegier, B.J.; Kimura, R.E. Calculating postnatal growth velocity in very low birth weight (VLBW) premature infants. J. Perinatol. 2009, 29, 618–622. [Google Scholar] [CrossRef]
- Fenton, T.R.; Lyon, A.W.; Rose, M.S. Cord blood calcium, phosphate, magnesium, and alkaline phosphatase gestational age-specific reference intervals for preterm infants. BMC Pediatr. 2011, 11, 76. [Google Scholar] [CrossRef]
- Noone, D.; Kieran, E.; Molloy, E.J. Serum magnesium in the first week of life in extremely low birth weight infants. Neonatology 2012, 101, 274–277. [Google Scholar] [CrossRef]
- Tinnion, R.J.; Embleton, N.D. How to use…alkaline phosphatase in neonatology. Arch. Dis. Child. Educ. Pract. Ed. 2012, 97, 157–163. [Google Scholar] [CrossRef]
- Pohlandt, F.; Mihatsch, W.A. Reference values for urinary calcium and phosphorus to prevent osteopenia of prematurity. Pediatr. Nephrol. 2004, 19, 1192–1193. [Google Scholar] [CrossRef]
- Matejek, T.; Navratilova, M.; Zaloudkova, L.; Malakova, J.; Maly, J.; Skalova, S.; Palicka, V. Parathyroid hormone–reference values and association with other bone metabolism markers in very low birth weight infants–pilot study. J. Matern. Neonatal Med. 2019, 32, 2860–2867. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Desci, T.; Domellöf, M.; Embleton, N.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition. Clin. Nutr. 2018, 37, 2303–2305. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.; Fewtrell, M.; Goulet, O.; Molgaard, C.; Picaud, J.-C.; Senterre, T.; ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Calcium, phosphorus and magnesium. Clin. Nutr. 2018, 37, 2360–2365. [Google Scholar] [CrossRef] [PubMed]
- Backström, M.C.; Kouri, T.; Kuusela, A.L.; Sievänen, H.; Koivisto, A.M.; Ikonen, R.S.; Mäki, M. Bone isoenzyme of serum alkaline phosphatase and serum inorganic phosphate in metabolic bone disease of prematurity. Acta Paediatr. Int. J. Paediatr. 2000, 89, 867–873. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.M.; Rogers, S.P.; Hicks, P.D.; Hawthorne, K.M.; Parker, B.R.; Abrams, S.A. High frequencies of elevated alkaline phosphatase activity and rickets exist in extremely low birth weight infants despite current nutritional support. BMC Pediatr. 2009, 9, 47. [Google Scholar] [CrossRef]
- Ukarapong, S.; Venkatarayappa, S.K.B.; Navarrete, C.; Berkovitz, G. Risk factors of metabolic bone disease of prematurity. Early Hum. Dev. 2017, 112, 29–34. [Google Scholar] [CrossRef]
- Figueras-Aloy, J.; Álvarez-Domínguez, E.; Pérez-Fernández, J.M.; Moretones-Suñol, G.; Vidal-Sicart, S.; Botet-Mussons, F. Metabolic bone disease and bone mineral density in very preterm infants. J. Pediatr. 2014, 164, 499–504. [Google Scholar] [CrossRef]
- Rigo, J.; Nyamugabo, K.; Picaud, J.C.; Gerard, P.; Pieltain, C.; De Curtis, M. Reference values of body composition obtained by dual energy X-ray absorptiometry in preterm and term neonates. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 184–190. [Google Scholar] [CrossRef]
- Ghirardello, S.; Dusi, E.; Cortinovis, I.; Villa, S.; Fumagalli, M.; Agosti, M.; Milani, S.; Mosca, F. Effects of Red Blood Cell Transfusions on the Risk of Developing Complications or Death: An Observational Study of a Cohort of Very Low Birth Weight Infants. Am. J. Perinatol. 2017, 34, 88–95. [Google Scholar] [CrossRef]
- Kirpalani, H.; Whyte, R.K.; Andersen, C.; Asztalos, E.V.; Heddle, N.; Blajchman, M.A.; Peliowski, A.; Rios, A.; LaCorte, M.; Connelly, R.; et al. The Premature Infants in Need of Transfusion (PINT) study: A randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J. Pediatr. 2006, 149, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Mahachoklertwattana, P.; Sirikulchayanonta, V.; Chuansumrit, A.; Karnsombat, P.; Choubtum, L.; Sriphrapradang, A.; Domrongkitchaiporn, S.; Sirisriro, R.; Rajatanavin, R. Bone histomorphometry in children and adolescents with beta-thalassemia disease: Iron-associated focal osteomalacia. J. Clin. Endocrinol. Metab. 2003, 88, 3966–3972. [Google Scholar] [CrossRef]
- Raffaeli, G.; Manzoni, F.; Cortesi, V.; Cavallaro, G.; Mosca, F.; Ghirardello, S. Iron Homeostasis Disruption and Oxidative Stress in Preterm Newborns. Nutrients 2020, 12, 1554. [Google Scholar] [CrossRef]
- Treviño-Báez, J.D.; Briones-Lara, E.; Alamillo-Velázquez, J.; Martínez-Moreno, M.I. Multiple red blood cell transfusions and iron overload in very low birth weight infants. Vox Sang. 2017, 112, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, Y. Citrate metabolism in blood transfusions and its relationship due to metabolic alkalosis and respiratory acidosis. Int. J. Clin. Exp. Med. 2015, 8, 6578–6584. [Google Scholar] [PubMed]
- Abdallah, E.A.A.; Said, R.N.; Mosallam, D.S.; Moawad, E.M.I.; Kamal, N.M.; Fathallah, M.G.E.-D. Serial serum alkaline phosphatase as an early biomarker for osteopenia of prematurity. Medicine 2016, 95, e4837. [Google Scholar] [CrossRef] [PubMed]
| Demographic and Perinatal Variables | Respiratory Support | ||
|---|---|---|---|
| Gestational age, week | 29.9 (1.9) (28.8–31.4) | Intubation in delivery room | 36 (16.5) |
| Female | 125 (57.3) | Surfactant | 85 (39) |
| Prenatal steroids (complete) | 183 (83.9) | NIV during admission | 209 (95.9) |
| Chorioamnionitis | 46 (21.1) | MV during admission | 59 (27) |
| Maternal arterial hypertension | 52 (23.9) | Duration of MV, h (n = 59) | 144.1 (213.1) (19–144) |
| Multiple birth | 83 (38.1) | Duration of NIV, h (n = 209) | 176.3 (188) (48–216) |
| IVF | 65 (29.8) | Duration of supplementary oxygen (hours) | 461.9 (661.5) (24–708) |
| Caesarean section | 169 (77.5) | Clinical outcomes | |
| Apgar 1 min | 6.8 (1.8) (6–8) | BPD | 41 (18.8) |
| Apgar 5 min | 8.2 (1.3) (8–9) | BPD moderate-severe | 18 (8.3) |
| Medically treated PDA | 25 (11.5) | ||
| Nutrition and growth | Surgically treated PDA | 12 (5.5) | |
| Birth weight, g | 1207.5 (245.3) (1011–1430) | GM-IVH (all grades) | 30 (13.8) |
| Birth weight, z-score | −0.47 (0.77) (−0.99–0.06) | IVH ≥ Grade 3 | 6 (2.8) |
| Small for gestational age | 15 (6.9) | Late-onset sepsis | 62 (28.4) |
| Weight at discharge, g | 2437.2 (365.3) (2230–2588) | NEC | 7 (3.2) |
| Weight at discharge, z-score | −1.3 (1.0) (−1.8–−0.7) | Surgical NEC | 4 (1.8) |
| Weight gain, g/kg/day | 14.5 (21.5) (11.4–14.8) | Leukomalacia | 15 (6.9) |
| Postnatal growth restriction | 89 (40.8) | ROP | 43 (14.6) |
| Parenteral nutrition | 218 (100) | ROP grade III-IV | 5 (2.4) |
| Days on parenteral nutrition | 10.4 (6.8) (6–12) | Intensive care stay, d | 24.0 (18.7) (10–34) |
| Start of enteral feeding, days of age | 1.9 (1.3) (1–2) | Hospital stay, d | 57.6 (53.1) (40–66) |
| Type of enteral feeding | Red blood cell transfusion | 96 (44) | |
| Breast milk | 117 (53.7) | ||
| Donor breast milk | 47 (21.6) | ||
| Formula | 54 (24.7) | ||
| Breast milk fortification | 186 (85.3) | Biochemical MBD screening | |
| Start of fortification, days of age (n = 186) | 12.2 (6.3) (9–14) | Age at biochemical screening, days | 19.7 (7.1) (15–24) |
| Serum creatinine, mg/dL | 0.42 (0.19) (0.27–0.56) | ||
| Medications | Serum phosphorus, mg/dL | 6.2 (1.0) (5.7–7) | |
| Caffeine | 214 (98.2) | 214 (98.2) | 9.8 (0.5) (9.5–10.2) |
| Inotropes | 16 (7.3) | Serum magnesium, mg/dL (n = 166) | 2.1 (0.5) (1.9–2.2) |
| Steroids for BPD | 21 (9.6) | Serum ALP, IU/L | 832 (354) (582–1019) |
| Start of steroids for BPD, days of age (n = 21) | 26.6 (16.5) (17–32) | PTH, pg/mL (n = 143) | 89.1 (67.7) (44–121) |
| Furosemide | 4 (1.8) | Serum ALP > 900 IU/L | 77 (35.3) |
| Thiazides | 9 (4.1) | Serum phosphorus > 5.5 mg/dL | 49 (22) |
| Spironolactone | 3 (1.4) | MBD | 27 (12.3) |
| Days on diuretics (n = 12) | 11.0 (18.5) (5–35) | High screening risk for MBD | 96 (44) |
| Oral phosphorus supplements | 44 (20.2) | Hyperparathyroidism | 59 (27.1) |
| Oral calcium supplements | 124 (56.9) | ||
| Oral vitamin D supplements | 218 (100) | ||
| MBD | High-Risk for MBD | |||||
|---|---|---|---|---|---|---|
| No N = 191 1 | Yes N = 27 1 | p-Value 2 | No N = 122 1 | Yes N = 96 1 | p-Value 2 | |
| Demographic and perinatal variables | ||||||
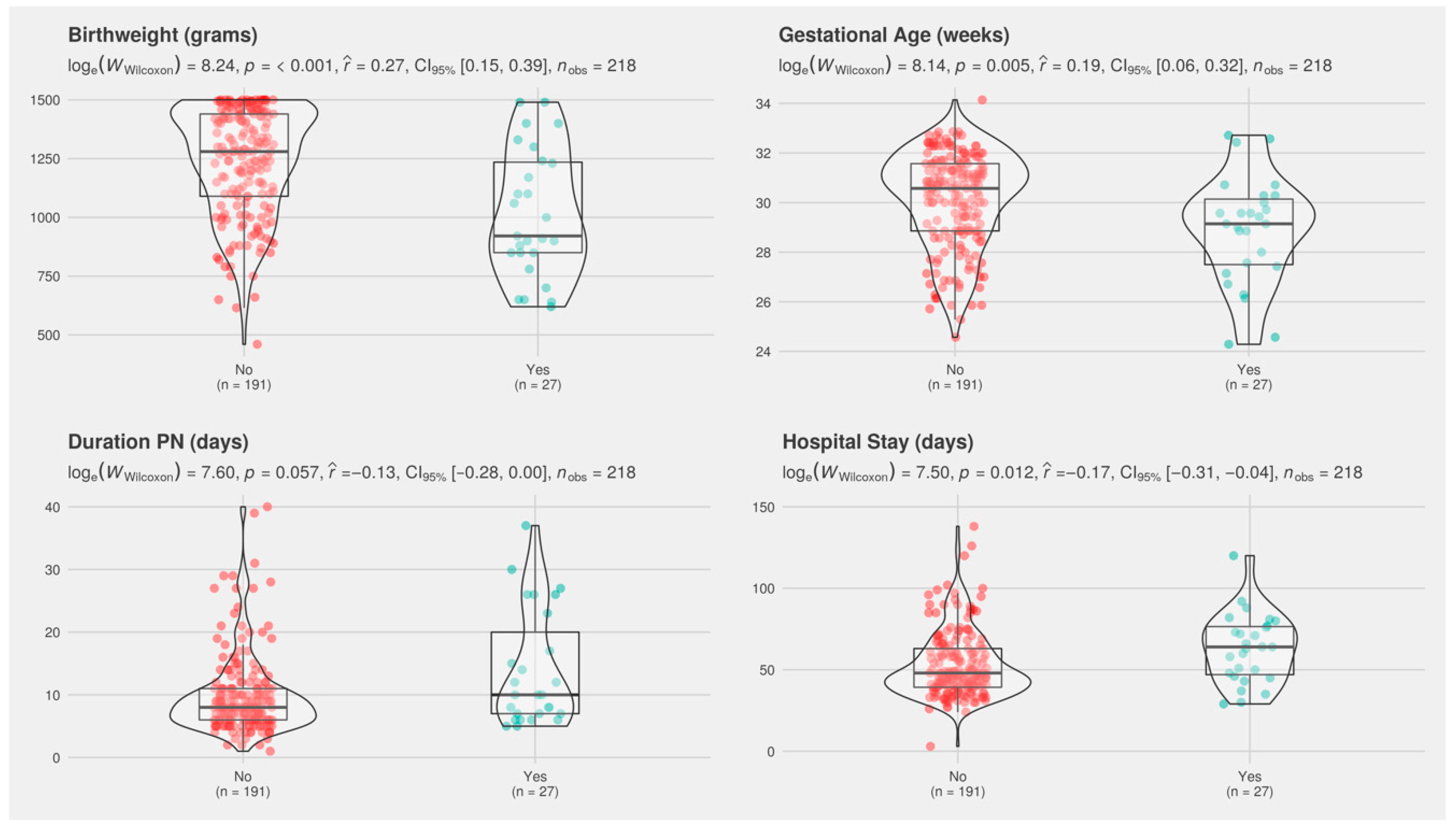
| Gestational age, week | 30.57 (28.86–31.57) | 29.14 (27.5–30.14) | 0.005 | 30.86 (29.28–31.71) | 29.57 (28.0–30.79) | <0.001 |
| Female | 113 (59%) | 12 (44%) | 0.2 | 74 (61%) | 51 (53%) | 0.3 |
| Prenatal steroids (complete) | 159 (83%) | 24 (89%) | 0.3 | 100 (82%) | 83 (86%) | 0.6 |
| Chorioamnionitis | 42 (22%) | 4 (15%) | 0.5 | 27 (22%) | 19 (20%) | 0.8 |
| Maternal hypertension | 44 (23%) | 8 (30%) | 0.6 | 26 (21%) | 26 (27%) | 0.4 |
| Multiple birth | 75 (39%) | 8 (30%) | 0.5 | 48 (39%) | 35 (36%) | 0.8 |
| IVF | 58 (30%) | 7 (26%) | 0.8 | 35 (29%) | 30 (31%) | 0.8 |
| Caesarean section | 147 (77%) | 22 (81%) | 0.8 | 93 (76%) | 76 (79%) | 0.7 |
| Apgar 1 min | 7 (6.00, 8.00) | 7 (5.00, 7.00) | 0.11 | 7 (6.00, 9.00) | 7 (6.00, 8.00) | 0.042 |
| Apgar 5 min | 8 (8.00, 9.00) | 8 (8.00, 8.00) | 0.095 | 8 (8.00, 9.00) | 8 (8.00, 9.00) | 0.064 |
| Nutrition and growth | ||||||
| Birth weight, g | 1280 (1099–1440) | 921 (850–1230) | <0.001 | 1330 (1150–1460) | 1120 (908–1300) | <0.001 |
| Birth weight, z-score | −0.46 (−0.96, 0.09) | −0.87 (−1.27, −0.49) | 0.024 | −0.43 (−0.92, 0.03) | −0.59 (−1.05, 0.08) | 0.4 |
| Small for gestational age | 13 (6.8%) | 2 (7.4%) | 0.9 | 6 (4.9%) | 9 (9.4%) | 0.3 |
| Maternal Body Mass Index | 25.57 | 27.27 | 0.2 | 25.59 | 26.04 | 0.6 |
| Weight at discharge, kg | 2.37 (2.24–2.59) | 2.32 (2.2–2.54) | 0.6 | 2.36 (2.24–2.54) | 2.36 (2.20–2.67) | 0.7 |
| Weight gain, g/kg/day | 14.07 (11.34, 14.62) | 14.56 (12.75, 15.75) | 0.8 | 13.35 (11.51, 14.84) | 13.06 (11.25, 14.83) | 0.7 |
| Postnatal growth restriction | 81 (42%) | 8 (30%) | 0.3 | 42 (34%) | 47 (49%) | 0.043 |
| Days on PN | 8 (6, 11) | 10 (7, 20) | 0.045 | 7 (6, 10) | 10 (7, 15) | <0.001 |
| Start of eteral feeding start, days of age | 2.00 (1–2) | 200 (1–2) | 0.8 | 2.00 (1–2) | 2.00 (1–2) | 0.4 |
| Breast milk | 141 (74%) | 23 (85%) | 0.3 | 90 (74%) | 74 (77%) | 0.7 |
| Breast milk fortification | 161 (84%) | 25 (93%) | 0.4 | 103 (84%) | 83 (86%) | 0.8 |
| Respiratory support | ||||||
| Intubation in delivery room | 29 (15%) | 7 (26%) | 0.2 | 17 (14%) | 19 (20%) | 0.3 |
| Surfactant | 73 (38%) | 12 (44%) | 0.7 | 40 (33%) | 45 (47%) | 0.048 (0.14) |
| NIV during admission | 182 (95.3%) | 27 (100%) | 0.4 | 115 (94.3%) | 94 (97.9%) | 0.3 |
| MV during admission | 50 (26%) | 9 (33%) | 0.6 | 26 (21%) | 33 (34%) | 0.045 (0.15) |
| Duration of MV, h (n = 59) | 48 (18, 120) | 24 (20, 324) | 0.9 | 27 (18, 85) | 72 (20, 324) | 0.3 |
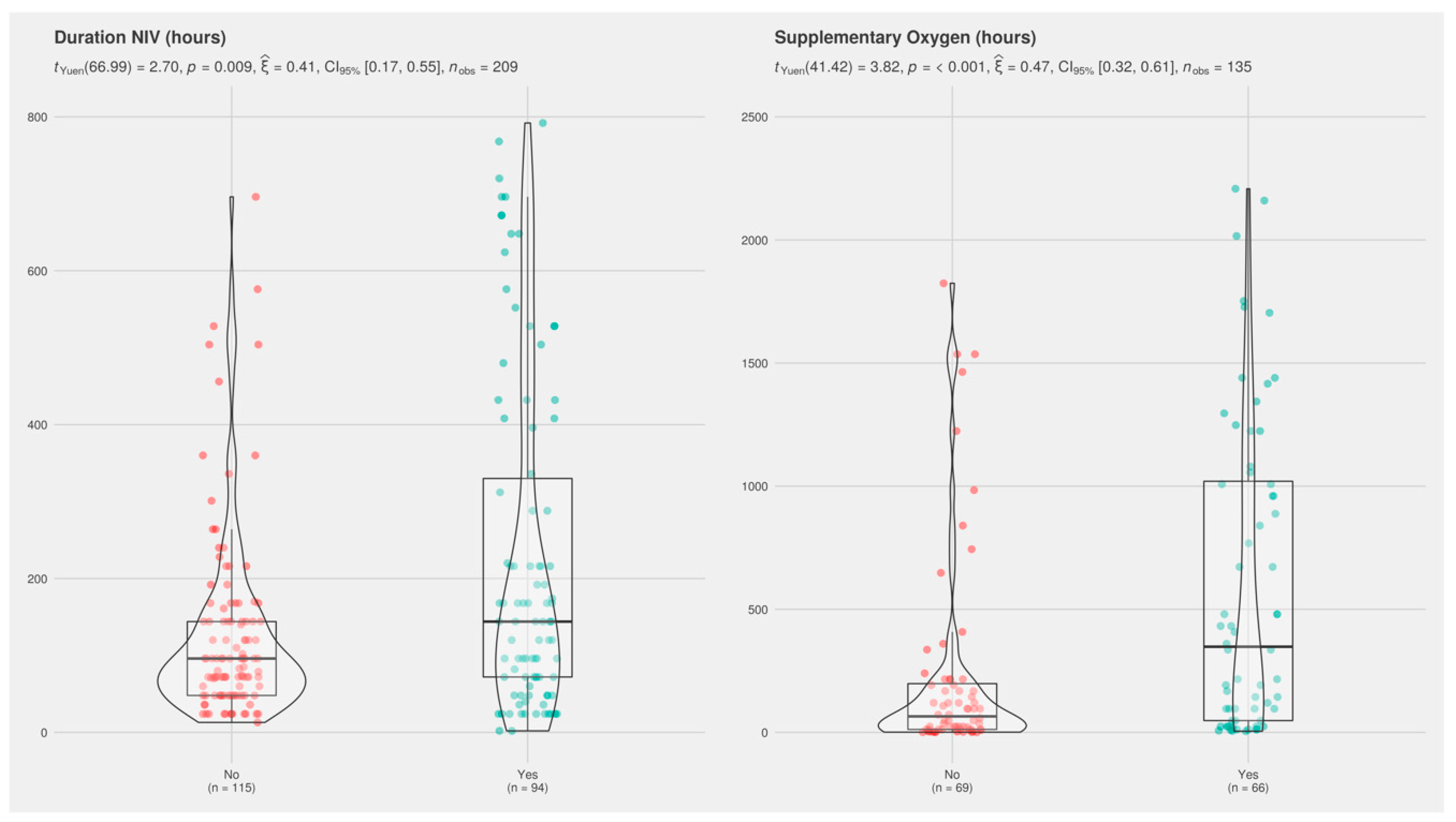
| Duration of NIV, h (n = 209) | 96 (51, 186) | 120 (60, 402) | 0.3 | 96 (48, 144) | 144 (72, 330) | 0.009 |
| Duration of supplementary oxygen, h (n = 135) | 120 (19, 654) | 408 (96, 888) | 0.052 | 72 (12, 216) | 384 (60, 1074) | <0.001 |
| Medications | ||||||
| Caffeine | 187 (98%) | 27 (100%) | >0.9 | 118 (97%) | 96 (100%) | 0.13 |
| Inotropes | 12 (6.3%) | 4 (15%) | 0.12 | 4 (3.3%) | 12 (12%) | 0.02 (0.18) |
| Steroids for BPD | 16 (8.4%) | 5 (19%) | 0.2 | 7 (5.7%) | 14 (15%) | 0.049 (0.15) |
| Start of steroids for BPD, days of age (n = 21) | 22 (16, 30) | 31 (23, 33) | 0.2 | 21 (16, 23) | 30 (21, 33) | 0.14 |
| Clinical outcomes | ||||||
| BPD | 33 (17%) | 8 (30%) | 0.2 | 14 (11%) | 27 (28%) | 0.003 (0.21) |
| BPD moderate-severe | 15 (7.9%) | 3 (11%) | 0.5 | 7 (5.7%) | 11 (11%) | 0.2 |
| Medically treated PDA | 171 (90%) | 22 (81%) | 0.2 | 115 (94%) | 78 (81%) | 0.005 (0.2) |
| IVH ≥ Grade 3 | 4 (2.1%) | 2 (7.4%) | 0.2 | 2 (1.6%) | 4 (4.2%) | 0.4 |
| Late-onset sepsis | 49 (26%) | 13 (48%) | 0.028 (0.16) | 26 (21%) | 36 (38%) | 0.013 (0.18) |
| NEC | 5 (2.6%) | 2 (7.4%) | 0.2 | 3 (2.5%) | 4 (4.2%) | 0.7 |
| Leukomalacia | 10 (5.2%) | 5 (19%) | 0.025 (0.17) | 9 (7.4%) | 6 (6.2%) | >0.9 |
| ROP | 14 (7.8%) | 2 (7.7%) | >0.9 | 5 (4.4%) | 11 (12%) | 0.075 |
| NICU stay, days | 20 (10, 34) | 18 (10, 31) | 0.9 | 20 (10, 32) | 20 (10, 34) | 0.5 |
| Hospital stay, days | 48 (40, 64) | 64 (47, 76) | 0.012 | 44 (36, 58) | 58 (46, 74) | <0.001 |
| Red blood cell transfusion | 76 (40%) | 20 (74%) | 0.002 (0.23) | 35 (29%) | 61 (64%) | <0.001 (0.35) |
| Biochemical MBD screening | ||||||
| Age at biochemical screening, days | 16 (15, 25) | 16 (15, 17) | 0.5 | 16 (15, 30) | 16 (15, 17) | 0.08 |
| Serum Ca, mg/dL | 9.90 (9.60, 10.28) | 9.60 (9.30, 9.95) | 0.002 | 10.00 (9.70, 10.3) | 9.70 (9.30, 10.03) | <0.001 |
| Serum Mg, mg/dL | 2.08 (1.94, 2.20) | 2.02 (1.90, 2.21) | 0.9 | 2.06 (1.95, 2.16) | 2.09 (1.91, 2.23) | 0.5 |
| PTH, pg/mL (n = 143) | 74 (47, 123) | 56 (34, 96) | 0.11 | 64 (47, 123) | 77 (40, 110) | 0.9 |
| 25-OH-D, ng/mL | 29 (21, 37) | 28 (22, 40) | 0.6 | 28 (20, 38) | 29 (24, 37) | 0.2 |
| Hyperparathyroidism | 53 (28%) | 6 (22%) | 0.7 | 30 (25%) | 29 (30%) | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Alvarez, A.; Urisarri, A.; Fuentes-Carballal, J.; Mandiá, N.; Sucasas-Alonso, A.; Couce, M.L. Metabolic Bone Disease of Prematurity: Risk Factors and Associated Short-Term Outcomes. Nutrients 2020, 12, 3786. https://doi.org/10.3390/nu12123786
Avila-Alvarez A, Urisarri A, Fuentes-Carballal J, Mandiá N, Sucasas-Alonso A, Couce ML. Metabolic Bone Disease of Prematurity: Risk Factors and Associated Short-Term Outcomes. Nutrients. 2020; 12(12):3786. https://doi.org/10.3390/nu12123786
Chicago/Turabian StyleAvila-Alvarez, Alejandro, Adela Urisarri, Jesús Fuentes-Carballal, Natalia Mandiá, Andrea Sucasas-Alonso, and María L. Couce. 2020. "Metabolic Bone Disease of Prematurity: Risk Factors and Associated Short-Term Outcomes" Nutrients 12, no. 12: 3786. https://doi.org/10.3390/nu12123786
APA StyleAvila-Alvarez, A., Urisarri, A., Fuentes-Carballal, J., Mandiá, N., Sucasas-Alonso, A., & Couce, M. L. (2020). Metabolic Bone Disease of Prematurity: Risk Factors and Associated Short-Term Outcomes. Nutrients, 12(12), 3786. https://doi.org/10.3390/nu12123786






