Effect of Korean Red Ginseng on Cholesterol Metabolites in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
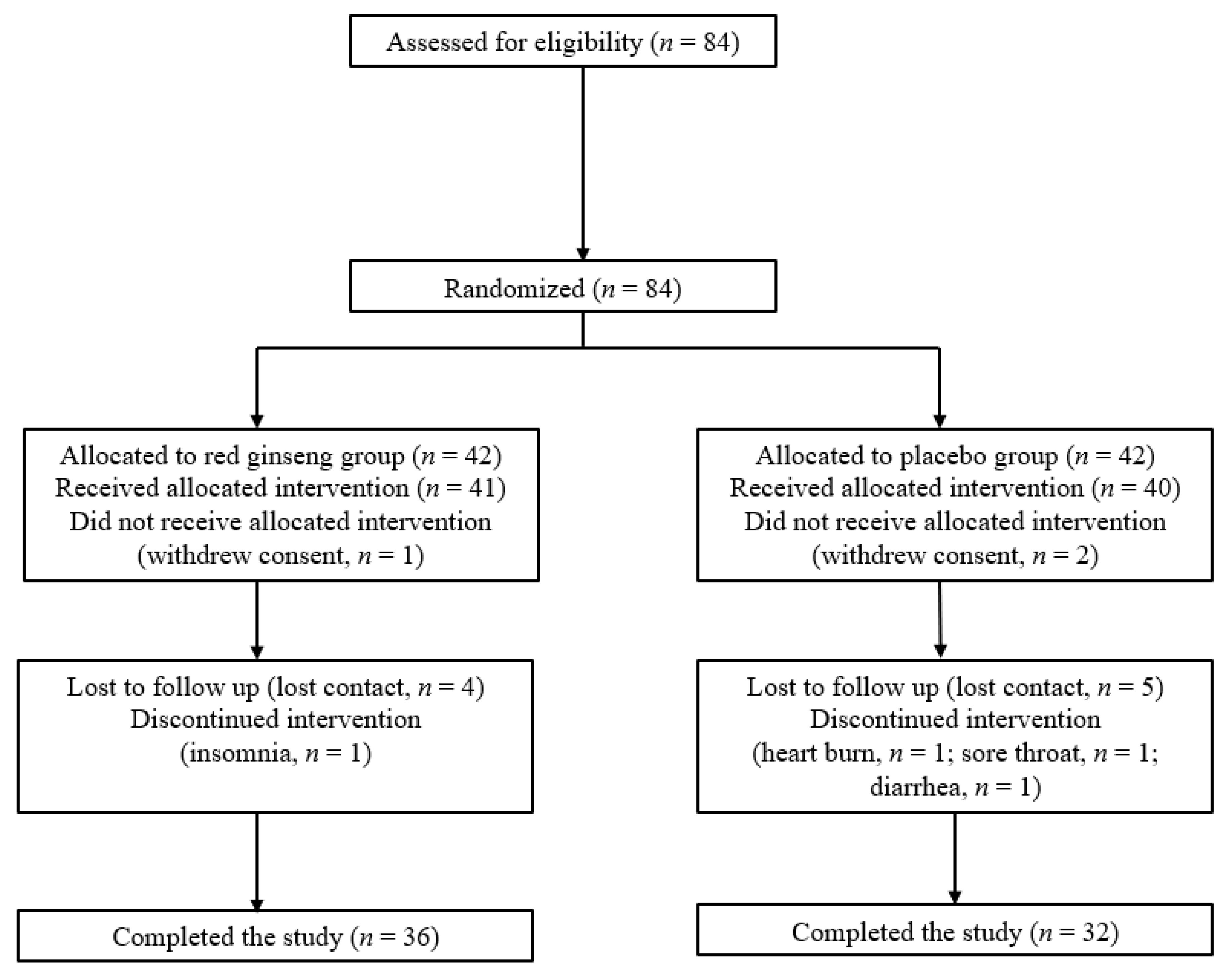
2.1. Study Design and Patients
2.2. Randomization and Masking
2.3. Procedures and Endpoints
2.4. Reagents
2.5. Lipid Extraction and LC-MS/MS Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Welty, F.K. Cardiovascular Disease and Dyslipidemia in Women. Arch. Intern. Med. 2001, 161, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Kreisberg, R.A.; Kasim, S. Cholesterol metabolism and aging. Am. J. Med. 1987, 82, 54–60. [Google Scholar] [CrossRef]
- Johnson, C.L.; Rifkind, B.M.; Sempos, C.T.; Carroll, M.D.; Bachorik, P.S.; Briefel, R.R.; Gordon, D.J.; Burt, V.L.; Brown, C.D.; Lippel, K.; et al. Declining serum total cholesterol levels among US adults. The National Health and Nutrition Examination Surveys. JAMA 1993, 269, 3002–3008. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Wilson, P.W. Risk factors that attenuate the female coronary disease advantage. Arch. Intern. Med. 1995, 155, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Exp. Med. Biol. 2017, 1043, 227–256. [Google Scholar] [CrossRef] [Green Version]
- Baeg, I.-H.; So, S.-H. The world ginseng market and the ginseng (Korea). J. Ginseng. Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, S.-H.; Lee, J.W.; Kim, Y.-S.; Hyun, S.H.; Han, C.-K. Red ginseng monograph. J. Ginseng. Res. 2018, 42, 549–561. [Google Scholar] [CrossRef]
- Yun, T.-K. Panax ginseng—A non-organ-specific cancer preventive? Lancet Oncol. 2001, 2, 49–55. [Google Scholar] [CrossRef]
- Hong, M.; Lee, Y.H.; Kim, S.; Suk, K.T.; Bang, C.S.; Yoon, J.H.; Baik, G.H.; Kim, D.J.; Kim, M.J. Anti-inflammatory and antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease. J. Ginseng. Res. 2016, 40, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Kim, J.-H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng. Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Seo, S.K.; Choi, Y.S.; Jeon, Y.E.; Lim, K.J.; Cho, S.; Lee, B.S. Effects of red ginseng supplementation on menopausal symptoms and cardiovascular risk factors in postmenopausal women: A double-blind randomized controlled trial. Menopause 2012, 19, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Ji, G.E. The effect of fermented red ginseng on depression is mediated by lipids. Nutr. Neurosci. 2013, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.Y.; Kang, H.J.; Kim, O.Y.; Lee, J.H. Beneficial effects of Korean red ginseng on lymphocyte DNA damage, antioxidant enzyme activity, and LDL oxidation in healthy participants: A randomized, double-blind, placebo-controlled trial. Nutr. J. 2012, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.; Kwak, J.H.; Ahn, H.Y.; Shin, D.Y.; Lee, J.H. Korean Red Ginseng Improves Glucose Control in Subjects with Impaired Fasting Glucose, Impaired Glucose Tolerance, or Newly Diagnosed Type 2 Diabetes Mellitus. J. Med. Food 2014, 17, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Dufourc, E.J. Sterols and membrane dynamics. J. Chem. Biol. 2008, 1, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Wollam, J.; Antebi, A. Sterol Regulation of Metabolism, Homeostasis, and Development. Annu. Rev. Biochem. 2011, 80, 885–916. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.S.; Choi, M.H. Cholesterol homeostasis in cardiovascular disease and recent advances in measuring cholesterol signatures. J. Steroid Biochem. Mol. Biol. 2015, 153, 72–79. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Rogers, M.A. Sterol Metabolism and Transport in Atherosclerosis and Cancer. Front. Endocrinol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organ. Tech. Rep. Ser. 1996, 866, 1–107. [Google Scholar]
- Peixoto, A.J. Acute Severe Hypertension. N. Engl. J. Med. 2019, 381, 1843–1852. [Google Scholar] [CrossRef]
- Johnson, W.; Nguyen, M.-L.; Patel, R. Hypertension Crisis in the Emergency Department. Cardiol. Clin. 2012, 30, 533–543. [Google Scholar] [CrossRef]
- Chen, S.; Hoene, M.; Li, J.; Li, Y.; Zhao, X.; Häring, H.-U.; Schleicher, E.D.; Weigert, C.; Xu, G.; Lehmann, R. Simultaneous extraction of metabolome and lipidome with methyl tert-butyl ether from a single small tissue sample for ultra-high performance liquid chromatography/mass spectrometry. J. Chromatogr. A 2013, 1298, 9–16. [Google Scholar] [CrossRef]
- Buré, C.; Ayciriex, S.; Testet, E.; Schmitter, J.-M. A single run LC-MS/MS method for phospholipidomics. Anal. Bioanal. Chem. 2012, 405, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Mok, H.J.; Lee, D.Y.; Park, S.C.; Kim, G.-S.; Lee, S.-E.; Lee, Y.-S.; Kim, K.P.; Kim, H.D. UPLC-QqQ/MS-Based Lipidomics Approach To Characterize Lipid Alterations in Inflammatory Macrophages. J. Proteome Res. 2017, 16, 1460–1469. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Abdel-Khalik, J.; Crick, P.J.; Yutuc, E.; Wang, Y. New methods for analysis of oxysterols and related compounds by LC–MS. J. Steroid Biochem. Mol. Biol. 2016, 162, 4–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBose-Boyd, R.A. Feedback regulation of cholesterol synthesis: Sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008, 18, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Wu, C.; Dou, D.; Chen, Y.; Ogihara, Y. Lipoprotein lipase activation by red Ginseng saponins in hyperlipidemia model animals. Phytomedicine 1999, 6, 257–265. [Google Scholar] [CrossRef]
- Saba, E.; Jeon, B.R.; Jeong, D.-H.; Lee, K.; Goo, Y.-K.; Kim, S.-H.; Sung, C.-K.; Roh, S.-S.; Kim, S.D.; Kim, H.-K.; et al. Black ginseng extract ameliorates hypercholesterolemia in rats. J. Ginseng Res. 2016, 40, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Lee, N.; Ahn, Y.; Lee, H. Study on improving blood flow with Korean red ginseng substances using digital infrared thermal imaging and Doppler sonography: Randomized, double blind, placebo-controlled clinical trial with parallel design. J. Tradit. Chin. Med. 2013, 33, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Jin, Y.; Lim, W.; Ji, S.; Choi, S.; Jang, S.; Lee, S. A ginsenoside-Rh1, a component of ginseng saponin, activates estrogen receptor in human breast carcinoma MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2003, 84, 463–468. [Google Scholar] [CrossRef]
- Cho, J.; Park, W.; Lee, S.; Ahn, W.; Lee, Y. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor-alpha and -beta, independent of ligand binding. J. Clin. Endocrinol. Metab. 2004, 89, 3510–3515. [Google Scholar] [CrossRef]
- Guetta, V.; Cannon, R.O. Cardiovascular Effects of Estrogen and Lipid-Lowering Therapies in Postmenopausal Women. Circulation 1996, 93, 1928–1937. [Google Scholar] [CrossRef]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.-C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.-S.; Kim, C.-T.; Kim, I.-H.; Kim, Y. Ginsenoside Rg3 Reduces Lipid Accumulation with AMP-Activated Protein Kinase (AMPK) Activation in HepG2 Cells. Int. J. Mol. Sci. 2012, 13, 5729–5739. [Google Scholar] [CrossRef] [Green Version]
- Guillemot-Legris, O.; Mutemberezi, V.; Muccioli, G.G. Oxysterols in Metabolic Syndrome: From Bystander Molecules to Bioactive Lipids. Trends Mol. Med. 2016, 22, 594–614. [Google Scholar] [CrossRef]
- Olkkonen, V.M.; Béaslas, O.; Nissilä, E. Oxysterols and Their Cellular Effectors. Biomolecules 2012, 2, 76–103. [Google Scholar] [CrossRef] [Green Version]
- Gargiulo, S.; Gamba, P.; Testa, G.; Leonarduzzi, G.M.; Poli, G. The role of oxysterols in vascular ageing. J. Physiol. 2016, 594, 2095–2113. [Google Scholar] [CrossRef] [Green Version]
- Björkhem, I.; Diczfalusy, U. Oxysterols: Friends, foes, or just fellow passengers? Arterioscler. Thromb. Vasc. Biol. 2002, 22, 734–742. [Google Scholar] [CrossRef]
- Song, Y.-B.; An, Y.R.; Kim, S.J.; Park, H.-W.; Jung, J.-W.; Kyung, J.-S.; Hwang, S.Y.; Kim, Y.-S. Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J. Sci. Food Agric. 2011, 92, 388–396. [Google Scholar] [CrossRef]
- Kim, D.Y.; Yuan, H.D.; Chung, I.K.; Chung, S.H. Compound K, Intestinal Metabolite of Ginsenoside, Attenuates Hepatic Lipid Accumulation via AMPK Activation in Human Hepatoma Cells. J. Agric. Food Chem. 2009, 57, 1532–1537. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Yoon, H.; Park, H.-M.; Song, B.C.; Yeum, K.-J. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J. Ginseng. Res. 2017, 41, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.-H.; Lee, Y.-J.; Kim, C.-B.; Kim, J.-Y.; Shin, S.-H.; Park, J.-K. Effects of ginseng on peripheral blood mitochondrial DNA copy number and hormones in men with metabolic syndrome: A randomized clinical and pilot study. Complement. Ther. Med. 2016, 24, 40–46. [Google Scholar] [CrossRef] [PubMed]
| Ginseng | Placebo | p-Value | |
|---|---|---|---|
| n | 36 | 32 | |
| Age, years | 55.9 ± 5.9 | 58.1 ± 4.7 | 0.093 |
| Physical measurement | |||
| Body mass index, kg/m2 | 24.3 ± 3.2 | 24.5 ± 3.7 | 0.741 |
| Waist circumference, cm | 82.5 ± 8.7 | 82.6 ± 10.2 | 0.950 |
| SBP (mmHg) | 119.8 ± 13.5 | 116.8 ± 16.5 | 0.409 |
| DBP (mmHg) | 76.7 ± 9.7 | 72.2 ± 9.3 | 0.065 |
| Heart rate (bpm) | 76.8 ± 9.6 | 75.4 ± 11.4 | 0.585 |
| WBC(×103 L) | 5.7 ± 1.4 | 5.8 ± 1.6 | 0.786 |
| AST (IU/L) | 24.5 ± 7.0 | 25.2 ± 6.0 | 0.654 |
| ALT (IU/L) | 21.7 ± 12.6 | 20.5 ± 7.8 | 0.629 |
| Comorbid condition, n (%) | |||
| Hypertension | 5 (13.9) | 5 (15.6) | 0.572 |
| Diabetes | 2 (5.6) | 1 (3.1) | 0.535 |
| Physical activity, n (%) | 15 (41.7) | 10 (31.3) | 0.374 |
| Smoking, n (%) | 2 (5.6) | 1 (3.1) | 0.534 |
| Alcohol consumption, n (%) | 9 (25.0) | 10(13.2) | 0.567 |
| Sterols | |||
| Cholesterol (nmol/mL) | 1634.5 ± 409.3 | 1510.6 ± 339.2 | 0.182 |
| Plant sterols (nmol/mL) | |||
| Sitosterol | 8.2 ± 8.0 | 6.0 ± 3.2 | 0.159 |
| Campesterol | 11.3 ± 12.6 | 8.5 ± 4.8 | 0.245 |
| Cholesterol precursor (nmol/mL) | |||
| Lanosterol | 2.9 ± 1.2 | 3.0 ± 1.2 | 0.626 |
| Desmosterol | 9.6 ± 2.4 | 10.7 ± 13.8 | 0.622 |
| 7-Dehydrocholesterol | 6.2 ± 1.7 | 9.4 ± 22.2 | 0.392 |
| Oxysterols (nmol/mL) | |||
| 7-Hydroxycholesterol | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.123 |
| Cholesterol esters (nmol/mL) | |||
| CE 14:0 | 14.6 ± 15.0 | 16.9 ± 19.0 | 0.572 |
| CE 20:4 | 111.9 ± 124.8 | 99.1 ± 112.3 | 0.659 |
| Ginseng | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolic Parameters | Baseline | After 4 Weeks | p † | Change | Baseline | After 4 Weeks | p † | Change | p ‡ |
| BMI (kg/m2) | 24.3 ± 3.2 | 24.4 ± 3.0 | 0.315 | 0.1 ± 0.8 | 24.5 ± 3.7 | 24.5 ± 4.0 | 0.986 | −0.00 ± 0.8 | 0.473 |
| WC (cm) | 82.5 ± 8.7 | 83.3 ± 8.8 | 0.145 | 0.9 ± 3.4 | 82.6 ± 10.2 | 83.1 ± 10.2 | 0.494 | 0.5 ± 3.7 | 0.648 |
| SBP (mmHg) | 119.8 ± 13.5 | 116.6 ± 15.1 | 0.056 | −3.2 ± 9.7 | 116.8 ± 16.5 | 116.8 ± 17.5 | 0.989 | 0.03 ± 12.6 | 0.239 |
| DBP (mmHg) | 76.7 ± 9.7 | 73.1 ± 8.8 | 0.017 | −3.6 ± 8.7 | 72.4 ± 9.3 | 71.6 ± 11.4 | 0.661 | −0.8 ± 10.0 | 0.212 |
| HR (bpm) | 76.8 ± 9.6 | 76.1 ± 11.2 | 0.555 | −0.3 ± 1.2 | 75.4 ± 11.4 | 74.7 ± 12.5 | 0.771 | −0.7 ± 7.0 | 0.988 |
| WBC L) | 5.7 ± 1.4 | 5.5 ± 1.4 | 0.828 | −0.05 ± 1.3 | 5.8 ± 1.6 | 5.5 ± 1.2 | 0.239 | −0.3 ± 1.2 | 0.487 |
| AST (IU/L) | 24.5 ± 7.0 | 25.9 ± 18.2 | 0.659 | 1.4 ± 19.5 | 25.2 ± 6.0 | 25.9 ± 7.9 | 0.602 | 0.7 ± 7.7 | 0.844 |
| ALT(IU/L) | 21.7 ± 12.6 | 21.8 ± 21.8 | 0.989 | 0.1 ± 23.9 | 20.5 ± 7.8 | 19.8 ± 10.5 | 0.720 | −0.7 ± 10.7 | 0.872 |
| Ginseng | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sterols (nmol/mL) | Baseline | After 4 Weeks | p † | Change | Baseline | After 4 Weeks | p † | Change | p ‡ | p |
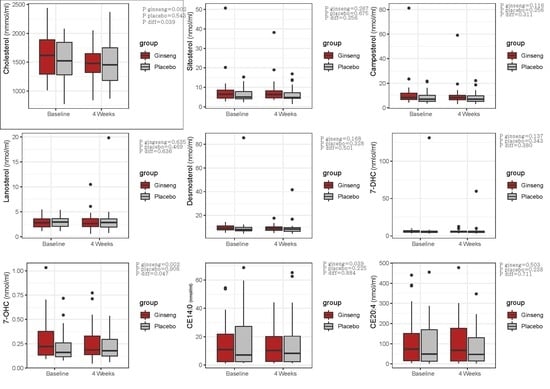
| Cholesterol | 1634.4 ± 409.4 | 1486.1 ± 312.8 | 0.002 | −148.3 ± 261.1 | 1510.6 ± 339.2 | 1486.6 ± 338.0 | 0.543 | −23.0 ± 220.5 | 0.039 | 0.047 |
| Sitosterol | 8.2 ± 8.0 | 7.7 ± 6.1 | 0.267 | −0.5 ± 0.09 | 6.0 ± 3.2 | 6.1 ± 3.6 | 0.675 | 0.1 ± 1.5 | 0.256 | 0.804 |
| Campesterol | 11.3 ± 12.6 | 10.1 ± 9.0 | 0.116 | −1.1 ± 4.2 | 8.5 ± 4.8 | 8.2 ± 4.6 | 0.256 | −0.3 ± 1.6 | 0.311 | 0.937 |
| Lanosterol | 2.9 ± 1.2 | 3.0 ± 1.7 | 0.635 | 0.1 ± 1.6 | 3.0 ± 1.2 | 3.4 ± 3.2 | 0.469 | 0.4 ± 0.3 | 0.636 | 0.568 |
| Desmosterol | 9.6 ± 2.4 | 9.1 ± 2.5 | 0.168 | −0.5 ± 2.0 | 10.7 ± 13.8 | 9.3 ± 6.3 | 0.328 | −1.4 ± 8.0 | 0.501 | 0.551 |
| 7-DHC | 6.2 ± 1.7 | 5.9 ± 1.8 | 0.137 | −0.3 ± 1.1 | 9.4 ± 22.2 | 7.2 ± 9.7 | 0.343 | −2.1 ± 12.7 | 0.380 | 0.829 |
| 7-OHC | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.002 | −0.05 ± 0.09 | 0.2 ± 0.6 | 0.2 ± 1.4 | 0.908 | −0.002 ± 0.1 | 0.047 | 0.063 |
| CE14:0 | 14.6 ± 14.9 | 13.1 ± 12.8 | 0.393 | −1.4 ± 9.9 | 16.9 ± 19.0 | 15.2 ± 17.4 | 0.225 | −1.8 ± 8.0 | 0.884 | 0.896 |
| CE20:4 | 111.9 ± 124.8 | 102.8 ± 107.6 | 0.503 | −9.1 ± 80.9 | 99.1 ± 112.3 | 82.9 ± 88.1 | 0.238 | −16.2 ± 76.2 | 0.711 | 0.459 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-J.; Jang, S.-N.; Liu, K.-H.; Jung, D.-H. Effect of Korean Red Ginseng on Cholesterol Metabolites in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial. Nutrients 2020, 12, 3423. https://doi.org/10.3390/nu12113423
Kwon Y-J, Jang S-N, Liu K-H, Jung D-H. Effect of Korean Red Ginseng on Cholesterol Metabolites in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial. Nutrients. 2020; 12(11):3423. https://doi.org/10.3390/nu12113423
Chicago/Turabian StyleKwon, Yu-Jin, Su-Nyeong Jang, Kwang-Hyeon Liu, and Dong-Hyuk Jung. 2020. "Effect of Korean Red Ginseng on Cholesterol Metabolites in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial" Nutrients 12, no. 11: 3423. https://doi.org/10.3390/nu12113423