In Utero HIV Exposure and the Early Nutritional Environment Influence Infant Neurodevelopment: Findings from an Evidenced Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Information Sources and Search Terms
2.3. Article Screening and Data Collection
2.3.1. Level One: Screening for Growth, Neurodevelopment, and Immune Outcomes
2.3.2. Level Two: Screening for Neurodevelopment
2.3.3. Level Three: Screening and Data Collection for the Neurodevelopmental Theme
2.4. Screening for Early Life Nutritional Factors Within the Neurodevelopmental Theme
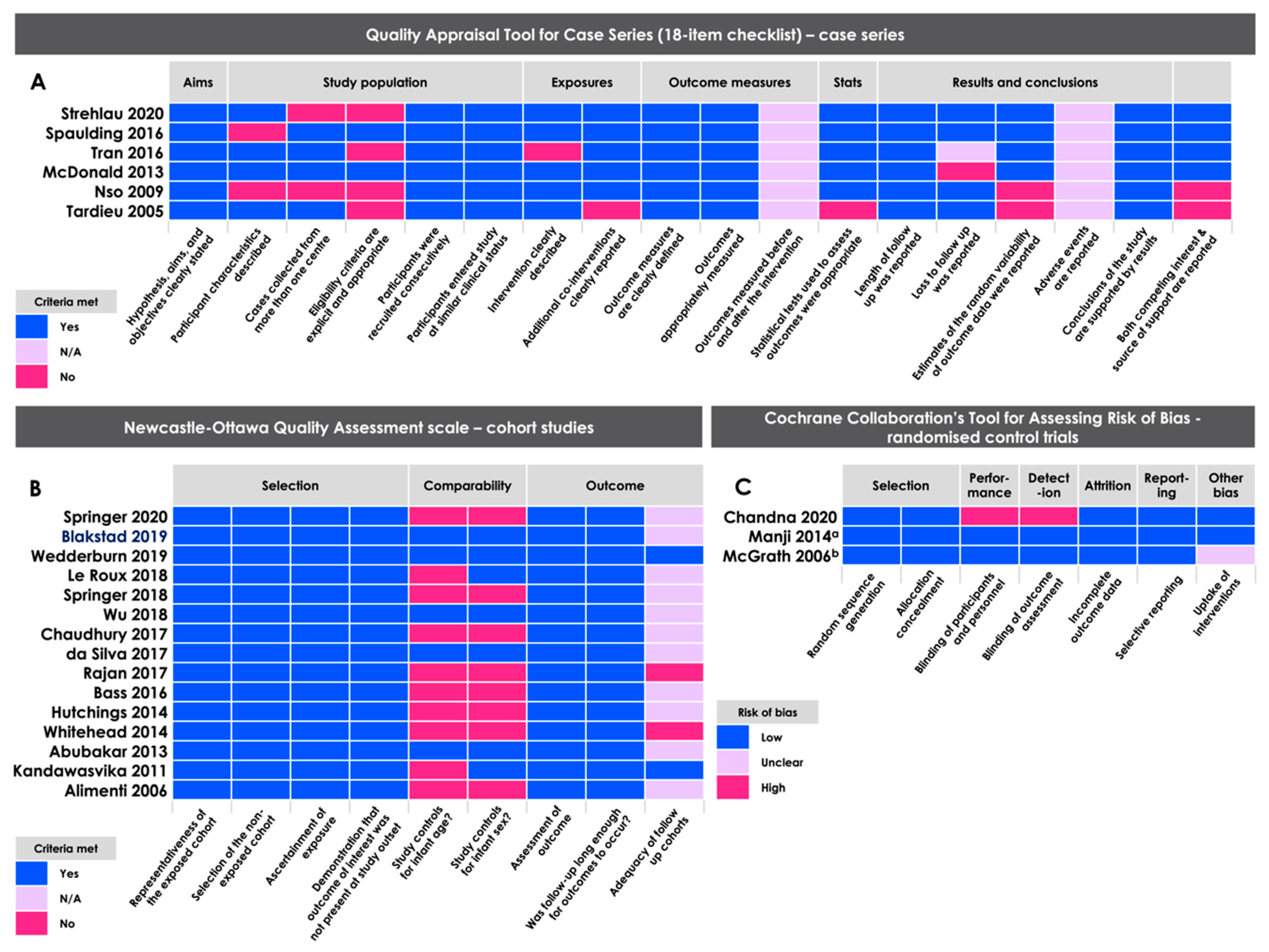
2.5. Methodological Quality Assessment
2.6. Data Analysis
3. Results
3.1. Study Location, Demographics, and Design
3.2. Study Measurement Tools
3.3. Methodological Quality Assessments
3.4. Infant HIV Exposure Status Associates with Neurodevelopmental Outcomes in the First 36 Months of Life
3.4.1. Cognitive Outcomes
3.4.2. Motor Outcomes
3.4.3. Language Outcomes
3.4.4. Behavioural Outcomes
3.4.5. Neurostructural Outcomes
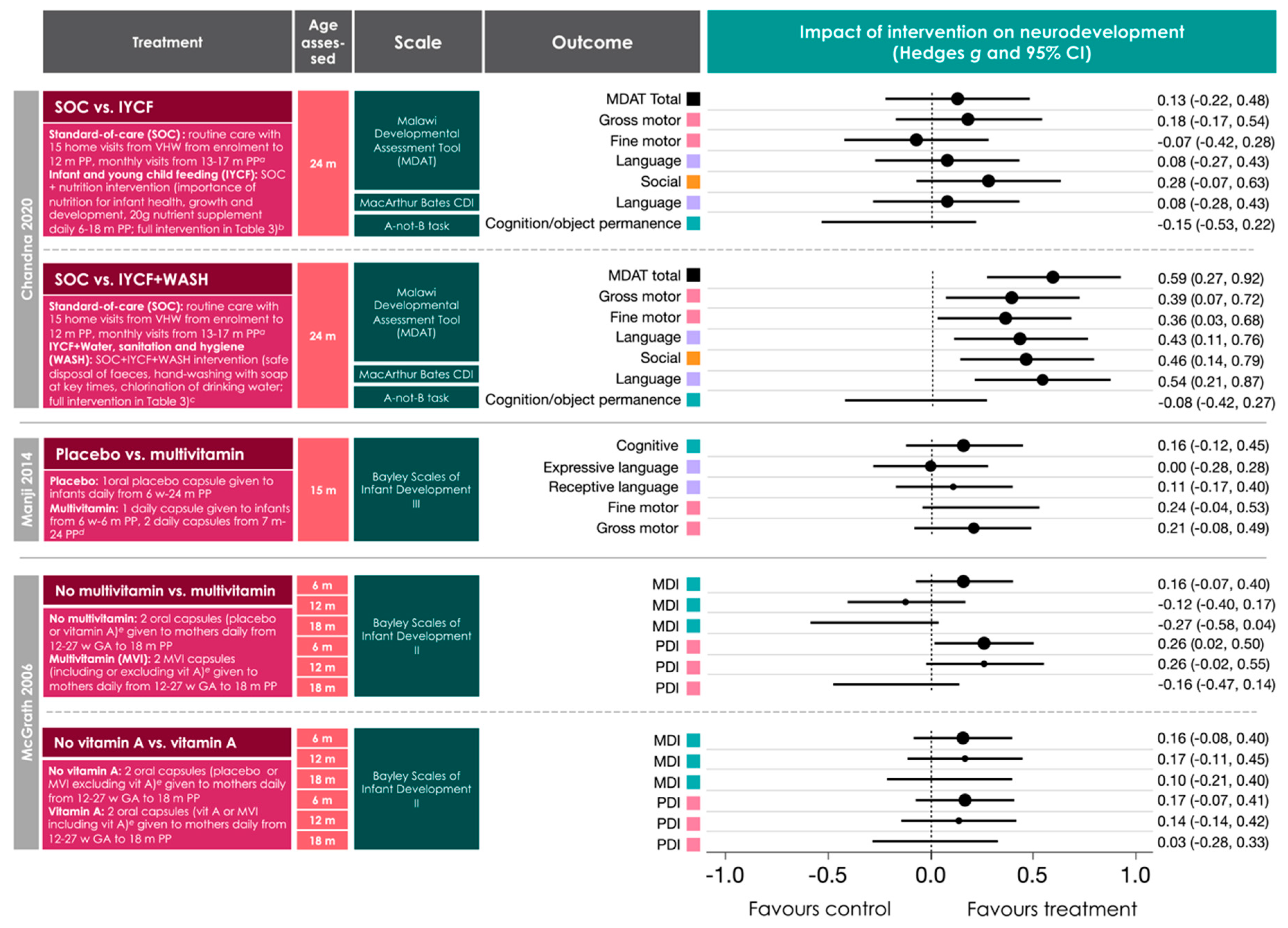
3.5. Relationships between Early Life Nutritional Factors and Neurodevelopmental Outcomes in Infants Perinatally Exposed to Maternal HIV Infection and ART
3.6. Sex Differences and Neurodevelopmental Outcomes in Infants Perinatally Exposed to Maternal HIV Infection and ART
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- UNAIDS; AIDSinfo. Elimination of Mother-to-Child Transmission: Pregnant Women Who Received ARV for PMTCT. Available online: https://aidsinfo.unaids.org/ (accessed on 6 August 2020).
- UNAIDS; AIDSinfo. Elimination of Mother-to-Child Transmission: HIV-Exposed Children Who Are Uninfected. Available online: https://aidsinfo.unaids.org/ (accessed on 6 August 2020).
- Guaraldi, G.; Stentarelli, C.; Da Silva, A.D.; Luzi, K.; Neri, I.; Cellini, M.; Petrella, E.; Garlassi, E.; Menozzi, M.; Facchinetti, F.; et al. Metabolic alterations in HIV-infected pregnant women: Moving to metabolic tailoring of antiretroviral drugs. AIDS Rev. 2014, 16, 14–22. [Google Scholar] [PubMed]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.D.; Graham, A.M.; Feczko, E.; Miranda-Dominguez, O.; Rasmussen, J.M.; Nardos, R.; Entringer, S.; Wadhwa, P.D.; Buss, C.; Fair, D.A. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 2018, 21, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Updates on HIV and Infant Feeding; WHO: Geneva, Swizerland, 2016. [Google Scholar]
- Asbjornsdottir, K.H.; Slyker, J.A.; Maleche-Obimbo, E.; Wamalwa, D.; Otieno, P.; Gichuhi, C.M.; John-Stewart, G. Breastfeeding Is Associated with Decreased Risk of Hospitalization among HIV-Exposed, Uninfected Kenyan Infants. J. Hum. Lact. 2015. [Google Scholar] [CrossRef] [Green Version]
- Anema, A.; Vogenthaler, N.; Frongillo, E.A.; Kadiyala, S.; Weiser, S.D. Food insecurity and HIV/AIDS: Current knowledge, gaps, and research priorities. Curr. Hiv Aids Rep. 2009, 6, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekar, V.; Kestens, D.; Spinosa-Guzman, S.; De Pauw, M.; De Paepe, E.; Vangeneugden, T.; Lefebvre, E.; Hoetelmans, R.M.W. The Effect of Different Meal Types on the Pharmacokinetics of Darunavir (TMC114)/Ritonavir in HIV-Negative Healthy Volunteers. J. Clin. Pharmacol. 2007, 47, 479–484. [Google Scholar] [CrossRef]
- WHO. Consultative Meeting on Nutrition Interventions for Improving the Prevention, Care and Management of HIV/AIDS; WHO: Geneva, Swizerland, 2003. [Google Scholar]
- Sherr, L.; Mueller, J.; Varrall, R. A systematic review of cognitive development and child human immunodeficiency virus infection. Psychol. Health Med. 2009, 14, 387–404. [Google Scholar] [CrossRef]
- Sherr, L.; Croome, N.; Parra Castaneda, K.; Bradshaw, K. A systematic review of psychological functioning of children exposed to HIV: Using evidence to plan for tomorrow‘s HIV needs. Aids Behav. 2014, 18, 2059–2074. [Google Scholar] [CrossRef]
- McHenry, M.S.; McAteer, C.I.; Oyungu, E.; McDonald, B.C.; Bosma, C.B.; Mpofu, P.B.; Deathe, A.R.; Vreeman, R.C. Neurodevelopment in Young Children Born to HIV-Infected Mothers: A Meta-analysis. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [Green Version]
- McHenry, M.S.; Dixit, A.; Vreeman, R.C. A Systematic Review of Nutritional Supplementation in HIV-Infected Children in Resource-Limited Settings. J. Int. Assoc. Provid. Aids Care 2015, 14, 313–323. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E.; Lancet Nutrition Interventions Review Group, Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Bale, T.L. The placenta and neurodevelopment: Sex differences in prenatal vulnerability. Dialogues Clin. Neurosci. 2016, 18, 459–464. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- WHO. Infant and Young Child Feeding. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 15 April 2020).
- Langa, L. Breast Is Always Best, Even for HIV-Positive Mothers; World Health Organization: Geneva, Swizerland, 2010; pp. 9–10. [Google Scholar]
- Fox, S.E.; Levitt, P.; Nelson, C.A. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010, 81, 28–40. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Health Sector Response to HIV, 2000–2015: Focus on Innovations in Africa: Progress Report; WHO: Geneva, Swizerland, 2015. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Health Research Institute: Ottawa, ON, Canada, 2009. [Google Scholar]
- Moga, C.; Guo, B.; Schopflocher, D.; Harstall, C. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique; Institute of Health Economics: Edmonton, AB, Canada, 2012. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef]
- Rosenkrantz, T.S.; Hussain, Z.; Fitch, R.H. Sex Differences in Brain Injury and Repair in Newborn Infants: Clinical Evidence and Biological Mechanisms. Front. Pediatr. 2019, 7, 211. [Google Scholar] [CrossRef]
- Hartling, L.; Hamm, M.; Milne, A.; Vandermeer, B.; Santaguida, P.L.; Ansari, M.; Tsertsvadze, A.; Hempel, S.; Shekelle, P.; Dryden, D.M. Validity and Inter-Rater Reliability Testing of Quality Assessment Instruments; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012.
- Bayley, N. Bayley Scales of Infant and Toddler Development, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 2006. [Google Scholar]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.; Turner, R. Power analysis for random-effects meta-analysis. Res. Synth. Methods 2017, 8, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hippel, P.T. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [Green Version]
- Spaulding, A.B.; Yu, Q.; Civitello, L.; Mussi-Pinhata, M.M.; Pinto, J.; Gomes, I.M.; Alarcón, J.O.; Siberry, G.K.; Harris, D.R.; Hazra, R. Neurologic Outcomes in HIV-Exposed/Uninfected Infants Exposed to Antiretroviral Drugs During Pregnancy in Latin America and the Caribbean. Aids Res. Hum. Retrovir. 2016, 32, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievert, K.; Hussain, S.M.; Page, M.J.; Wang, Y.; Hughes, H.J.; Malek, M.; Cicuttini, F.M. Effect of breakfast on weight and energy intake: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 364, l42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, N.; Potterton, J.; Coovadia, A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. Aids Care 2014, 26, 497–504. [Google Scholar] [CrossRef]
- McDonald, C.M.; Manji, K.P.; Kupka, R.; Bellinger, D.C.; Spiegelman, D.; Kisenge, R.; Msamanga, G.; Fawzi, W.W.; Duggan, C.P. Stunting and wasting are associated with poorer psychomotor and mental development in HIV-exposed Tanzanian infants. J. Nutr. 2013, 143, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandawasvika, G.Q.; Ogundipe, E.; Gumbo, F.Z.; Kurewa, E.N.; Mapingure, M.P.; Stray-Pedersen, B. Neurodevelopmental impairment among infants born to mothers infected with human immunodeficiency virus and uninfected mothers from three peri-urban primary care clinics in Harare, Zimbabwe. Dev. Med. Child. Neurol. 2011, 53, 1046–1052. [Google Scholar] [CrossRef] [Green Version]
- Nso Roca, A.P.; García-Bermejo, C.G.-B.; Larru, B.R.M.; Muñoz Fernández, M.A.; de José, M.I. Pathology in children of HIV women. Indian J. Pediatr. 2009, 76, 1125–1130. [Google Scholar] [CrossRef]
- Rajan, R.; Seth, A.; Mukherjee, S.B.; Chandra, J. Development assessment of HIV exposed children aged 6-18 months: A cohort study from North India. Aids Care 2017, 29, 1404–1409. [Google Scholar] [CrossRef]
- Da Silva, K.M.; de Sá, C.d.S.C.; Carvalho, R. Evaluation of motor and cognitive development among infants exposed to HIV. Early Hum. Dev. 2017, 105, 7–10. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Li, Y.; Loo, K.K.; Yang, H.; Wang, Q.; Duan, R.; Xiao, X.; Song, X.; Yang, S.; et al. Neurodevelopmental outcomes in young children born to HIV-positive mothers in rural Yunnan, China. Pediatr. Int. Off. J. Jpn. Pediatric Soc. 2018, 60, 618–625. [Google Scholar] [CrossRef]
- Bass, J.K.; Nakasujja, N.; Familiar-Lopez, I.; Sikorskii, A.; Murray, S.M.; Opoka, R.; Augustinavicius, J.; Boivin, M.J. Association of caregiver quality of care with neurocognitive outcomes in HIV-affected children aged 2–5 years in Uganda. Aids Care 2016, 28 (Suppl. 1), 76–83. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.T.; Roos, A.; Fouche, J.-P.; Koen, N.; Woods, R.P.; Zar, H.J.; Narr, K.L.; Stein, D.J.; Donald, K.A. White Matter Microstructural Integrity and Neurobehavioral Outcome of HIV-Exposed Uninfected Neonates. Medicine 2016, 95, e2577. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, J.; Potterton, J. Developmental delay in HIV-exposed infants in Harare, Zimbabwe. Vulnerable Child. Youth Stud. 2014, 9, 43–55. [Google Scholar] [CrossRef]
- Abubakar, A.; Holding, P.; Van Baar, A.; Newton, C.R.J.C.; Van de Vijver, F.J.R.; Espy, K.A. The performance of children prenatally exposed to HIV on the A-not-B task in Kilifi, Kenya: A preliminary study. Int. J. Environ. Res. Public Health 2013, 10, 4132–4142. [Google Scholar] [CrossRef] [Green Version]
- Alimenti, A.; Forbes, J.C.; Oberlander, T.F.; Money, D.M.; Grunau, R.E.; Papsdorf, M.P.; Maan, E.; Cole, L.J.; Burdge, D.R. A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics 2006, 118, e1139–e1145. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, M.; Brunelle, F.; Raybaud, C.; Ball, W.; Barret, B.; Pautard, B.; Lachassine, E.; Mayaux, M.-J.; Blanche, S. Cerebral MR imaging in uninfected children born to HIV-seropositive mothers and perinatally exposed to zidovudine. Ajnr. Am. J. Neuroradiol. 2005, 26, 695–701. [Google Scholar]
- Springer, P.E.; Slogrove, A.L.; Laughton, B.; Bettinger, J.A.; Saunders, H.H.; Molteno, C.D.; Kruger, M. Neurodevelopmental outcome of HIV-exposed but uninfected infants in the Mother and Infants Health Study, Cape Town, South Africa. Trop. Med. Int. Health 2018, 23, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Wedderburn, C.J.; Yeung, S.; Rehman, A.M.; Stadler, J.A.M.; Nhapi, R.T.; Barnett, W.; Myer, L.; Gibb, D.M.; Zar, H.J.; Stein, D.J.; et al. Neurodevelopment of HIV-exposed uninfected children in South Africa: Outcomes from an observational birth cohort study. Lancet Child Adolesc. Health 2019, 3, 803–813. [Google Scholar] [CrossRef] [Green Version]
- McGrath, N.; Bellinger, D.; Robins, J.; Msamanga, G.I.; Tronick, E.; Fawzi, W.W. Effect of maternal multivitamin supplementation on the mental and psychomotor development of children who are born to HIV-1-infected mothers in Tanzania. Pediatrics 2006, 117, e216–e225. [Google Scholar] [CrossRef] [Green Version]
- Chandna, J.; Ntozini, R.; Evans, C.; Kandawasvika, G.; Chasekwa, B.; Majo, F.; Mutasa, K.; Tavengwa, N.; Mutasa, B.; Mbuya, M.; et al. Effects of improved complementary feeding and improved water, sanitation and hygiene on early child development among HIV-exposed children: Substudy of a cluster randomised trial in rural Zimbabwe. BMJ Glob. Health 2020, 5, e001718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springer, P.E.; Slogrove, A.L.; Kidd, M.; Kalk, E.; Bettinger, J.A.; Esser, M.M.; Cotton, M.F.; Zunza, M.; Molteno, C.D.; Kruger, M. Neurodevelopmental and behavioural outcomes of HIV-exposed uninfected and HIV-unexposed children at 2-3 years of age in Cape Town, South Africa. Aids Care 2020, 32, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Strehlau, R.; van Aswegen, T.; Burke, M.; Kuhn, L.; Potterton, J. A description of early neurodevelopment in a cohort of HIV-exposed uninfected children. Aids Care 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blakstad, M.M.; Smith, E.R.; Etheredge, A.; Locks, L.M.; McDonald, C.M.; Kupka, R.; Kisenge, R.; Aboud, S.; Bellinger, D.; Sudfeld, C.R.; et al. Nutritional, Socioeconomic, and Delivery Characteristics Are Associated with Neurodevelopment in Tanzanian Children. J. Pediatr. 2019, 207, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, S.M.; Donald, K.A.; Brittain, K.; Phillips, T.K.; Zerbe, A.; Nguyen, K.K.; Strandvik, A.; Kroon, M.; Abrams, E.J.; Myer, L. Neurodevelopment of breastfed HIV-exposed uninfected and HIV-unexposed children in South Africa. AIDS 2018, 32, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Williams, P.L.; Mayondi, G.K.; Leidner, J.; Holding, P.; Tepper, V.; Nichols, S.; Magetse, J.; Sakoi, M.; Moabi, K.; et al. Neurodevelopment of HIV-Exposed and HIV-Unexposed Uninfected Children at 24 Months. Pediatrics 2017, 140, e20170988. [Google Scholar] [CrossRef] [Green Version]
- Manji, K.P.; McDonald, C.M.; Kupka, R.; Bosch, R.J.; Kisenge, R.; Aboud, S.; Bellinger, D.C.; Fawzi, W.W.; Duggan, C.P. Effect of multivitamin supplementation on the neurodevelopment of HIV-exposed Tanzanian infants: A randomized, double-blind, placebo-controlled clinical trial. J. Trop. Pediatr. 2014, 60, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Bayley, N. Manual for the Bayley Scales of Infant Development; The Psychological Corporation: San Antonio, TX, USA, 1969. [Google Scholar]
- Bayley, N. Bayley Scales of Infant Development, 2nd ed.; Manual; The Psychological Corporation: San Antonio, TX, USA, 1993. [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-For-Age, Weight-For-Age, Weight-for-Length, Weight-For-Height and Body Mass Index-For-Age: Methods and Development; WHO: Geneva, Switzerland, 2006; p. 312. [Google Scholar]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [Green Version]
- Gordon-Lipkin, E.; Gentner, M.B.; German, R.; Leppert, M.L. Neurodevelopmental Outcomes in 22 Children With Microcephaly of Different Etiologies. J. Child Neurol. 2017, 32, 804–809. [Google Scholar] [CrossRef]
- Sherr, L.; Croome, N.; Bradshaw, K.; Parra Castaneda, K. A systematic review examining whether interventions are effective in reducing cognitive delay in children infected and affected with HIV. Aids Care 2014, 26 (Suppl. 1), S70–S77. [Google Scholar] [CrossRef]
- UNAIDS. Global AIDS Monitoring 2018; UNAIDS: Geneva, Swizerland, 2018. [Google Scholar]
- South African National Aids Council. Let Our Actions Count: National Strategic Plan. on HIV, TB and STIs (2017–2022); South African National Aids Council: Pretoria, South African, 2018. [Google Scholar]
- UNAIDS. Country Factsheets South Africa 2018. Available online: https://www.unaids.org/en/regionscountries/countries/southafrica (accessed on 19 February 2020).
- Mirza, M.A.; Ritzel, R.; Xu, Y.; McCullough, L.D.; Liu, F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J. Neuroinflamm. 2015, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Ardalan, M.; Chumak, T.; Vexler, Z.; Mallard, C. Sex-Dependent Effects of Perinatal Inflammation on the Brain: Implication for Neuro-Psychiatric Disorders. Int. J. Mol. Sci. 2019, 20, 2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrer, H.F.; Cambiasso, M.J. Sexual differentiation of the brain: Genes, estrogen, and neurotrophic factors. Cell. Mol. Neurobiol. 2002, 22, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Roth, E.; Lo, Y.J.; Hurst, C.; Vollner, J.; Kendell, A. In poor families, mothers’ milk is richer for daughters than sons: A test of Trivers-Willard hypothesis in agropastoral settlements in Northern Kenya. Am. J. Phys. Anthropol. 2012, 149, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.H.; Song, J.H.; Song, S.; Kang, N.M. Do gender and birth height of infant affect calorie of human milk? An association study between human milk macronutrient and various birth factors. J. Matern. Fetal. Neonatal. Med. 2017, 30, 1608–1612. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Wang, Y.; Guyer, B. Breastfeeding in rural China: Association between knowledge, attitudes, and practices. J. Hum. Lact. 2008, 24, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Wanjohi, M.; Griffiths, P.; Wekesah, F.; Muriuki, P.; Muhia, N.; Musoke, R.N.; Fouts, H.N.; Madise, N.J.; Kimani-Murage, E.W. Sociocultural factors influencing breastfeeding practices in two slums in Nairobi, Kenya. Int. Breastfeed. J. 2016, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darvesh, N.; Das, J.K.; Vaivada, T.; Gaffey, M.F.; Rasanathan, K.; Bhutta, Z.A.; Team, S.D.o.H.S. Water, sanitation and hygiene interventions for acute childhood diarrhea: A systematic review to provide estimates for the Lives Saved Tool. BMC Public Health 2017, 17, 776. [Google Scholar] [CrossRef]
- Ngure, F.M.; Reid, B.M.; Humphrey, J.H.; Mbuya, M.N.; Pelto, G.; Stoltzfus, R.J. Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: Making the links. Ann. N. Y. Acad Sci. 2014, 1308, 118–128. [Google Scholar] [CrossRef]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, R.L.; Lockman, S.; Kim, S.; Smeaton, L.; Rahkola, J.T.; Thior, I.; Wester, C.; Moffat, C.; Arimi, P.; Ndase, P.; et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J. Infect. Dis. 2007, 196, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.H.; Wilkinson, A.L.; Andreasen, A.; Kinung’hi, S.M.; Urassa, M.; Michael, D.; Todd, J.; Changalucha, J.; McDermid, J.M. Longitudinal analysis of mature breastmilk and serum immune composition among mixed HIV-status mothers and their infants. Clin. Nutr. 2016, 35, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, L.; Kim, H.-Y.; Hsiao, L.; Nissan, C.; Kankasa, C.; Mwiya, M.; Thea, D.M.; Aldrovandi, G.M.; Bode, L. Oligosaccharide Composition of Breast Milk Influences Survival of Uninfected Children Born to HIV-Infected Mothers in Lusaka, Zambia. J. Nutr. 2015, 145, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef] [Green Version]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Le Doare, K.; Bland, R.; Newell, M.L. Neurodevelopment in Children Born to HIV-Infected Mothers by Infection and Treatment Status. Pediatrics 2012, 130, e1326–e1344. [Google Scholar] [CrossRef] [Green Version]
- Laughton, B.; Cornell, M.; Grove, D.; Kidd, M.; Springer, P.E.; Dobbels, E.; van Rensburg, A.J.; Violari, A.; Babiker, A.G.; Madhi, S.A.; et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS 2012, 26, 1685–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goeden, N.; Velasquez, J.; Arnold, K.A.; Chan, Y.; Lund, B.T.; Anderson, G.M.; Bonnin, A. Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain. J. Neurosci. 2016, 36, 6041–6049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elovitz, M.A.; Brown, A.G.; Breen, K.; Anton, L.; Maubert, M.; Burd, I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2011, 29, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Hackman, D.A.; Farah, M.J.; Meaney, M.J. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010, 11, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.; Tough, S.; Whitfield, H. Prenatal and postpartum maternal psychological distress and infant development: A systematic review. Child. Psychiatry Hum. Dev. 2012, 43, 683–714. [Google Scholar] [CrossRef]
- Kapetanovic, S.; Dass-Brailsford, P.; Nora, D.; Talisman, N. Mental health of HIV-seropositive women during pregnancy and postpartum period: A comprehensive literature review. Aids Behav. 2014, 18, 1152–1173. [Google Scholar] [CrossRef] [PubMed]
- Mebrahtu, H.; Simms, V.; Chingono, R.; Mupambireyi, Z.; Weiss, H.A.; Ndlovu, P.; Malaba, R.; Cowan, F.M.; Sherr, L. Postpartum maternal mental health is associated with cognitive development of HIV-exposed infants in Zimbabwe: A cross-sectional study. AIDS Care 2018, 30, 74–82. [Google Scholar] [CrossRef]
- Koss, C.A.; Natureeba, P.; Plenty, A.; Luwedde, F.; Mwesigwa, J.; Ades, V.; Charlebois, E.D.; Clark, T.D.; Achan, J.; Ruel, T.; et al. Risk Factors for Preterm Birth Among HIV-Infected Pregnant Ugandan Women Randomized to Lopinavir/Ritonavir- or Efavirenz-Based Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2014, 67, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpino, C.; Compagnone, E.; Montanaro, M.L.; Cacciatore, D.; De Luca, A.; Cerulli, A.; Di Girolamo, S.; Curatolo, P. Preterm birth and neurodevelopmental outcome: A review. Childs Nerv. Syst. 2010, 26, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 2013, 136, 1692–1707. [Google Scholar] [CrossRef] [Green Version]
- Berlot, R.; Metzler-Baddeley, C.; Ikram, M.A.; Jones, D.K.; O’Sullivan, M.J. Global Efficiency of Structural Networks Mediates Cognitive Control in Mild Cognitive Impairment. Front. Aging Neurosci. 2016, 8, 292. [Google Scholar] [CrossRef] [Green Version]
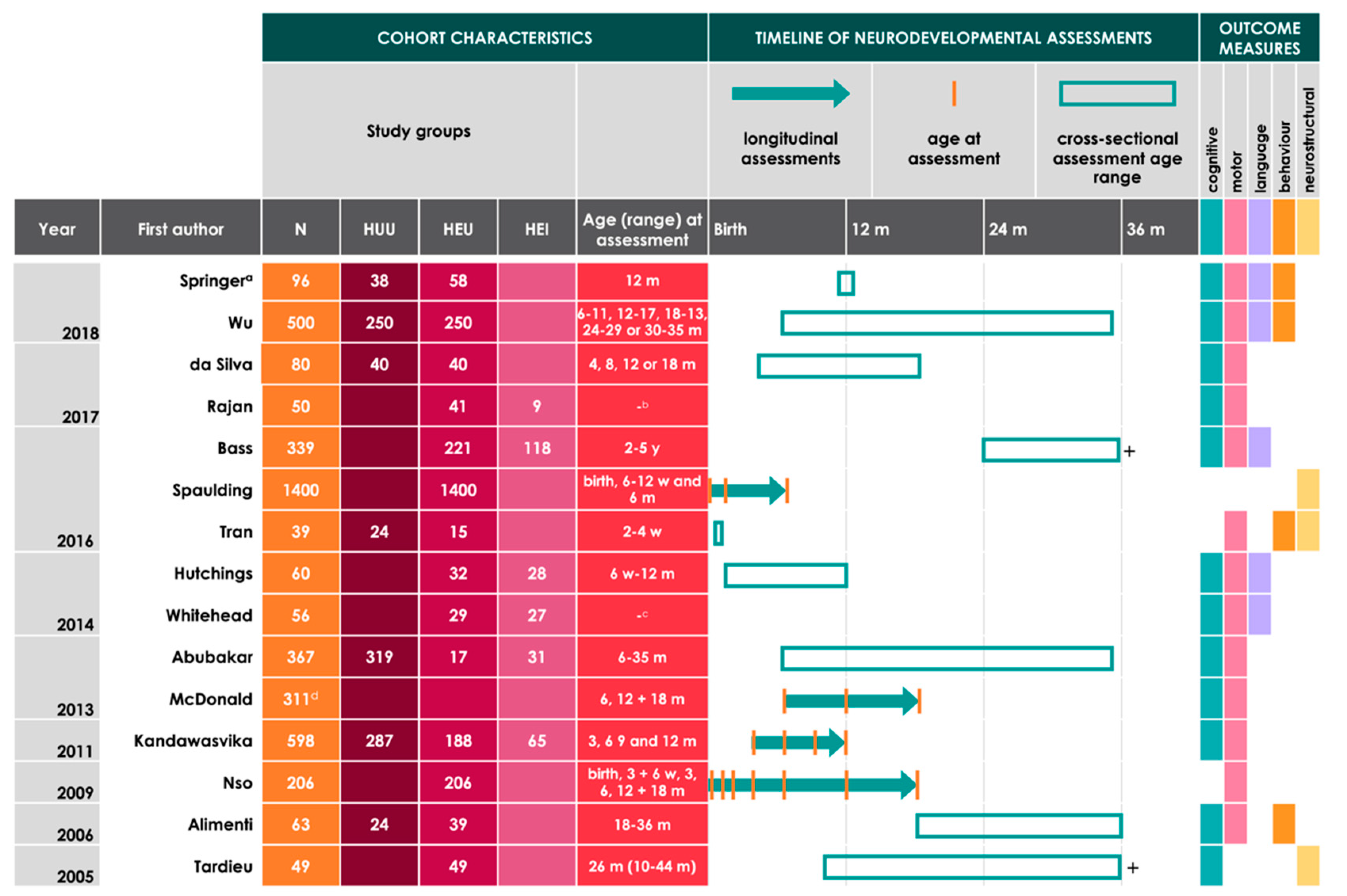

| Study | Location | Composition of Cohort by Infant HIV Status | Neurodevelopmental Assessment Tools Used | Age Assessed | Key Findings |
|---|---|---|---|---|---|
| Springer 2018 [49] | South Africa | 96 (58 HEU, 38 HUU) |
| 12 m |
|
| Wu 2018 [42] | China | 500 (250 HEU, 250 HUU) |
| 6–11, 12–17, 18–13, 24–29, or 30–35 m |
|
| da Silva 2017 [41] | Brazil | 80 (40 HEU, 40 HUU) |
| 4, 8, 12, or 18 m |
|
| Rajan 2017 [40] | India | 50 (9 HEI, 41 HEU) |
| - b |
|
| Bass 2016 c [43] | Uganda | 339 (118 HEI, 221 HEU) |
| 2–5 y |
|
| Spaulding 2016 [34] | Brazil, Argentina, Mexico, Peru, Bahamas, and Jamaica | 1400 HEU |
| Birth, 6–12 w, and 6 m |
|
| Tran 2016 [44] | South Africa | 39 (15 HEU, 24 HUU) |
| 2–4 w |
|
| Hutchings 2014 [45] | Zimbabwe | 60 (28 HEI, 32 HEU) |
| 6 w–12 m |
|
| Whitehead 2014 [36] | South Africa | 56 (27 HEI, 29 HEU; Note c) |
| - f |
|
| Abubakar 2013 [46] | Kenya | 367 (31 HEI, 17 HEU, 319 HUU) |
| 6–35 m |
|
| McDonald 2013 g [37] | Tanzania | 311 HE h |
| 6, 12, and 18 m |
|
| Kandawasvika 2011 [38] | Zimbabwe | 598 (65 HEI, 188 HEU, 287 HUU, 58 HIV-exposed/status unknown) |
| 3, 6, 9, and 12 m |
|
| Nso 2009 [39] | Spain | 206 HEU |
| Birth, 3 and 6 w, 3, 6, 12, and 18 m |
|
| Alimenti 2006 [47] | Canada | 63 (39 HEU, 24 HUU) |
| 18–36 m |
|
| Tardieu 2005 [48] | France | 49 HEU |
| 26 m (10–44 m) |
|
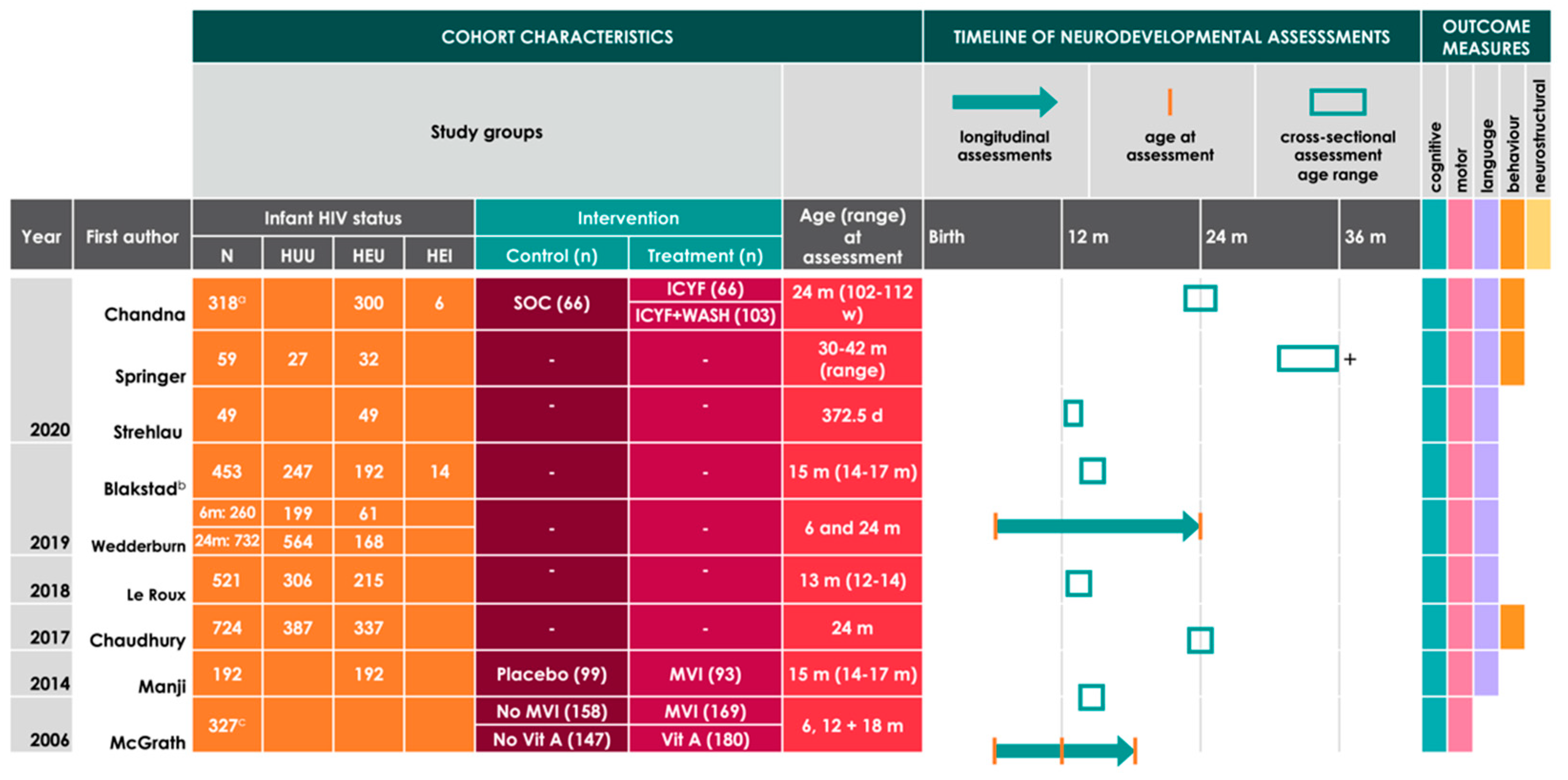
| Study | Location | Composition of Cohort Assessed for Neurodevelopment | Neurodevelopmental Assessment Tools Used | Age Assessed | Key Findings | |
|---|---|---|---|---|---|---|
| Infant HIV Status | Treatment Groups a | |||||
| Chandna 2020 [52] | Zimbabwe | 318 HIV-exposed (6 HEI, 300 HEU, 12 with unknown status) |
|
| 24 m (range 102–112 w) |
|
| Springer 2020 [53] | South Africa | 59 (27 HUU, 32 HEU) | - |
| 30–42 m (range) |
|
| Strehlau 2020 [54] | South Africa | 49 HEU | - |
| 12 m |
|
| Blakstad 2019 b [55] | Tanzania | 453 (206 HEU c, 247 HUU) | - |
| 15 m (range 14–17 m) |
|
| Wedderburn 2019 [50] | South Africa | 6 m: 260 (61 HEU, 199 HUU)24 m: 732 (168 HEU, 564 HUU) | - |
| 6 and 24 m |
|
| Le Roux 2018 [56] | South Africa | 521 (306 HUU, 215 HEU) | - |
| 13 m (range 12–14) |
|
| Chaudhury 2017 [57] | Botswana | 724 (387 HUU, 337 HEU) | - |
| 24 m |
|
| Manji 2014 [58] | Tanzania | 192 HEU b |
|
| 15 m (range 14–17 m) |
|
| McGrath 2006 [51] | Tanzania | 327 HE i |
|
| 6, 12 and 18 m |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, M.; Connor, K.L. In Utero HIV Exposure and the Early Nutritional Environment Influence Infant Neurodevelopment: Findings from an Evidenced Review and Meta-Analysis. Nutrients 2020, 12, 3375. https://doi.org/10.3390/nu12113375
White M, Connor KL. In Utero HIV Exposure and the Early Nutritional Environment Influence Infant Neurodevelopment: Findings from an Evidenced Review and Meta-Analysis. Nutrients. 2020; 12(11):3375. https://doi.org/10.3390/nu12113375
Chicago/Turabian StyleWhite, Marina, and Kristin L. Connor. 2020. "In Utero HIV Exposure and the Early Nutritional Environment Influence Infant Neurodevelopment: Findings from an Evidenced Review and Meta-Analysis" Nutrients 12, no. 11: 3375. https://doi.org/10.3390/nu12113375
APA StyleWhite, M., & Connor, K. L. (2020). In Utero HIV Exposure and the Early Nutritional Environment Influence Infant Neurodevelopment: Findings from an Evidenced Review and Meta-Analysis. Nutrients, 12(11), 3375. https://doi.org/10.3390/nu12113375




