Exogenous Ketone Supplements Improved Motor Performance in Preclinical Rodent Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ketogenic Compounds
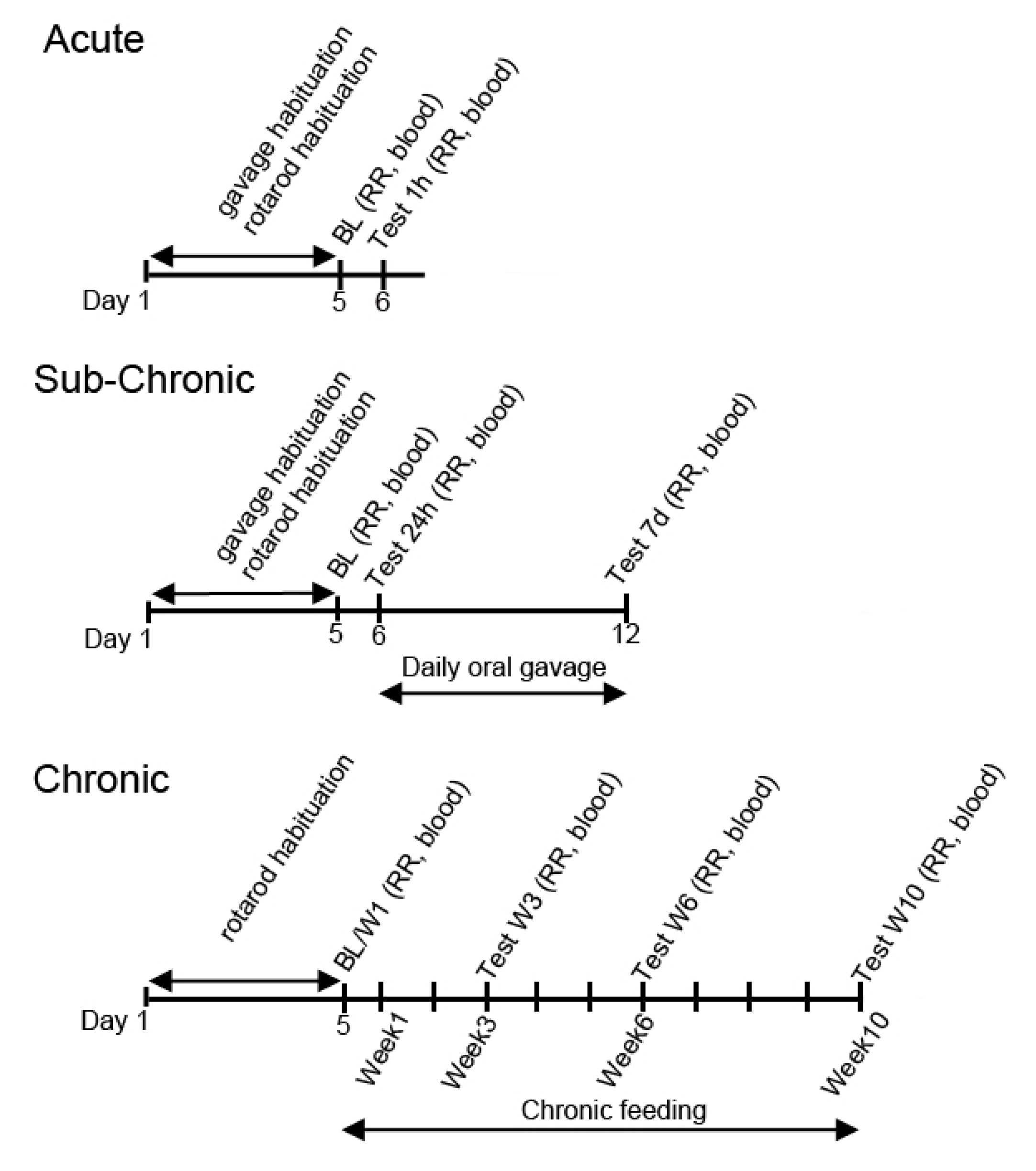
2.3. Exposure Schedule
2.4. Treatment Groups
2.5. Motor Performance Testing
2.6. Blood Glucose and R-βHB
2.7. Statistics
3. Results
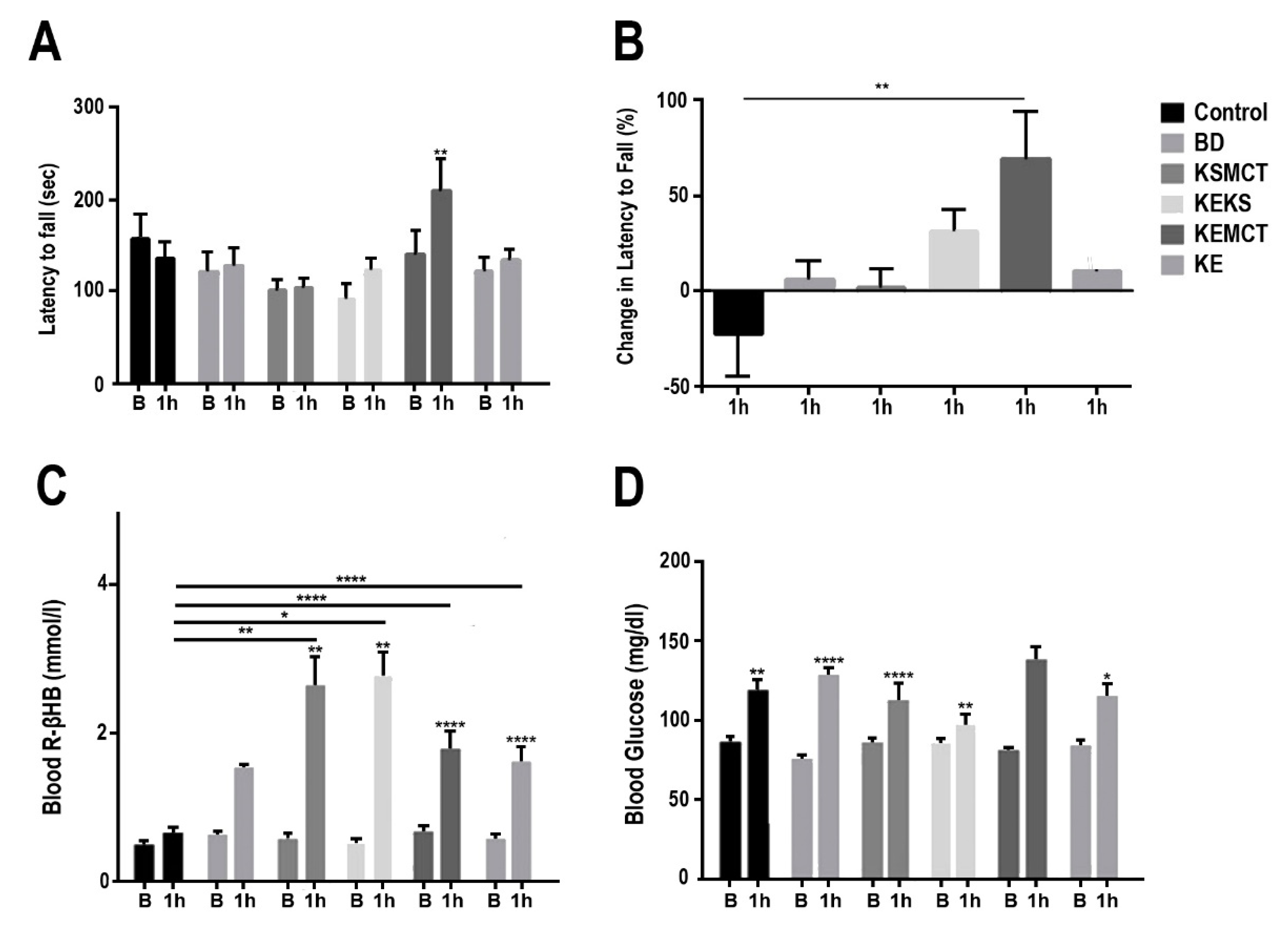
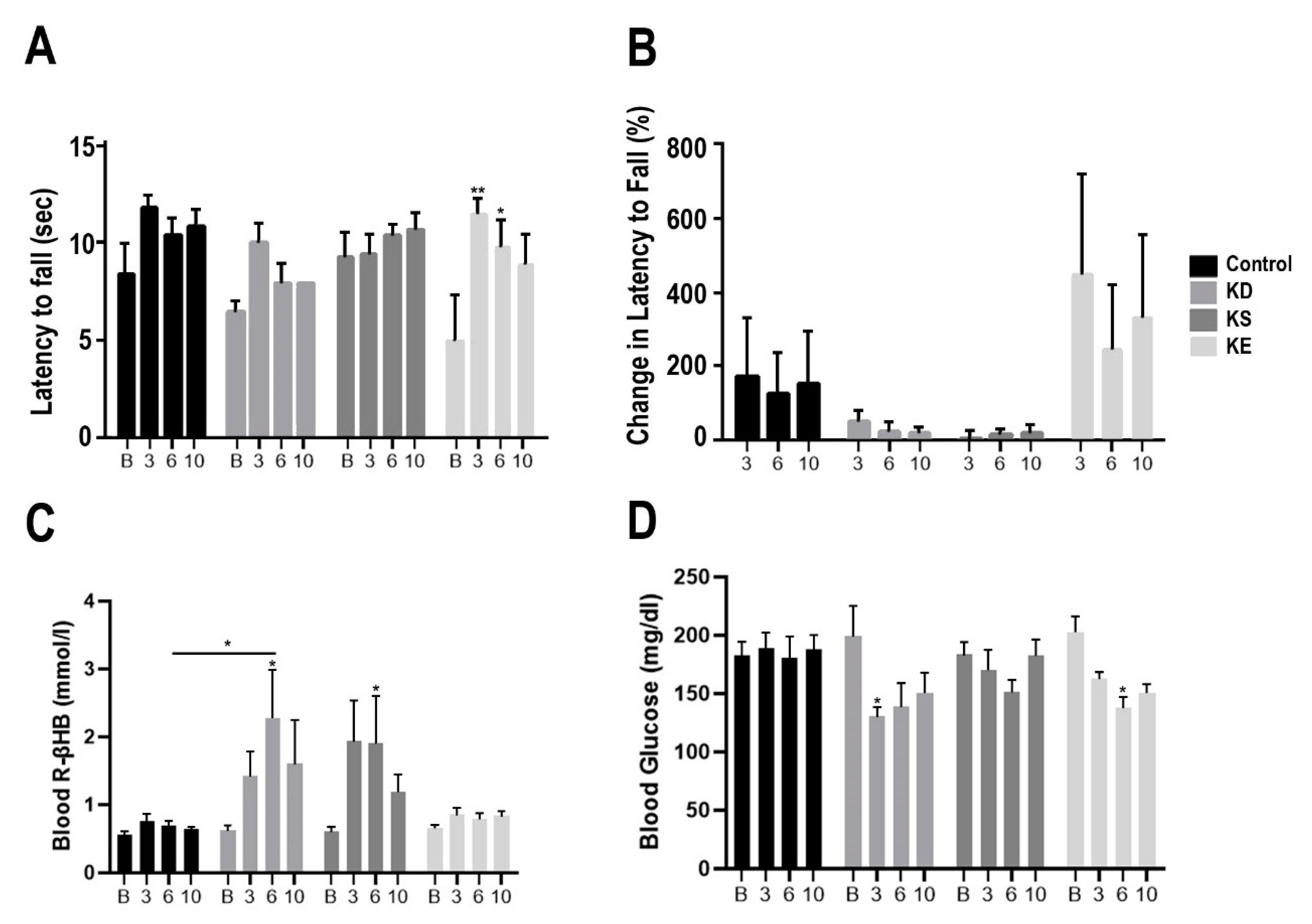
3.1. Changes in Motor Performance, Blood Glucose, and R-βHB Levels in SPD Rats with Acute Exposure
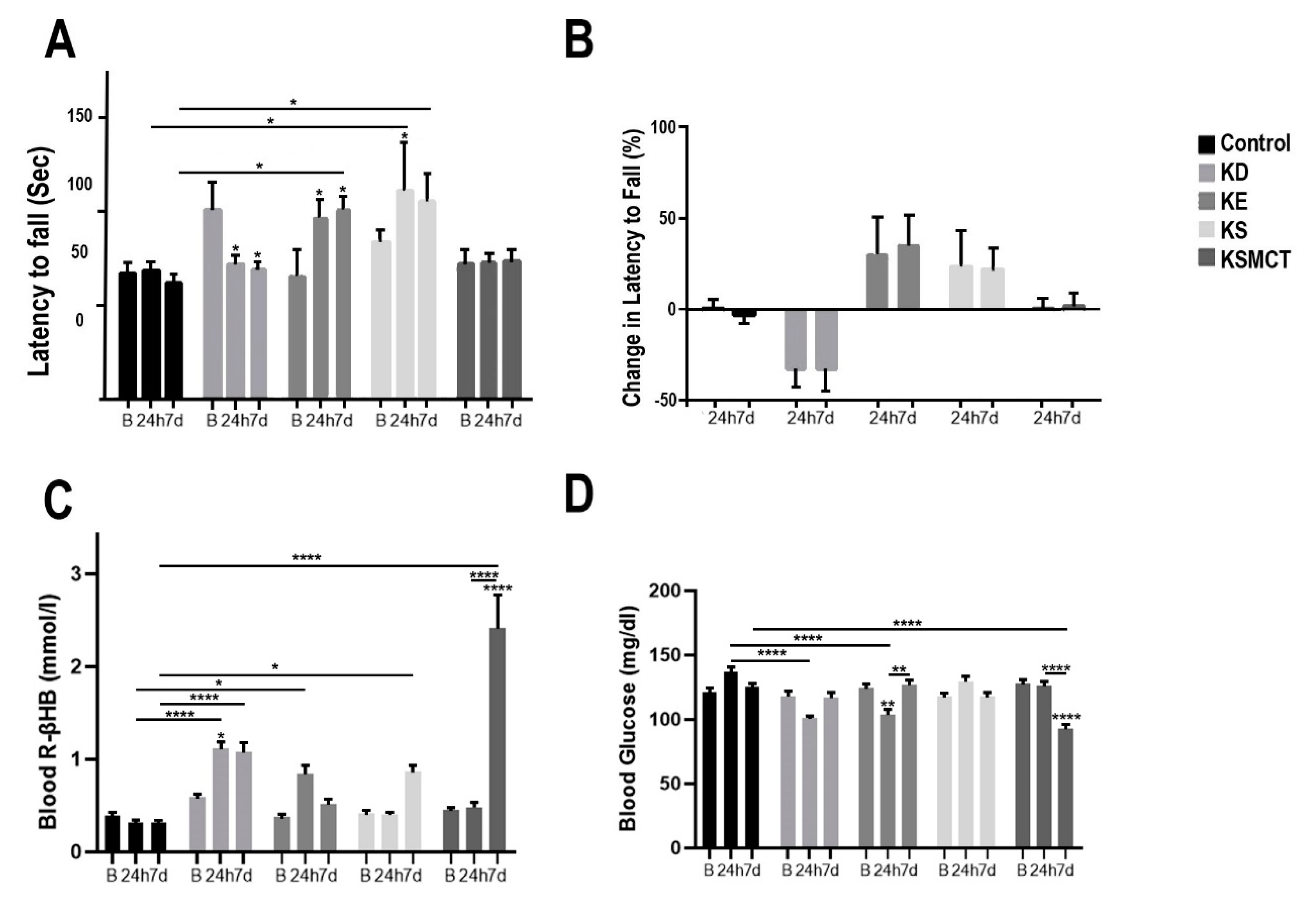
3.2. Changes in Motor Performance, Blood Glucose, and R-βHB Levels in SPD Rats with Subchronic Exposure
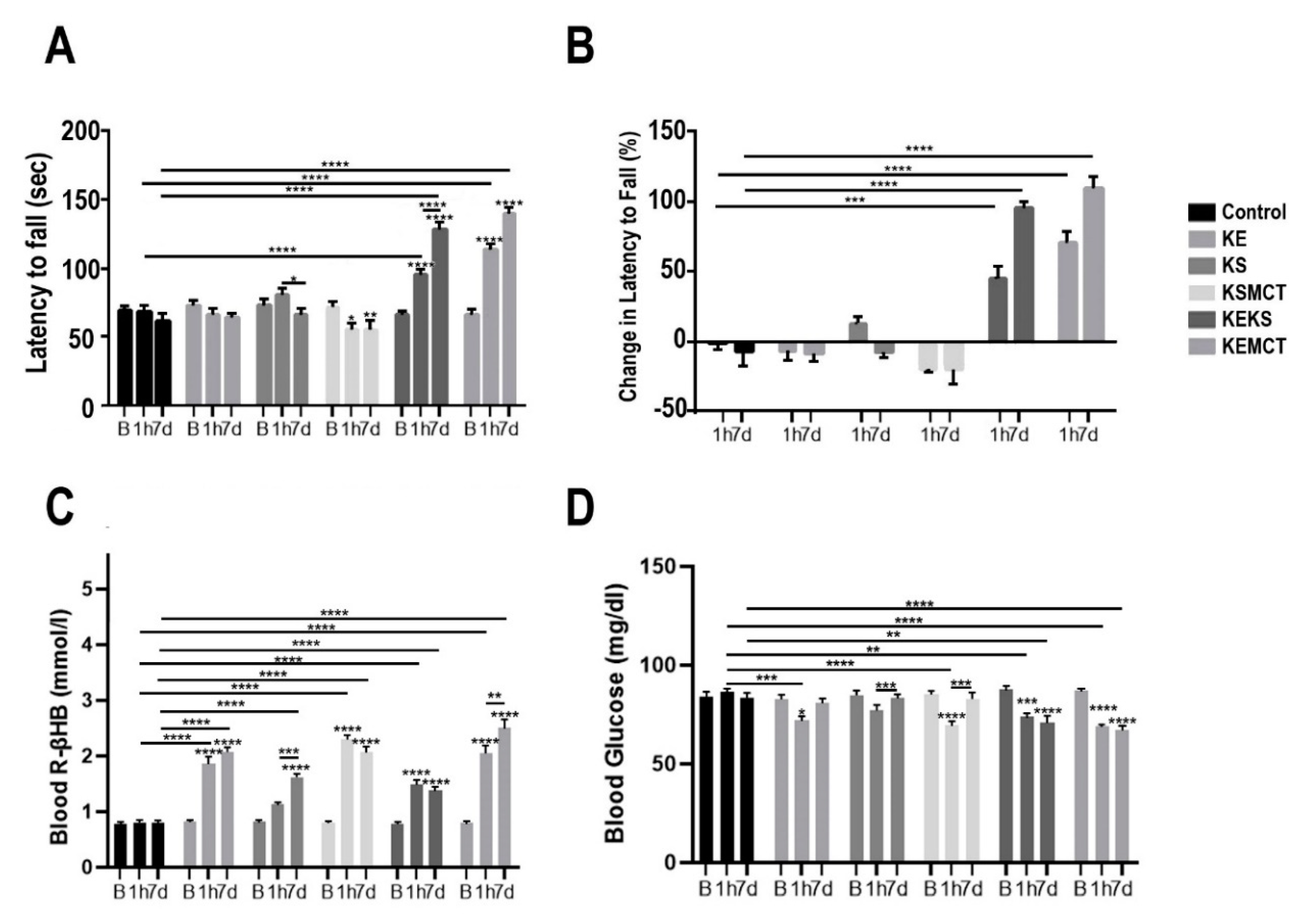
3.3. Changes in Motor Performance, Blood Glucose, and R-βHB Levels in WR Rats with Acute and Subchronic Exposure
3.4. Changes in Motor Performance, Blood Glucose, and R-βHB Levels in G1D Mice with Chronic Exposure
4. Discussion
Limitations and Inconsistencies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koppel, S.J.; Swerdlow, R.H. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem. Int. 2018, 117, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lange, D.J.; Voustianiouk, A.; MacGrogan, D.; Ho, L.; Suh, J.; Humala, N.; Thiyagarajan, M.; Wang, J.; Pasinetti, G.M. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006, 7, 29. [Google Scholar] [CrossRef]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr. Metab. (Lond.) 2005, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Vanitallie, T.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S. Treatment of Parkinson disease with diet-induced hyperketonemia: A feasibility study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef]
- Klepper, J. Glucose transporter deficiency syndrome (GLUT1DS) and the ketogenic diet. Epilepsia 2008, 49, 46–49. [Google Scholar] [CrossRef]
- De la Monte, S.M. Insulin resistance and Alzheimer’s disease. BMB Rep. 2009, 42, 475–481. [Google Scholar] [CrossRef]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. 2009, 6, 31. [Google Scholar] [CrossRef]
- Yin, J.X.; Maalouf, M.; Han, P.; Zhao, M.; Gao, M.; Dharshaun, T.; Ryan, C.; Whitelegge, J.; Wu, J.; Eisenberg, D.; et al. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol. Aging 2016, 39, 25–37. [Google Scholar] [CrossRef]
- Allen, F.M.; Stillman, E.; Fitz, R. Total Dietary Regulation in the Treatment of Diabetes; Rockefeller Institute for Medical Research: New York, NY, USA, 1919. [Google Scholar]
- Coppola, G.; Veggiotti, P.; Cusmai, R.; Bertoli, S.; Cardinali, S.; Dionisi-Vici, C.; Elia, M.; Lispi, M.L.; Sarnelli, C.; Tagliabue, A.; et al. The ketogenic diet in children, adolescents and young adults with refractory epilepsy: An Italian multicentric experience. Epilepsy Res. 2002, 48, 221–227. [Google Scholar] [CrossRef]
- Hemingway, C.; Freeman, J.M.; Pillas, D.J.; Pyzik, P.L. The Ketogenic Diet: A 3- to 6-Year Follow-Up of 150 Children Enrolled Prospectively. Pediatrics 2001, 108, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, H.H.; Thiele, E.A. Low-glycemic-index treatment: A liberalized ketogenic diet for treatment of intractable epilepsy. Neurology 2005, 65, 1810–1812. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Kovács, Z.; Juhasz, G.; Murdun, C.; Goldhagen, C.R.; Koutnik, A.P.; Poff, A.M.; Kesl, S.L.; D’Agostino, D.P. Exogenous Ketone Supplements Reduce Anxiety-Related Behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk Rats. Front. Mol. Neurosci. 2016, 9. [Google Scholar] [CrossRef]
- Ari, C.; Kovács, Z.; Murdun, C.; Koutnik, A.P.; Goldhagen, C.R.; Rogers, C.; Diamond, D.; D’Agostino, D.P. Nutritional ketosis delays the onset of isoflurane induced anesthesia. BMC Anesthesiol. 2018, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Koutnik, A.P.; DeBlasi, J.; Landon, C.; Rogers, C.Q.; Vallas, J.; Bharwani, S.; Puchowicz, M.; Bederman, I.; Diamond, D.M.; et al. Delaying latency to hyperbaric oxygen-induced CNS oxygen toxicity seizures by combinations of exogenous ketone supplements. Physiol. Rep. 2019, 7, e13961. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, D.P.; Pilla, R.; Held, H.E.; Landon, C.S.; Puchowicz, M.; Brunengraber, H.; Ari, C.; Arnold, P.; Dean, J.B. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R829–R836. [Google Scholar] [CrossRef]
- Kesl, S.L.; Poff, A.M.; Ward, N.P.; Fiorelli, T.N.; Ari, C.; Van Putten, A.J.; Sherwood, J.W.; Arnold, P.; D’Agostino, D.P. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague–Dawley rats. Nutr. Metab. 2016, 13, 9. [Google Scholar] [CrossRef]
- Kovács, Z.; D’Agostino, D.P.; Diamond, D.; Kindy, M.S.; Rogers, C.; Ari, C. Therapeutic Potential of Exogenous Ketone Supplement Induced Ketosis in the Treatment of Psychiatric Disorders: Review of Current Literature. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef]
- Adlercreutz, H. Western diet and Western diseases: Some hormonal and biochemical mechanisms and associations. Scand. J. Clin. Lab. Investig. 1990, 50, 3–23. [Google Scholar] [CrossRef]
- Cahill, G.F. Fuel Metabolism in Starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef]
- Lieberman, M.; Peet, A.; Chansky, M. Marks’ Basic Medical Biochemistry: A Clinical Approach, 5e|Medical Education|Health Library; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2018; ISBN 978-1-4963-2481-8. [Google Scholar]
- Grabacka, M.; Pierzchalska, M.; Dean, M.; Reiss, K. Regulation of Ketone Body Metabolism and the Role of PPARα. Int. J. Mol. Sci. 2016, 17, 2093. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Williamson, D.H.; Krebs, H.A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem. J. 1971, 122, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.L. Cerebral Metabolic Adaptation and Ketone Metabolism after Brain Injury. J. Cereb. Blood Flow Metab. 2008, 28, 1–16. [Google Scholar] [CrossRef]
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F. Brain Metabolism during Fasting. J. Clin. Investig. 1967, 46, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Murdun, C.; Koutnik, A.P.; Goldhagen, C.R.; Rogers, C.; Park, C.; Bharwani, S.; Diamond, D.M.; Kindy, M.S.; D’Agostino, D.P.; et al. Exogenous Ketones Lower Blood Glucose Level in Rested and Exercised Rodent Models. Nutrients 2019, 11, 2330. [Google Scholar] [CrossRef]
- D’Agostino, D.P.; Putnam, R.W.; Dean, J.B. Superoxide (O2−) Production in CA1 Neurons of Rat Hippocampal Slices Exposed to Graded Levels of Oxygen. J. Neurophysiol. 2007, 98, 1030–1041. [Google Scholar] [CrossRef]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; Knight, N.S.; Murray, A.J.; Cochlin, L.E.; King, M.T.; Wong, A.W.; Roberts, A.; et al. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul. Toxicol. Pharmacol. 2012, 63, 196–208. [Google Scholar] [CrossRef]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; Todd King, M.; Musa-Veloso, K.; Ho, M.; Roberts, A.; Robertson, J.; VanItallie, T.B.; et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 2012, 63, 401–408. [Google Scholar] [CrossRef]
- Kovács, Z.; D’Agostino, D.P.; Dobolyi, A.; Ari, C. Adenosine A1 Receptor Antagonism Abolished the Anti-seizure Effects of Exogenous Ketone Supplementation in Wistar Albino Glaxo Rijswijk Rats. Front. Mol. Neurosci. 2017, 10, 235. [Google Scholar] [CrossRef]
- Masino, S.A. Ketogenic Diet and Metabolic Therapies: Expanded Roles in Health and Disease; Oxford University Press: Oxford, UK, 2016; ISBN 978-0-19-049800-9. [Google Scholar]
- Brownlow, M.L.; Benner, L.; D’Agostino, D.; Gordon, M.N.; Morgan, D. Ketogenic Diet Improves Motor Performance but Not Cognition in Two Mouse Models of Alzheimer’s Pathology. PLoS ONE 2013, 8, e75713. [Google Scholar] [CrossRef]
- Ciarlone, S.L.; Grieco, J.C.; D’Agostino, D.P.; Weeber, E.J. Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model. Neurobiol. Dis. 2016, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Varghese, M.; Vempati, P.; Dzhun, A.; Cheng, A.; Wang, J.; Lange, D.; Bilski, A.; Faravelli, I.; Pasinetti, G.M. Caprylic Triglyceride as a Novel Therapeutic Approach to Effectively Improve the Performance and Attenuate the Symptoms Due to the Motor Neuron Loss in ALS Disease. PLoS ONE 2012, 7, e49191. [Google Scholar] [CrossRef] [PubMed]
- Coenen, A.M.L.; van Luijtelaar, E.L.J.M. Genetic Animal Models for Absence Epilepsy: A Review of the WAG/Rij Strain of Rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef] [PubMed]
- Marin-Valencia, I.; Good, L.B.; Ma, Q.; Duarte, J.; Bottiglieri, T.; Sinton, C.M.; Heilig, C.W.; Pascual, J.M. Glut1 deficiency (G1D): Epilepsy and metabolic dysfunction in a mouse model of the most common human phenotype. Neurobiol. Dis. 2012, 48, 92–101. [Google Scholar] [CrossRef]
- Citraro, R.; Iannone, M.; Leo, A.; De Caro, C.; Nesci, V.; Tallarico, M.; Abdalla, K.; Palma, E.; Arturi, F.; De Sarro, G.; et al. Evaluation of the effects of liraglutide on the development of epilepsy and behavioural alterations in two animal models of epileptogenesis. Brain Res. Bull. 2019, 153, 133–142. [Google Scholar] [CrossRef]
- Sarkisova, K.; van Luijtelaar, G. The WAG/Rij strain: A genetic animal model of absence epilepsy with comorbidity of depression [corrected]. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 854–876. [Google Scholar] [CrossRef]
- Sarkisova, K.Y.; Midzianovskaia, I.S.; Kulikov, M.A. Depressive-like behavioral alterations and c-fos expression in the dopaminergic brain regions in WAG/Rij rats with genetic absence epilepsy. Behav. Brain Res. 2003, 144, 211–226. [Google Scholar] [CrossRef]
- Friedman, J.R.L.; Thiele, E.A.; Wang, D.; Levine, K.B.; Cloherty, E.K.; Pfeifer, H.H.; Vivo, D.C.D.; Carruthers, A.; Natowicz, M.R. Atypical GLUT1 deficiency with prominent movement disorder responsive to ketogenic diet. Mov. Disord. 2006, 21, 241–244. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Kim, D.Y.; Davis, L.M.; Sullivan, P.G.; Maalouf, M.; Simeone, T.A.; van Brederode, J.; Rho, J.M. Ketone bodies are protective against oxidative stress in neocortical neurons. J. Neurochem. 2007, 101, 1316–1326. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; Toniolo, L.; Canato, M.; Bianco, A.; Fratter, A. Nutrition and acne: Therapeutic potential of ketogenic diets. Ski. Pharm. Physiol. 2012, 25, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.-C.; Yan, S.-D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, B. Neuroprotective and Anti-inflammatory Activities of Ketogenic Diet on MPTP-induced Neurotoxicity. J. Mol. Neurosci. 2010, 42, 145–153. [Google Scholar] [CrossRef]
- Lim, S.; Chesser, A.S.; Grima, J.C.; Rappold, P.M.; Blum, D.; Przedborski, S.; Tieu, K. D-β-Hydroxybutyrate Is Protective in Mouse Models of Huntington’s Disease. PLoS ONE 2011, 6, e24620. [Google Scholar] [CrossRef] [PubMed]
- Veyrat-Durebex, C.; Reynier, P.; Procaccio, V.; Hergesheimer, R.; Corcia, P.; Andres, C.R.; Blasco, H. How Can a Ketogenic Diet Improve Motor Function? Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Gano, L.B.; Patel, M.; Rho, J.M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014, 55, 2211–2228. [Google Scholar] [CrossRef]
- Maalouf, M.; Rho, J.M.; Mattson, M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009, 59, 293–315. [Google Scholar] [CrossRef]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; Hayek, L.E.; Haidar, E.A.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body b- hydroxybutyrate. Cell Biol. 2016, 21. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-hydroxybutyrate: Much more than a metabolite. Diabetes Res. Clin. Pract. 2014, 106, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour—epigenetic regulation of the gut–brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Hao, J.; Liu, R.; Turner, G.; Shi, F.-D.; Rho, J.M. Inflammation-Mediated Memory Dysfunction and Effects of a Ketogenic Diet in a Murine Model of Multiple Sclerosis. PLoS ONE 2012, 7, e35476. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Meng, J.; Li, L.; Han, W.; Li, C.; Zhong, R.; Miao, X.; Cai, J.; Zhang, Y.; Zhu, D. Acetoacetate Accelerates Muscle Regeneration and Ameliorates Muscular Dystrophy in Mice. J. Biol. Chem. 2016, 291, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.S.; Welle, S.L.; Halliday, D.; Campbell, R.G. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J. Clin. Investig. 1988, 82, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Vandoorne, T.; De Smet, S.; Ramaekers, M.; Van Thienen, R.; De Bock, K.; Clarke, K.; Hespel, P. Intake of a Ketone Ester Drink during Recovery from Exercise Promotes mTORC1 Signaling but Not Glycogen Resynthesis in Human Muscle. Front. Physiol. 2017, 8, 310. [Google Scholar] [CrossRef]
- Thomsen, H.H.; Rittig, N.; Johannsen, M.; Møller, A.B.; Jørgensen, J.O.; Jessen, N.; Møller, N. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: Anticatabolic impact of ketone bodies. Am. J. Clin. Nutr. 2018, 108, 857–867. [Google Scholar] [CrossRef]
- Koutnik, A.P.; D’Agostino, D.P.; Egan, B. Anticatabolic Effects of Ketone Bodies in Skeletal Muscle. Trends Endocrinol. Metab. 2019, 30, 227–229. [Google Scholar] [CrossRef]
- Koutnik, A.P.; Poff, A.M.; Ward, N.P.; DeBlasi, J.M.; Soliven, M.A.; Romero, M.A.; Roberson, P.A.; Fox, C.D.; Roberts, M.D.; D’Agostino, D.P. Ketone Bodies Attenuate Wasting in Models of Atrophy. J. Cachexia Sarcopenia Muscle 2020. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Calderón, N.; Betancourt, L.; Hernández, L.; Rada, P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: A microdialysis study. Neurosci. Lett. 2017, 642, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Erecińska, M.; Nelson, D.; Daikhin, Y.; Yudkoff, M. Regulation of GABA level in rat brain synaptosomes: Fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J. Neurochem. 1996, 67, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; D’Agostino, D.P.; Ari, C. Anxiolytic Effect of Exogenous Ketone Supplementation Is Abolished by Adenosine A1 Receptor Inhibition in Wistar Albino Glaxo/Rijswijk Rats. Front. Behav. Neurosci. 2018, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Masino, S.A.; Li, T.; Theofilas, P.; Sandau, U.S.; Ruskin, D.N.; Fredholm, B.B.; Geiger, J.D.; Aronica, E.; Boison, D. A ketogenic diet suppresses seizures in mice through adenosine A₁ receptors. J. Clin. Investig. 2011, 121, 2679–2683. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J. Int. Soc. Sports Nutr. 2012, 9, 34. [Google Scholar] [CrossRef]
- Gerhart, D.Z.; Enerson, B.E.; Zhdankina, O.Y.; Leino, R.L.; Drewes, L.R. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am. J. Physiol. 1997, 273, E207–E213. [Google Scholar] [CrossRef]
- Ito, K.; Uchida, Y.; Ohtsuki, S.; Aizawa, S.; Kawakami, H.; Katsukura, Y.; Kamiie, J.; Terasaki, T. Quantitative membrane protein expression at the blood-brain barrier of adult and younger cynomolgus monkeys. J. Pharm. Sci. 2011, 100, 3939–3950. [Google Scholar] [CrossRef]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef]
- Achanta, L.B.; Rae, C.D. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef]
- Leong, S.F.; Lai, J.C.; Lim, L.; Clark, J.B. Energy-metabolizing enzymes in brain regions of adult and aging rats. J. Neurochem. 1981, 37, 1548–1556. [Google Scholar] [CrossRef]
- Page, M.A.; Williamson, D.H. Enzymes of Ketone-Body Utilisation in Human Brain. Lancet 1971, 298, 66–68. [Google Scholar] [CrossRef]
- Williamson, D.H.; Bates, M.W.; Page, M.A.; Krebs, H.A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem. J. 1971, 121, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.N. Circadian rhythms, diet, and neuronal excitability. Epilepsia 2008, 49, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, G.; Thorell, J.O.; Ingvar, M.; Grill, V.; Widén, L.; Stone-Elander, S. Use of R-beta-[1-11C]hydroxybutyrate in PET studies of regional cerebral uptake of ketone bodies in humans. Am. J. Physiol. 1995, 269, E948–E959. [Google Scholar] [CrossRef]
- Hawkins, R.A.; Biebuyck, J.F. Ketone bodies are selectively used by individual brain regions. Science 1979, 205, 325–327. [Google Scholar] [CrossRef]
- Van Rijt, W.J.; Jager, E.A.; Allersma, D.P.; Aktuğlu Zeybek, A.Ç.; Bhattacharya, K.; Debray, F.-G.; Ellaway, C.J.; Gautschi, M.; Geraghty, M.T.; Gil-Ortega, D.; et al. Efficacy and safety of D,L-3-hydroxybutyrate (D,L-3-HB) treatment in multiple acyl-CoA dehydrogenase deficiency. Genet. Med. 2020, 22, 908–916. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef]
- Desrochers, S.; David, F.; Garneau, M.; Jetté, M.; Brunengraber, H. Metabolism of R- and S-1,3-butanediol in perfused livers from meal-fed and starved rats. Biochem. J. 1992, 285, 647–653. [Google Scholar] [CrossRef]
- Li, R.-J.; Liu, Y.; Liu, H.-Q.; Li, J. Ketogenic diets and protective mechanisms in epilepsy, metabolic disorders, cancer, neuronal loss, and muscle and nerve degeneration. J. Food Biochem. 2020, 44, e13140. [Google Scholar] [CrossRef]
- Marchiò, M.; Roli, L.; Giordano, C.; Trenti, T.; Guerra, A.; Biagini, G. Decreased ghrelin and des-acyl ghrelin plasma levels in patients affected by pharmacoresistant epilepsy and maintained on the ketogenic diet. Clin. Nutr. 2019, 38, 954–957. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Löscher, W.; Rho, J.M. Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, M.; Bruehl, C.; Voskuyl, R.A.; Kang, J.X.; Leaf, A.; Wadman, W.J. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 12559–12563. [Google Scholar] [CrossRef] [PubMed]
- Mazier, M.; Jones, P. Diet fat saturation and feeding state modulate rates of cholesterol synthesis in normolipidemic men. J. Nutr. 1997, 127, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Gasior, M.; Vining, E.P.G.; Rogawski, M.A. The Neuropharmacology of the Ketogenic Diet. Pediatr. Neurol. 2007, 36, 281–292. [Google Scholar] [CrossRef] [PubMed]
| Standard Diet (SD) | Ketogenic Diet (KD) | |
|---|---|---|
| Protein, % of kcal | 24 | 22.4 |
| Carbohydrate, 5 of kcal | 62 | 0.5 |
| Fat, % by kcal | 14 | 77.1 |
| kcal/g | 3.0 | 4.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ari, C.; Murdun, C.; Goldhagen, C.; Koutnik, A.P.; Bharwani, S.R.; Diamond, D.M.; Kindy, M.; D’Agostino, D.P.; Kovacs, Z. Exogenous Ketone Supplements Improved Motor Performance in Preclinical Rodent Models. Nutrients 2020, 12, 2459. https://doi.org/10.3390/nu12082459
Ari C, Murdun C, Goldhagen C, Koutnik AP, Bharwani SR, Diamond DM, Kindy M, D’Agostino DP, Kovacs Z. Exogenous Ketone Supplements Improved Motor Performance in Preclinical Rodent Models. Nutrients. 2020; 12(8):2459. https://doi.org/10.3390/nu12082459
Chicago/Turabian StyleAri, Csilla, Cem Murdun, Craig Goldhagen, Andrew P. Koutnik, Sahil R. Bharwani, David M. Diamond, Mark Kindy, Dominic P. D’Agostino, and Zsolt Kovacs. 2020. "Exogenous Ketone Supplements Improved Motor Performance in Preclinical Rodent Models" Nutrients 12, no. 8: 2459. https://doi.org/10.3390/nu12082459
APA StyleAri, C., Murdun, C., Goldhagen, C., Koutnik, A. P., Bharwani, S. R., Diamond, D. M., Kindy, M., D’Agostino, D. P., & Kovacs, Z. (2020). Exogenous Ketone Supplements Improved Motor Performance in Preclinical Rodent Models. Nutrients, 12(8), 2459. https://doi.org/10.3390/nu12082459










