Diet and Cardiovascular Disease Risk Among Individuals with Familial Hypercholesterolemia: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Types of Studies
2.1.2. Study Participants
2.1.3. Interventions
2.2. Outcomes
2.3. Information Sources
2.4. Data Collection and Analysis
2.4.1. Selection of Studies
2.4.2. Data Extraction and Management
- Dietary interventions to reduce fat content.
- Supplementation with omega-3 fatty acids compared with placebo.
- Dietary interventions modifying unsaturated fat content.
- Cholesterol-lowering diet compared with dietary interventions increasing intake of plant stanols.
- Cholesterol-lowering diet compared with dietary interventions increasing intake of plant sterols.
- Dietary interventions increasing intake of plant stanols compared with plant sterols.
- Dietary interventions modifying protein content.
- Dietary interventions increasing intake of dietary fiber.
2.5. Assessment of Risk of Bias in Included Studies
2.6. Measurements of Treatment Effect
2.7. Synthesis of Results
2.7.1. Missing Data
2.7.2. Assessment of Heterogeneity
2.8. Assessment of Reporting Biases
2.9. Subgroup Analysis and Investigation of Heterogeneity
3. Results
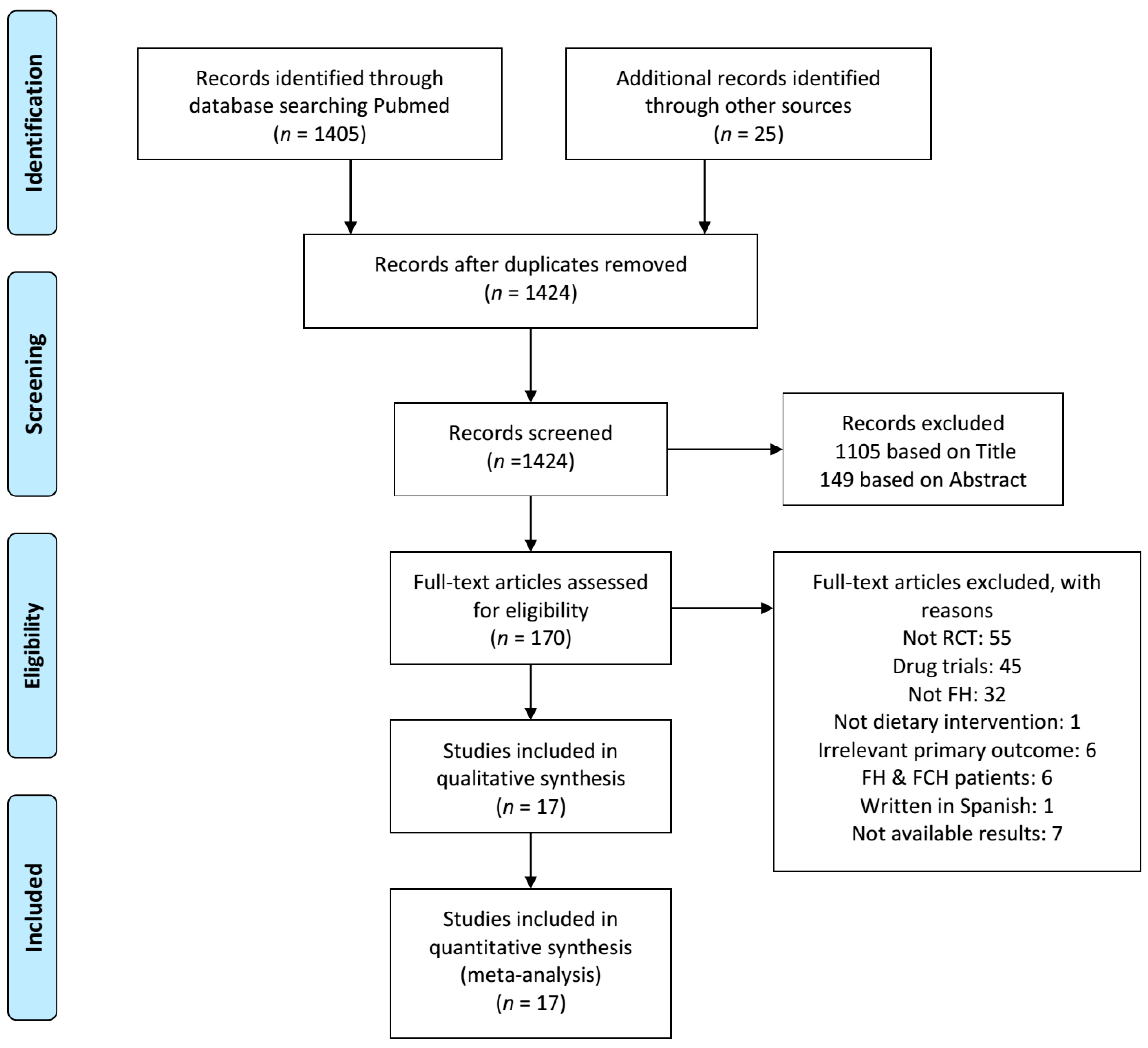
3.1. Study Selection
3.2. Study Characteristics
- Only one study evaluated the impact of cholesterol-lowering diet in adults with FH, who were treated with simvastatin [21].
- Three trials compared the effect of treatment with omega-3 fatty acids in comparison with placebo [11,13,20]. The daily supplementation of omega-3 fatty acids was 5.1 g with a ratio of eicosapentaenoic acid/ docosahexaenoic acid (EPA/DHA) of 1:1 in the oldest trial [11], whereas the treatment arm in the rest RCTs comprised of 4 g/d of EPA/DHA (46% EPA and 38% DHA) [13,20]. All of these trials included adults taking lipid-lowering therapy [11,13,20] and only one reported that its subjects adhered to cholesterol-lowering diet [11].
- Two trials evaluated the impact of modified fat on FH patients. The former compared 2 low-fat diets enriched with either monounsaturated fatty acids (MUFAs) by rapeseed oil or polyunsaturated fatty acids (PUFAs) by sunflower oil in children with FH [19]. The second trial assigned its subjects to 2 cholesterol-lowering diets differing with regard to polyunsaturated:saturated values (2.0 and 1.3, respectively) [25].
- Two RCTs investigated the dietary interventions increasing the intake of plant stanols. The first study compared the addition of 3 g sitostanol dissolved in margarine to cholesterol-lowering diet with placebo in children with FH [16]. The second one evaluated the addition of 2 g plant stanols to cholesterol-lowering diet in a fortified yogurt in comparison with placebo in children with FH [14].
- Four trials evaluated the addition of plant sterols to cholesterol-lowering diet compared with placebo in FH patients [10,12,15,22]. Plants sterols were administered in a fortified margarine spread at a dose ranging 1.6-2.5 g/d. Two of the trials included children with FH [10,12] and the rest studies included FH adults receiving lipid-lowering drugs [15,22]. One trial compared the addition of 2 g/d plant stanols with 2 g/d plant sterols in FH adults who adhered to cholesterol-lowering diet and were on lipid-lowering therapy [23].
- Three RCTs evaluated dietary interventions modifying the protein content of the diet in FH patients [17,18,26]. Two of these trials manipulated protein content by increasing the consumption of soy protein [17,18]. The former compared 2 different cholesterol-lowering diets with high-protein content in which 35% of the protein was consumed as dairy source, either from soy beverage or cow milk [18]. The latter RCT investigated the addition of soy-protein to a diet high in unsaturated and low in saturated fats compared with placebo [17]. Both of these RCTs referred to children with FH [17,18]. The third trial investigated the increase in protein intake on top of a cholesterol-lowering diet in FH adults [26].
3.3. Bias Risk within Studies
3.3.1. Allocation
3.3.2. Blinding
3.3.3. Incomplete Outcome Data
3.3.4. Selective Reporting
3.4. Effects of Interventions
3.4.1. Dietary Interventions Reducing Fat Intake
3.4.2. Supplementation with Omega-3 Fatty Acids Compared with Placebo
3.4.3. Dietary Interventions Modifying Unsaturated Fat Content
Low-Fat Diet Regimes Enriched with either Monounsaturated Fatty Acids or Polyunsaturated Fatty Acids
Cholesterol-Lowering Diets Differing with Regard to Polyunsaturated:Saturated Values
3.4.4. Cholesterol-Lowering Diet Compared with Dietary Interventions Increasing Intake of Plant Stanols
3.4.5. Cholesterol-Lowering Diet Compared with Dietary Interventions Increasing Intake of Plant Sterols
3.4.6. Dietary Interventions Increasing Intake of Plant Stanols Compared with Plant Sterols
3.4.7. Dietary Interventions Modifying Protein Content
Soy Protein as a Form of Dietary Intervention Compared to Another Form or no Intervention
Dietary Intervention to Increase Protein Intake
3.4.8. Dietary Interventions to Increase Intake of Dietary Fiber
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Section/Topic | # | Checklist Item | Reported on Page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes and study design (PICOS). | 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | N/A |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 2 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 3 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 3 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 3–4 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 4 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 4 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 4 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 4–5 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 5–7 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 8 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 8–14 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 8–14 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 8–14 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression (see Item 16)). | 8–14 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users and policy makers). | 14–17 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 16–17 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 17 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 17 |
References
- Austin, M.A.; Hutter, C.M.; Zimmern, R.L.; Humphries, S.E. Genetic causes of monogenic heterozygous familial hypercholesterolemia: A HuGE prevalence review. Am. J. Epidemiol. 2004, 160, 407–420. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Kondapally Seshasai, S.R.; Cole, D.; Hovingh, G.K.; Kastelein, J.J.; Mata, P.; Raal, F.J.; Santos, R.D.; Soran, H.; Watts, G.F.; et al. Familial hypercholesterolaemia: A global call to arms. Atherosclerosis 2015, 243, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Barkas, F.; Liberopoulos, E.; Liamis, G.; Elisaf, M. Familial hypercholesterolemia is undertreated in clinical practice. Hellenic J. Atheroscler. 2016, 7, 120–130. [Google Scholar]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the national lipid association expert panel on familial hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [CrossRef]
- Raal, F.J.; Hovingh, G.K.; Catapano, A.L. Familial hypercholesterolemia treatments: Guidelines and new therapies. Atherosclerosis 2018, 277, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegard, L.; Jessup, W.; Jones, P.J.; Lutjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef]
- Gidding, S.S. Special commentary: Is diet management helpful in familial hypercholesterolemia? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 135–140. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Amundsen, A.L.; Ose, L.; Nenseter, M.S.; Ntanios, F.Y. Plant sterol ester-enriched spread lowers plasma total and LDL cholesterol in children with familial hypercholesterolemia. Am. J. Clin. Nutr. 2002, 76, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, G.P.; Maffi, V.; Sleiman, I.; Spandrio, S.; Di Stefano, O.; Salvi, A.; Scalvini, T. Fish oil supplementation in patients with heterozygous familial hypercholesterolemia. Recenti Prog. Med. 1996, 87, 102–105. [Google Scholar] [PubMed]
- de Jongh, S.; Vissers, M.N.; Rol, P.; Bakker, H.D.; Kastelein, J.J.; Stroes, E.S. Plant sterols lower LDL cholesterol without improving endothelial function in prepubertal children with familial hypercholesterolaemia. J. Inherit. Metab. Dis. 2003, 26, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Hande, L.N.; Thunhaug, H.; Enebakk, T.; Ludviksen, J.; Pettersen, K.; Hovland, A.; Lappegard, K.T. Addition of marine omega-3 fatty acids to statins in familial hypercholesterolemia does not affect in vivo or in vitro endothelial function. J. Clin. Lipidol. 2019, 13, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Jakulj, L.; Vissers, M.N.; Rodenburg, J.; Wiegman, A.; Trip, M.D.; Kastelein, J.J. Plant stanols do not restore endothelial function in pre-pubertal children with familial hypercholesterolemia despite reduction of low-density lipoprotein cholesterol levels. J. Pediatr. 2006, 148, 495–500. [Google Scholar] [CrossRef]
- Neil, H.A.; Meijer, G.W.; Roe, L.S. Randomised controlled trial of use by hypercholesterolaemic patients of a vegetable oil sterol-enriched fat spread. Atherosclerosis 2001, 156, 329–337. [Google Scholar] [CrossRef]
- Gylling, H.; Siimes, M.A.; Miettinen, T.A. Sitostanol ester margarine in dietary treatment of children with familial hypercholesterolemia. J. Lipid Res. 1995, 36, 1807–1812. [Google Scholar]
- Helk, O.; Widhalm, K. Effects of a low-fat dietary regimen enriched with soy in children affected with heterozygous familial hypercholesterolemia. Clin. Nutr. ESPEN 2020, 36, 150–156. [Google Scholar] [CrossRef]
- Laurin, D.; Jacques, H.; Moorjani, S.; Steinke, F.H.; Gagne, C.; Brun, D.; Lupien, P.J. Effects of a soy-protein beverage on plasma lipoproteins in children with familial hypercholesterolemia. Am. J. Clin. Nutr. 1991, 54, 98–103. [Google Scholar] [CrossRef]
- Negele, L.; Schneider, B.; Ristl, R.; Stulnig, T.M.; Willfort-Ehringer, A.; Helk, O.; Widhalm, K. Effect of a low-fat diet enriched either with rapeseed oil or sunflower oil on plasma lipoproteins in children and adolescents with familial hypercholesterolaemia. Results of a pilot study. Eur. J. Clin. Nutr. 2015, 69, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.C.; Pang, J.; Barrett, P.H.; Sullivan, D.R.; Burnett, J.R.; van Bockxmeer, F.M.; Watts, G.F. Omega-3 fatty acid ethyl esters diminish postprandial lipemia in familial hypercholesterolemia. J. Clin. Endocrinol. Metab. 2016, 101, 3732–3739. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, A.; Sutherland, W.; Ball, M. The effect of dietary fat content on plasma noncholesterol sterol concentrations in patients with familial hypercholesterolemia treated with simvastatin. Metabolism 1994, 43, 310–314. [Google Scholar] [CrossRef]
- Fuentes, F.; Lopez-Miranda, J.; Garcia, A.; Perez-Martinez, P.; Moreno, J.; Cofan, M.; Caballero, J.; Paniagua, J.A.; Ros, E.; Perez-Jimenez, F. Basal plasma concentrations of plant sterols can predict LDL-C response to sitosterol in patients with familial hypercholesterolemia. Eur J. Clin. Nutr. 2008, 62, 495–501. [Google Scholar] [CrossRef]
- Ketomaki, A.; Gylling, H.; Miettinen, T.A. Non-cholesterol sterols in serum, lipoproteins, and red cells in statin-treated FH subjects off and on plant stanol and sterol ester spreads. Clin. Chim. Acta 2005, 353, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Wirth, A.; Middelhoff, G.; Braeuning, C.; Schlierf, G. Treatment of familial hypercholesterolemia with a combination of bezafibrate and guar. Atherosclerosis 1982, 45, 291–297. [Google Scholar] [CrossRef]
- Gustafsson, I.B.; Boberg, J.; Karlstrom, B.; Lithell, H.; Vessby, B. Similar serum lipoprotein reductions by lipid-lowering diets with different polyunsaturated:saturated fat values. Br. J. Nutr. 1983, 50, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, B.M.; Giovannetti, P.M. High protein diet complements resin therapy of familial hypercholesterolemia. Clin. Invest. Med. 1992, 15, 349–359. [Google Scholar]
- Austin, M.A.; Hutter, C.M.; Zimmern, R.L.; Humphries, S.E. Familial hypercholesterolemia and coronary heart disease: A HuGE association review. Am. J. Epidemiol. 2004, 160, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Hutter, C.M.; Austin, M.A.; Humphries, S.E. Familial hypercholesterolemia, peripheral arterial disease, and stroke: A HuGE minireview. Am. J. Epidemiol. 2004, 160, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Barkas, F.; Liberopoulos, E.; Kei, A.; Makri, A.; Megapanou, E.; Pantazi, A.; Elisaf, M.; Liamis, G. Clinical application of PCSK9 inhibitors in a specialized lipid clinic. HJM 2018, 120, 229–237. [Google Scholar] [CrossRef]
- Vuorio, A.; Kuoppala, J.; Kovanen, P.T.; Humphries, S.E.; Tonstad, S.; Wiegman, A.; Drogari, E.; Ramaswami, U. Statins for children with familial hypercholesterolemia. Cochrane Database Syst. Rev. 2017, 7, CD006401. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Judd, J.T.; Clevidence, B.A.; Muesing, R.A.; Wittes, J.; Sunkin, M.E.; Podczasy, J.J. Dietary trans fatty acids: Effects on plasma lipids and lipoproteins of healthy men and women. Am. J. Clin. Nutr. 1994, 59, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Ausman, L.M.; Jalbert, S.M.; Schaefer, E.J. Effects of different forms of dietary hydrogenated fats on serum lipoprotein cholesterol levels. N. Engl. J. Med. 1999, 340, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Diamond, D.M.; Alabdulgader, A.A.; de Lorgeril, M.; Harcombe, Z.; Kendrick, M.; Malhotra, A.; O’Neill, B.; Ravnskov, U.; Sultan, S.; Volek, J.S. Dietary recommendations for familial hypercholesterolaemia: An evidence-free zone. BMJ Evid. Based Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, N.; Vinknes, K.J.; Veierod, M.B.; Retterstol, K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2016, 115, 466–479. [Google Scholar] [CrossRef]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of low-fat vs. low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: The DIETFITS randomized clinical trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Broekhuizen, K.; van Poppel, M.N.; Koppes, L.L.; Kindt, I.; Brug, J.; van Mechelen, W. Can multiple lifestyle behaviours be improved in people with familial hypercholesterolemia? Results of a parallel randomised controlled trial. PLoS ONE 2012, 7, e50032. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [Green Version]
- Ursoniu, S.; Sahebkar, A.; Serban, M.C.; Antal, D.; Mikhailidis, D.P.; Cicero, A.; Athyros, V.; Rizzo, M.; Rysz, J.; Banach, M.; et al. Lipid-modifying effects of krill oil in humans: Systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2017, 75, 361–373. [Google Scholar] [CrossRef]
- Pan, A.; Yu, D.; Demark-Wahnefried, W.; Franco, O.H.; Lin, X. Meta-analysis of the effects of flaxseed interventions on blood lipids. Am. J. Clin. Nutr. 2009, 90, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Langlois, M.R.; Langsted, A.; Chapman, M.J.; Aakre, K.M.; Baum, H.; Boren, J.; Bruckert, E.; Catapano, A.; Cobbaert, C.; et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: Consensus-based recommendations from EAS and EFLM. Atherosclerosis 2020, 294, 46–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astrup, A.; Dyerberg, J.; Elwood, P.; Hermansen, K.; Hu, F.B.; Jakobsen, M.U.; Kok, F.J.; Krauss, R.M.; Lecerf, J.M.; LeGrand, P.; et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: Where does the evidence stand in 2010? Am. J. Clin. Nutr. 2011, 93, 684–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.; Duchateau, G.S.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Rideout, T.C.; Chan, Y.M.; Harding, S.V.; Jones, P.J. Low and moderate-fat plant sterol fortified soymilk in modulation of plasma lipids and cholesterol kinetics in subjects with normal to high cholesterol concentrations: Report on two randomized crossover studies. Lipids Health Dis. 2009, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Vuorio, A.; Kovanen, P.T. Decreasing the Cholesterol Burden in Heterozygous Familial Hypercholesterolemia Children by Dietary Plant Stanol Esters. Nutrients 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.A.; Blanco Mejia, S.; Chiavaroli, L.; Viguiliouk, E.; Li, S.S.; Kendall, C.W.C.; Vuksan, V.; Sievenpiper, J.L. Cumulative meta-analysis of the soy effect over time. J. Am. Heart Assoc. 2019, 8, e012458. [Google Scholar] [CrossRef] [Green Version]
- Jovanovski, E.; Yashpal, S.; Komishon, A.; Zurbau, A.; Blanco Mejia, S.; Ho, H.V.T.; Li, D.; Sievenpiper, J.; Duvnjak, L.; Vuksan, V. Effect of psyllium (Plantago ovata) fiber on LDL cholesterol and alternative lipid targets, non-HDL cholesterol and apolipoprotein B: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2018, 108, 922–932. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Poustie, V.J.; Rutherford, P. Dietary treatment for familial hypercholesterolaemia. Cochrane Database Syst. Rev. 2001. [Google Scholar] [CrossRef]
- Shafiq, N.; Singh, M.; Kaur, S.; Khosla, P.; Malhotra, S. Dietary treatment for familial hypercholesterolaemia. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Malhotra, A.; Shafiq, N.; Arora, A.; Singh, M.; Kumar, R.; Malhotra, S. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Engler, M.M.; Engler, M.B.; Malloy, M.; Chiu, E.; Besio, D.; Paul, S.; Stuehlinger, M.; Morrow, J.; Ridker, P.; Rifai, N.; et al. Docosahexaenoic acid restores endothelial function in children with hyperlipidemia: Results from the EARLY study. Int J. Clin. Pharmacol. Ther. 2004, 42, 672–679. [Google Scholar] [CrossRef]
- Nigon, F.; Serfaty-Lacrosniere, C.; Beucler, I.; Chauvois, D.; Neveu, C.; Giral, P.; Chapman, M.J.; Bruckert, E. Plant sterol-enriched margarine lowers plasma LDL in hyperlipidemic subjects with low cholesterol intake: Effect of fibrate treatment. Clin. Chem. Lab. Med. 2001, 39, 634–640. [Google Scholar] [CrossRef]
- Ketomaki, A.; Gylling, H.; Miettinen, T.A. Removal of intravenous Intralipid in patients with familial hypercholesterolemia during inhibition of cholesterol absorption and synthesis. Clin. Chim. Acta 2004, 344, 83–93. [Google Scholar] [CrossRef]








| Trial | Study Design (Duration) | Participants | Interventions |
|---|---|---|---|
| Amundsen 2002 [10] | Double-blind, placebo-controlled randomized, cross-over (8w) | 41 children with FH (aged 10.5 ± 1.7 yrs old, mean BMI 18.9 kg/m2) | Low-fat/low-cholesterol diet and 1.60 ± 0.13 g plant sterols in a fortified spread (18.2 ± 1.5 g/d) vs. low-fat/low-cholesterol diet and placebo |
| Balestrieri 1996 [11] | Double-blind, randomized, cross-over (4w) | 16 adults with FH treated with simvastatin (aged 45.2 ± 15 yrs old) | Cholesterol-lowering diet and 6 g/d fish oil ethyl ester vs. cholesterol-lowering diet and placebo (olive oil) |
| Chan 2016 [20] | Open-label, placebo-controlled randomized, cross-over (8w) | 22 adults with FH taking lipid-lowering therapy (aged 53.3 ± 3 yrs old, mean BMI 27 ± 1.4 kg/m2) | 4 g/d omega-3 fatty acid ethyl ester (46% eicosapentaenoic acid and 38% docosahexaenoic acid) vs. placebo |
| Chisholm 1994 [21] | Randomized, cross-over (8w) | 19 adults with FH treated with simvastatin (aged 51 ± 10 yrs old, mean BMI 28.7 ± 1.2 kg/m2) | Low-fat/low-cholesterol diet vs. a higher-fat/higher-cholesterol diet |
| De Jongh 2003 [12] | Double-blind, placebo-controlled randomized, cross-over (4w) | 41 children with FH (aged 9.2 ± 1.6 yrs old, mean BMI 17.7 kg/m2) and 20 controls (aged 8.2 ± 2.2 yrs old, mean BMI 17.5 kg/m2) | Low-fat/low-cholesterol diet and 2.3 g plant sterols in a fortified spread (15 g/d) vs. low-fat/low-cholesterol diet and placebo |
| Fuentes 2008 [22] | Randomized, cross-over (4w) | 30 adults with FH taking lipid-lowering therapy (aged 42 ± 18 yrs old, mean BMI 26.5 ± 3.7 kg/m2) | 4 low-fat diets with different content of cholesterol (<150 or 300 mg/d) and sitosterol (<1 or 2 g/d) |
| Gustafsson 1983 [25] | Randomized, cross-over (3w) | 20 hyperlipoproteinemic adults: 6 with type IIa (aged 30–60 yrs old), 8 with type IIb (aged 41–65 yrs old) and 6 with type IV hyperlipoproteinemia (aged 51–66 yrs old) | 2 low-cholesterol diets differing in polyunsaturated:saturated fat ratio (2.0 vs. 1.3) |
| Gylling 1995 [16] | Double-blind, placebo-controlled randomized, cross-over (6w) | 14 children with heterozygous FH (aged 9.1 ± 1.1 yrs old, mean BMI 17.7 ± 0.9 kg/m2) | Low-fat/low-cholesterol diet and 3 g sitostanol ester dissolved in rapeseed oil margarine vs. low-fat/low-cholesterol diet and placebo |
| Hande 2019 [13] | Double-blind, placebo-controlled randomized, cross-over (3m) | 34 patients with FH on lipid-lowering treatment (aged 46.6 (18–71) yrs old, mean BMI 27.6 ± 5 kg/m2) | 4 g/d omega-3 fatty acids in a 1000 mg capsule consisting of 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid (administered twice a day) vs. placebo (capsules with olive oil) |
| Helk 2019 [17] | Placebo-controlled randomized (13w) | 26 children with FH (aged 8.7 ± 3.8 yrs old, mean BMI 16.3 ± 3.1 kg/m2) | Diet high in unsaturated fats, low in saturated fats and enriched with soy-protein vs. diet high in unsaturated fats and low in saturated fats |
| Jakulj 2006 [14] | Double-blind, placebo-controlled randomized, cross-over (4w) | 42 children with FH (aged 9.8 ± 1.5 yrs old, mean BMI 17.7 ± 2.8 kg/m2) | Low-fat/low-cholesterol diet and 2 g plant stanols in a low-fat fortified yogurt (500 mL/d) vs. low-fat/low-cholesterol diet and placebo |
| Ketomaki 2005 [23] | Double-blind randomized, cross-over (4w) | 18 adults with FH taking lipid-lowering therapy (aged 48 ± 2 yrs old) | Low-fat diet and 2 g plant stanols (25 g spread/d) vs. low-fat diet and 2 g plant sterols (25 g spread/d) |
| Laurin 1991 [18] | Randomized, cross-over (4w) | 10 children with FH (aged 8 ± 1 yrs old, mean BMI 16.7 ± 0.9 kg/m2) | 2 different Low-fat/low-cholesterol/high-protein diets: about one-third (35%) of the protein energy was consumed as a dairy source, either from cow milk or a soy beverage |
| Negele 2015 [19] | Double-blind, randomized pilot trial (13w) | 21 children with FH (aged 11.1 ± 3.4 yrs old, mean BMI: 19.1 ± 3.5 kg/m2) | Low-fat/low-cholesterol diet and monounsaturated fatty acids by rapeseed oil vs. low-fat/low-cholesterol diet and polyunsaturated fatty acids by sunflower oil |
| Neil 2001 [15] | Double-blind, placebo-controlled randomized, cross-over (8w) | 62 adults with heterozygous FH (30 were statin-treated) (aged 51.6 (33.3–62.3) yrs old, mean BMI 25.9 ± 3.5 kg/m2) | Low-cholesterol diet and 2.5 g plant sterols in a fortified spread (25 g/d) vs. low-cholesterol diet and placebo |
| Wirth 1982 [24] | Randomized cross-over (2m) | 12 adults with FH treated with fibrate (aged 51.7 (31–60) yrs old, mean BMI 26.6 kg/m2) | Bezafibrate vs. bezafibrate and 5.2 g guar |
| Wolfe 1992 [26] | Randomized, cross-over (4-5w) | 10 adults with familial hypercholesterolemia (2 of those had possibly FCH) (aged 50 ± 5 yrs old, with mean BMI 24.4 kg/m2) | Low-fat/low-cholesterol/high-protein (23%) diet vs. low-fat/low-cholesterol/low-protein (11%) diet |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkas, F.; Nomikos, T.; Liberopoulos, E.; Panagiotakos, D. Diet and Cardiovascular Disease Risk Among Individuals with Familial Hypercholesterolemia: Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2436. https://doi.org/10.3390/nu12082436
Barkas F, Nomikos T, Liberopoulos E, Panagiotakos D. Diet and Cardiovascular Disease Risk Among Individuals with Familial Hypercholesterolemia: Systematic Review and Meta-Analysis. Nutrients. 2020; 12(8):2436. https://doi.org/10.3390/nu12082436
Chicago/Turabian StyleBarkas, Fotios, Tzortzis Nomikos, Evangelos Liberopoulos, and Demosthenes Panagiotakos. 2020. "Diet and Cardiovascular Disease Risk Among Individuals with Familial Hypercholesterolemia: Systematic Review and Meta-Analysis" Nutrients 12, no. 8: 2436. https://doi.org/10.3390/nu12082436






