Effect of Supplementation with Saccharomyces Boulardii on Academic Examination Performance and Related Stress in Healthy Medical Students: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
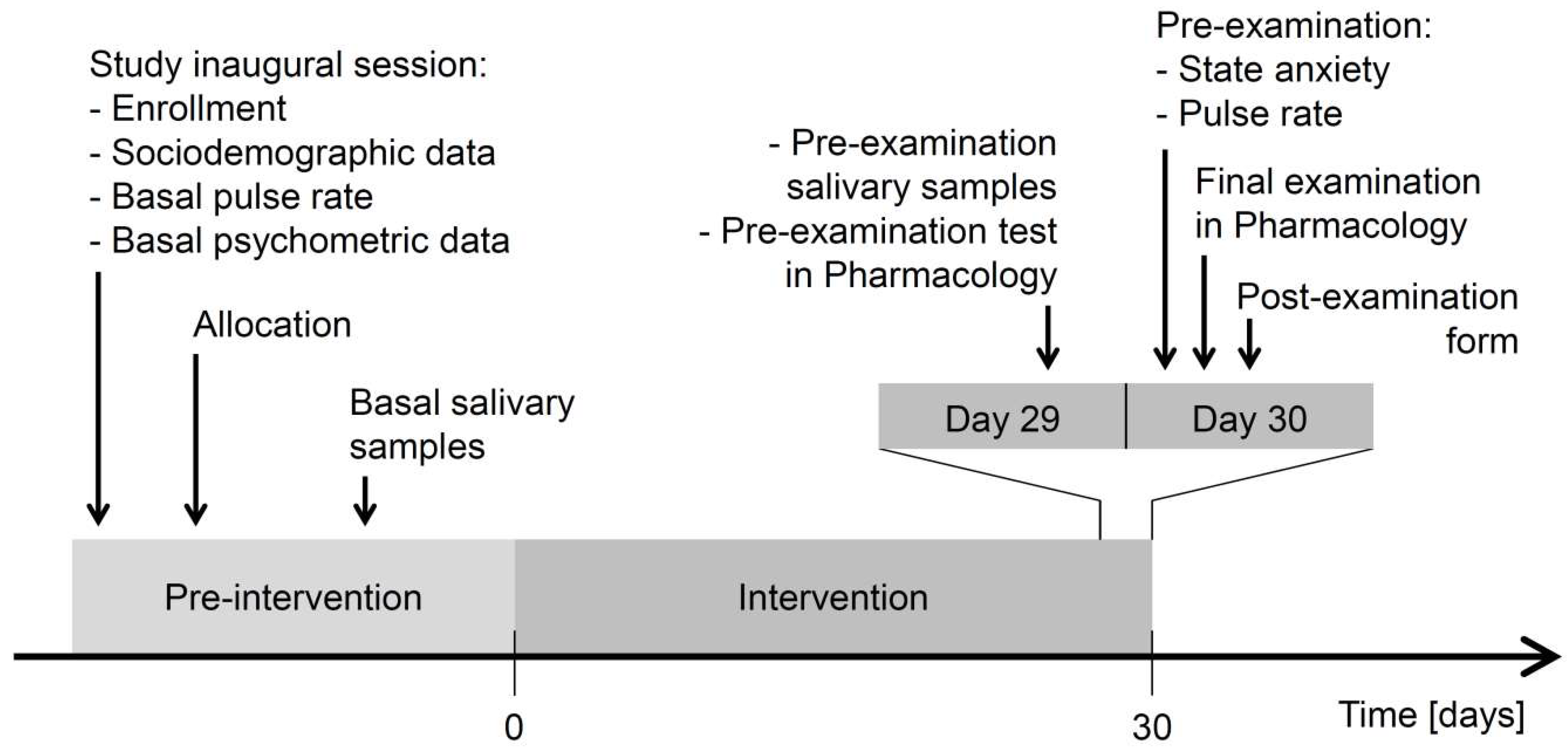
2.1. Study Design
2.2. Ethical Considerations
2.3. Participants
- being a third-year medical student of the Faculty of Medicine or Faculty of Military Medicine, Medical University of Lodz, Poland
- age 18–30 years
- formal inability to sit the first attempt of the final examination of Pharmacology
- chronic diseases: neurological, psychiatric, cardiological, gastroenterological, immunological, endocrine, or infectious
- state of immunosuppression
- history of hospitalization (up to three months before entrance to the study)
- presence of central venous catheter
- parenteral nutrition
- current pregnancy or intention to become pregnant within three months from the entrance to the study
- current lactation
- allergic reaction (up to three months before entrance to the study)
- hypersensitivity to yeast, maltodextrins, potato starch, magnesium stearate, hypromellose, gelatin, glycerol, or titanium dioxide
- body mass index over 30
- chronic medication use (up to three months before entrance to the study; “chronic” was defined from a frequency perspective as “at least 90 days a year on average”; pharmacological contraceptives were not considered “medication” and were allowed in the study)
- systemic antibacterial or antifungal medication use (up to three months before entrance to the study)
- overuse of alcohol (defined according to the Polish standards [25] as ≥20 g and ≥40 g per day for females and males, respectively) or psychoactive substances (up to three months before entrance to the study)
- tobacco smoking—more than 5 cigarettes (or equivalents) a day (up to three months before entrance to the study)
- pro- or prebiotic preparations intake (up to three months before entrance to the study)
- vegan or other atypical diet
- doing professional or extreme sports
2.4. Intervention
- LacidoEnter capsules (Institut Rosell, Montreal, Canada; batch numbers HG09241 and HI17731; expiry date 01/2017 and 03/2017, respectively) containing lyophilized Saccharomyces boulardii CNCM I-1079 in a declared amount of 5 × 109 CFU per dose,
- Dicoflor 60 capsules (Dicofarm, Rome, Italy; batch numbers SM513 and SM514; expiry date of both batches 03/2017) containing Lactobacillus rhamnosus GG (ATCC 53103) in a declared amount of 6 × 109 CFU per dose.
2.5. Outcomes
2.6. Procedure
2.7. Determination of Salivary Cortisol and Metanephrine
2.8. Data Analysis
3. Results
3.1. Flow of the Participants
3.2. Basal Characteristics of the Participants and Dropouts
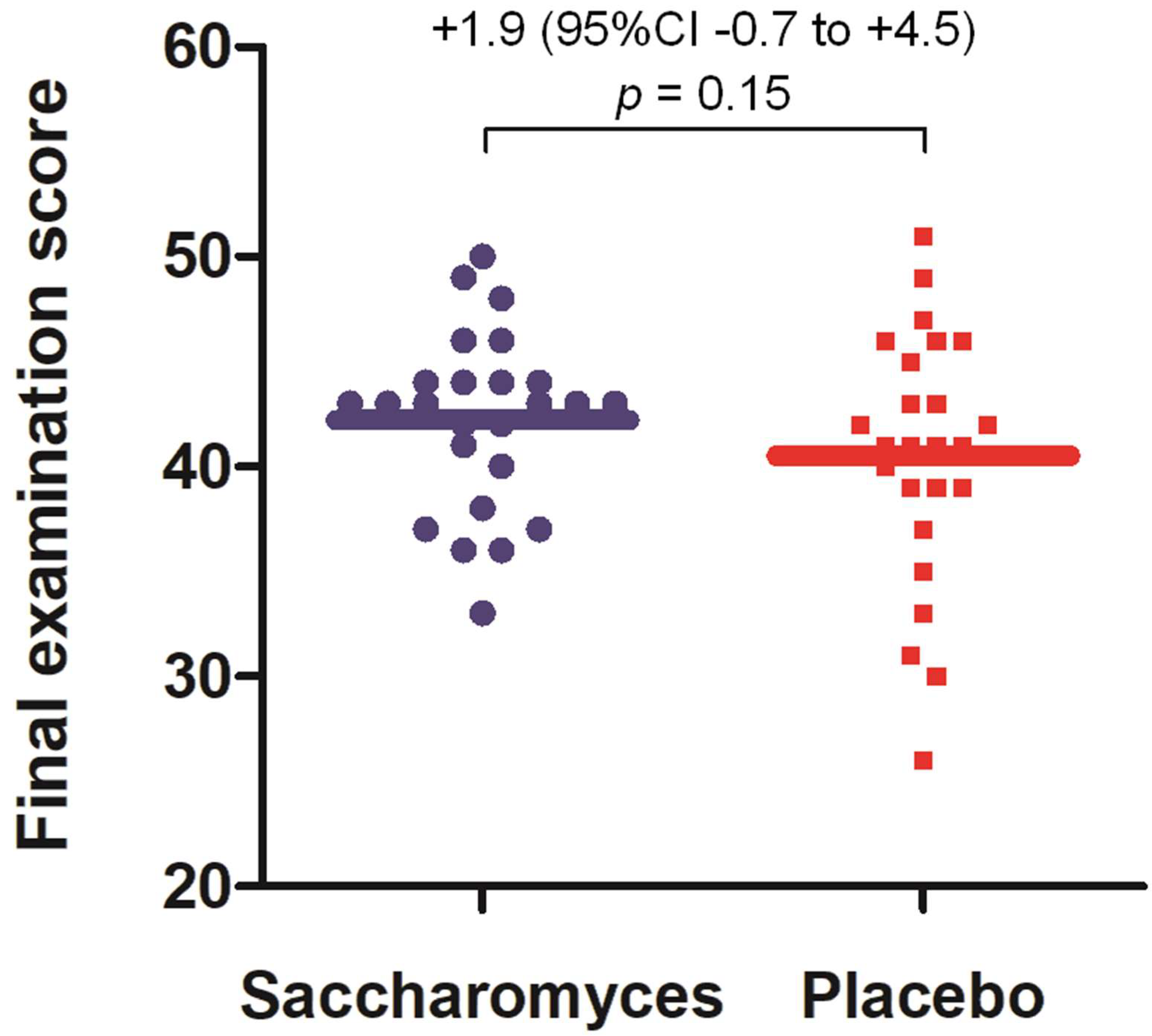
3.3. Performance in Academic Examination
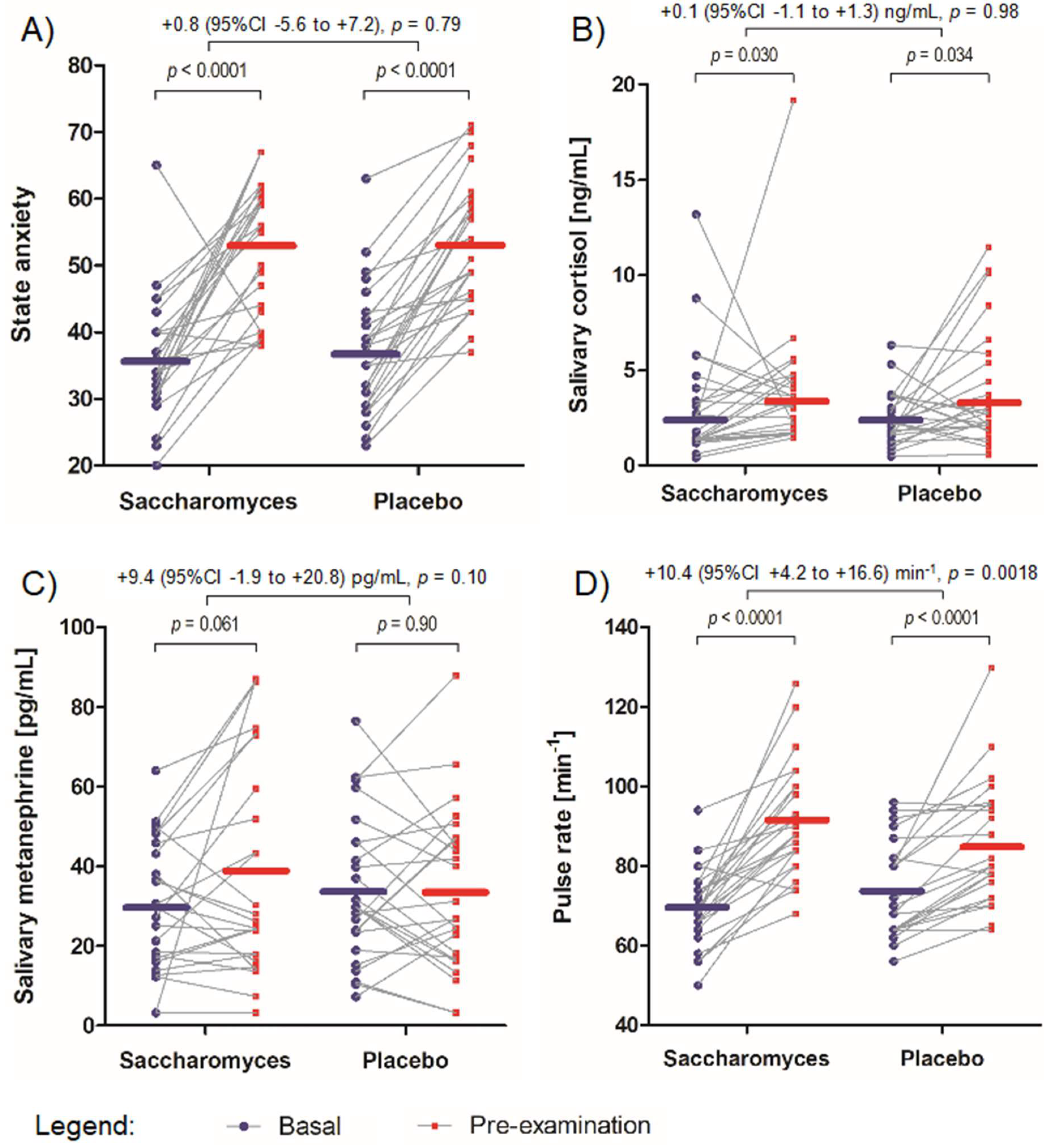
3.4. State Anxiety
3.5. Salivary Cortisol
3.6. Salivary Metanephrine
3.7. Pulse Rate
3.8. Ancillary Analyses
3.9. Harms
- cold—experienced by 11 participants (14%) for the median number of days of 3 (1st–3rd quartile: 2–5) in 30-day follow-up; no significant difference in the number of days with cold were reported between the study groups (Kruskal–Wallis H test, χ2(2) = 0.82, p = 0.66);
- fever—experienced by two participants (3%) for the median number of days of 1.5 (1st–3rd quartile: 1–2) in 30-day follow-up; both cases were in the Lactobacillus group; however, the difference in the number of days with cold were found statistically insignificant between the study groups (Kruskal–Wallis H test, χ2(2) = 3.97, p = 0.14);
- gastrointestinal symptoms—experienced by 22 participants (29%) for the median number of days of 2 (1st–3rd quartile: 1–3) in 30-day follow-up; no significant difference in the number of days with cold were reported between the study groups (Kruskal–Wallis H test, χ2(2) = 0.13, p = 0.94).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fortino, A.; Lowrance, R. Practice Makes Perfect: Memory Retrieval Strategies to Improve Student Academic Performance. Acad. Manag. 2019, 2019. [Google Scholar] [CrossRef]
- Kuncel, N.R.; Hezlett, S.A.; Ones, D.S. Academic Performance, Career Potential, Creativity, and Job Performance: Can One Construct Predict Them All? J. Pers. Soc. Psychol. 2004, 86, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.W.; Tranel, D.; Adolphs, R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn. Mem. 2006, 13, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kar, M. Evaluation of Examination Stress and Its Effect on Cognitive Function among First Year Medical Students. J. Clin. Diagn. Res. 2014, 8, BC05–BC07. [Google Scholar] [CrossRef]
- Dyrbye, L.N.; Thomas, M.R.; Shanafelt, T.D. Medical Student Distress: Causes, Consequences, and Proposed Solutions. Mayo Clin. Proc. 2005, 80, 1613–1622. [Google Scholar] [CrossRef]
- Kötter, T.; Wagner, J.; Brüheim, L.; Voltmer, E. Perceived Medical School stress of undergraduate medical students predicts academic performance: An observational study. BMC Med Educ. 2017, 17, 256. [Google Scholar] [CrossRef]
- Melaku, L.; Mossie, A.; Negash, A. Stress among Medical Students and Its Association with Substance Use and Academic Performance. J. Biomed. Educ. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Hill, M.R.; Goicochea, S.; Merlo, L.J. In their own words: Stressors facing medical students in the millennial generation. Med Educ. Online 2018, 23, 1530558. [Google Scholar] [CrossRef] [PubMed]
- Oaten, M.; Cheng, K. Academic Examination Stress Impairs Self–Control. J. Soc. Clin. Psychol. 2005, 24, 254–279. [Google Scholar] [CrossRef]
- Barry, V.; E Stout, M.; Lynch, M.E.; Mattis, S.; Tran, D.Q.; Antun, A.; Ribeiro, M.J.; Stein, S.F.; Kempton, C.L. The effect of psychological distress on health outcomes: A systematic review and meta-analysis of prospective studies. J. Health Psychol. 2019, 25, 227–239. [Google Scholar] [CrossRef]
- Leblanc, V.R. The Effects of Acute Stress on Performance: Implications for Health Professions Education. Acad. Med. 2009, 84, S25–S33. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ning, H.; Yang, L.; Jia, C.; Yang, F.; Xu, G.; Tan, H. Efficacy of Probiotics on Anxiety: A Meta-analysis of Randomized Controlled Trials. Neuropsychiatry 2017, 7, 862–871. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes 2016, 7, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. ProbioticLactobacillus caseistrain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Gondo, Y.; Kikuchi-Hayakawa, H.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Kuwano, Y.; Miyazaki, K.; et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: A double-blind, randomised, placebo-controlled trial. Benef. Microbes 2017, 8, 153–162. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Sugawara, T.; Aoki, Y.; Fujiwara, S.; Rokutan, K. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods 2017, 36, 112–121. [Google Scholar] [CrossRef]
- Papalini, S.; Michels, F.; Kohn, N.; Wegman, J.; Van Hemert, S.; Roelofs, K.; Vasquez, A.A.; Aarts, E. Stress matters: Randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol. Stress 2018, 10, 100141. [Google Scholar] [CrossRef]
- Hindal, H.; Reid, N.; Badgaish, M. Working memory, performance and learner characteristics. Res. Sci. Technol. Educ. 2009, 27, 187–204. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Haberer, P.; Geisen, R.; Bjorkroth, J.; Schillinger, U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001, 73 (Suppl. 2), 365s–373s. [Google Scholar] [CrossRef]
- McFarland, L.V. Systematic review and meta-analysis ofSaccharomyces boulardiiin adult patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, R.; Labrie, S.; Fliss, I. Antimicrobial and Probiotic Properties of Yeasts: From Fundamental to Novel Applications. Front. Microbiol. 2012, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Biology of Eukaryotic Probiotics. In Probiotics; Springer: Berlin/Heidelberg, Germany, 2011; Volume 21, pp. 29–55. [Google Scholar]
- Schulz, K.F.; Altman, U.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Kalinowski, A.; Humphreys, K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction 2016, 111, 1293–1298. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Reid, G.; Beuerman, D.; Heinemann, C.; Bruce, A.W. ProbioticLactobacillusdose required to restore and maintain a normal vaginal flora. FEMS Immunol. Med Microbiol. 2001, 32, 37–41. [Google Scholar] [CrossRef]
- Saxelin, M. Effective Dose of Lactobacillus GG. Rapid Response to: Effect of Long Term Consumption of Probiotic Milk on Infections in Children Attending Day Care Centres: Double Blind, Randomised Trial. BMJ 2001, 322, 1327. [Google Scholar] [CrossRef]
- Bozovic, D.; Racic, M.; Ivkovic, N. Salivary cortisol levels as a biological marker of stress reaction. Med. Arch. 2013, 67, 374. [Google Scholar] [CrossRef]
- Kantorovich, V.; Eisenhofer, G.; Pacak, K. Pheochromocytoma: An Endocrine Stress Mimicking Disorder. Ann. N. Y. Acad. Sci. 2008, 1148, 462–468. [Google Scholar] [CrossRef]
- Woods, D.R.; O’Hara, J.P.; Boos, C.J.; Hodkinson, P.D.; Tsakirides, C.; Hill, N.E.; Jose, D.; Hawkins, A.; Phillipson, K.; Hazlerigg, A.; et al. Markers of physiological stress during exercise under conditions of normoxia, normobaric hypoxia, hypobaric hypoxia, and genuine high altitude. Eur. J. Appl. Physiol. 2017, 117, 893–900. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Kopin, I.; Goldstein, D.S. Catecholamine Metabolism: A Contemporary View with Implications for Physiology and Medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J. Stress and the adrenocortical control of epinephrine synthesis. Metab. Clin. Exp. 2002, 51, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Raffesberg, W.; Raber, W.; Bernroider, E.; Niederle, B.; Waldhäusl, W.; Gasic, S. Quantification of Unconjugated Metanephrines in Human Plasma without Interference by Acetaminophen. Clin. Chem. 2001, 47, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Osinga, T.E.; Van Der Horst-Schrivers, A.N.; Van Faassen, M.; Kerstens, M.N.; Dullaart, R.P.; Pacak, K.; Links, T.P.; Kema, I. Mass spectrometric quantification of salivary metanephrines-A study in healthy subjects. Clin. Biochem. 2016, 49, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Stefanescu, A.-M. Salivary Free Catecholamines Metabolites as Possbile Biochemical Markers in Pheochromocytoma Diagnosis. Acta Endocrinol. 2011, 7, 431–439. [Google Scholar] [CrossRef]
- Garner, D.M.; Garfinkel, P.E. The Eating Attitudes Test: An index of the symptoms of anorexia nervosa. Psychol. Med. 1979, 9, 273–279. [Google Scholar] [CrossRef]
- Włodarczyk-Bisaga, K.; Dolan, B. A two-stage epidemiological study of abnormal eating attitudes and their prospective risk factors in Polish schoolgirls. Psychol. Med. 1996, 26, 1021–1032. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561. [Google Scholar] [CrossRef]
- Parnowski, T.; Jernajczyk, W. Inwentarz Depresji Becka w Ocenie Nastroju Osób Zdrowych i Chorych Na Choroby Afektywne (Ocena Pilotażowa). Psychiatr. Pol. 1977, 11, 417–425. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R.; Mermelstein, T.K. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385. [Google Scholar] [CrossRef]
- Juczyński, Z.; Ogińska-Bulik, N. PSS-10—Skala Odczuwanego Stresu | Pracownia Testów Psychologicznych. Available online: https://www.practest.com.pl/pss-10-skala-odczuwanego-stresu (accessed on 24 March 2020).
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Test manual for the State-Trait Anxiety Inventory; Consulting Psychologist Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Spielberger, C.D.; Strelau, J.; Tysarczyk, M.; Wrześniewski, K. STAI—Inwentarz Stanu i Cechy Lęku STAI | Pracownia Testów Psychologicznych. Available online: https://www.practest.com.pl/stai-inwentarz-stanu-i-cechy-leku-stai (accessed on 24 March 2020).
- Keizer, R.J.; Jansen, R.S.; Rosing, H.; Thijssen, B.; Beijnen, J.H.; Schellens, J.H.M.; Huitema, A.D.R. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol. Res. Perspect. 2015, 3, e00131. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, U.; Perret-Liaudet, A.; Doorn, L.J.C.V.W.V.; Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; Genc, S.; Kruse, N.; Kuiperij, H.B.; et al. A Practical Guide to Immunoassay Method Validation. Front. Neurol. 2015, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Jaedicke, K.; Taylor, J.; Preshaw, P. Validation and quality control of ELISAs for the use with human saliva samples. J. Immunol. Methods 2012, 377, 62–65. [Google Scholar] [CrossRef] [PubMed]
- White, I.R.; Horton, N.J.; Carpenter, J.; Statistics, R.I.M.A.S.; Pocock, S.J. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011, 342, d40. [Google Scholar] [CrossRef] [PubMed]
- Stowell, J. Use and Abuse of Academic Examinations in Stress Research. Psychosom. Med. 2003, 65, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Kuder, G.F.; Richardson, M.W. The theory of the estimation of test reliability. Psychometrika 1937, 2, 151–160. [Google Scholar] [CrossRef]
- Liu, B.; He, Y.; Wang, M.; Liu, J.; Ju, Y.; Zhang, Y.; Liu, T.; Li, L.; Li, Q. Efficacy of probiotics on anxiety-A meta-analysis of randomized controlled trials. Depress. Anxiety 2018, 35, 935–945. [Google Scholar] [CrossRef]
- Reis, D.J.; Ilardi, S.S.; Punt, S.E.W. The anxiolytic effect of probiotics: A systematic review and meta-analysis of the clinical and preclinical literature. PLoS ONE 2018, 13, e0199041. [Google Scholar] [CrossRef]
- Romijn, A.R.; Rucklidge, J.J. Systematic review of evidence to support the theory of psychobiotics. Nutr. Rev. 2015, 73, 675–693. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Berntson, G.G.; Malarkey, W.B.; Kiecolt-Glaser, J.K.; Sheridan, J.F.; Poehlmann, K.M.; Burleson, M.H.; Ernst, J.M.; Hawkley, L.C.; Glaser, R. Autonomic, Neuroendocrine, and Immune Responses to Psychological Stress: The Reactivity Hypothesisa. Ann. N. Y. Acad. Sci. 1998, 840, 664–673. [Google Scholar] [CrossRef]
- Rincón-Cortés, M.; Herman, J.P.; Lupien, S.; Maguire, J.L.; Shansky, R.M. Stress: Influence of sex, reproductive status and gender. Neurobiol. Stress 2019, 10, 100155. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.D.N.; Wanner, S.; Morais, E.S.S.; Hudson, A.S.R.; Martins, F.; Cardoso, V.N. Supplementation with Saccharomyces boulardii Increases the Maximal Oxygen Consumption and Maximal Aerobic Speed Attained by Rats Subjected to an Incremental-Speed Exercise. Nutrients 2019, 11, 2352. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Loomis, C.W.; Bieger, D. Effects of vagal cooling on esophageal cardiovascular reflex responses in the rat. Neurosci. Lett. 2000, 287, 89–92. [Google Scholar] [CrossRef]
- Pothoulakis, C. Review article: Anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2008, 30, 826–833. [Google Scholar] [CrossRef]
- Papaioannou, V.; Pneumatikos, I.; Maglaveras, N. Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: Current strengths and limitations. Front. Physiol. 2013, 4, 4. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Ritter, J.M.; Flower, R.J.; Henderson, G.; Loke, Y.K.; MacEwan, D.; Rang, H.P. Rang & Dale’s Pharmacology, 9th ed.; Elsevier: Edinburgh, Scotland, 2019. [Google Scholar]
- Walker, C.E. Effects of Catecholamines on Gut Microflora and Potential for Beta- Adrenergic Agonists to Impact Ruminal Fermentation. Open Agric. J. 2012, 6, 57–66. [Google Scholar] [CrossRef]
- West, C.; Stanisz, A.M.; Wong, A.; A Kunze, W. Effects ofSaccharomyces cerevisiaeorboulardiiyeasts on acute stress induced intestinal dysmotility. World J. Gastroenterol. 2016, 22, 10532–10544. [Google Scholar] [CrossRef]
- Mazurak, N.; Seredyuk, N.; Sauer, H.; Teufel, M.; Enck, P. Heart rate variability in the irritable bowel syndrome: A review of the literature. Neurogastroenterol. Motil. 2012, 24, 206–216. [Google Scholar] [CrossRef]
- Nieuwenhuys, A.; Oudejans, R.R.D. Anxiety and perceptual-motor performance: Toward an integrated model of concepts, mechanisms, and processes. Psychol. Res. 2011, 76, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Sänger, J.; Bechtold, L.; Schoofs, D.; Blaszkewicz, M.; Wascher, E. The influence of acute stress on attention mechanisms and its electrophysiological correlates. Front. Behav. Neurosci. 2014, 8, 8. [Google Scholar] [CrossRef]
- Cook, S.; Togni, M.; Schaub, M.C.; Wenaweser, P.; Hess, O.M. High heart rate: A cardiovascular risk factor? Eur. Hear. J. 2006, 27, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Hanes, D.A.; Schafer, M.B.; Mikolai, J.; Zwickey, H. Effect of the ProbioticSaccharomyces boulardiion Cholesterol and Lipoprotein Particles in Hypercholesterolemic Adults: A Single-Arm, Open-Label Pilot Study. J. Altern. Complement. Med. 2015, 21, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Moré, M.I.; Swidsinski, A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis—A review. Clin. Exp. Gastroenterol. 2015, 8, 237–255. [Google Scholar] [CrossRef]
- Brussow, H. Probiotics and prebiotics in clinical tests: An update. F1000Research 2019, 8, 1157. [Google Scholar] [CrossRef]
- Gerreth, K.; Chlapowska, J.; Lewicka-Panczak, K.; Sniatala, R.; Ekkert, M.; Borysewicz-Lewicka, M. Self-Evaluation of Anxiety in Dental Students. BioMed Res. Int. 2019, 2019, 6436750. [Google Scholar] [CrossRef]
- Silva, V.; Costa, M.J.; Pereira, I.; Faria, R.; Salgueira, A.; Costa, M.J.; Sousa, N.; Cerqueira, J.J.; Morgado, P. Depression in medical students: Insights from a longitudinal study. BMC Med Educ. 2017, 17, 184. [Google Scholar] [CrossRef]
- Pagnin, D.; De Queiroz, V. Comparison of quality of life between medical students and young general populations. Educ. Health 2015, 28, 209. [Google Scholar] [CrossRef]
- Kaczmarek, E. How to distinguish medicalization from over-medicalization? Med. Health Care Philos. 2018, 22, 119–128. [Google Scholar] [CrossRef]
- Wager, E.; Williams, P. “Hardly worth the effort”? Medical journals’ policies and their editors’ and publishers’ views on trial registration and publication bias: Quantitative and qualitative study. BMJ 2013, 347, 347. [Google Scholar] [CrossRef] [PubMed]
- Office for Registration of Medicinal Products, Medical Devices and Biocidal Products. Rejestracja nowego badania klinicznego produktu leczniczego | Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych. Available online: http://www.urpl.gov.pl/pl/produkty-lecznicze/badania-kliniczne/rejestracja-nowego-badania-klinicznego-produktu-leczniczego (accessed on 24 March 2020).
- De Angelis, C.; Drazen, J.M.; A Frizelle, F.; Haug, C.; Hoey, J.; Horton, R.; Kotzin, S.; Laine, C.; Marusic, A.; Overbeke, A.J.P.; et al. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. Lancet 2004, 364, 911–912. [Google Scholar] [CrossRef]

| Variable | Arm of the Trial | Test Statistics and p-Values for the Comparison of | |||
|---|---|---|---|---|---|
| Saccharomyces (n = 31) | Lactobacillus (n = 32) | Placebo (n = 29) | Saccharomyces vs. Placebo | Lactobacillus vs. Placebo | |
| Sex | |||||
| Male | 15 (48%) | 14 (44%) | 8 (28%) | χ2(1) = 2.74, p = 0.098a | χ2(1) = 1.72, p = 0.19a |
| Female | 16 (52%) | 18 (56%) | 21 (72%) | ||
| Age | |||||
| (years) | 22.7 (1.4) | 22.5 (1.1) | 22.8 (1.4) | t(58) = −0.22, p = 0.83b | t(59) = −0.90, p = 0.37b |
| Body mass index | |||||
| (kg × m−2) | 22.4 (2.6) | 22.1 (2.3) | 22.2 (2.5) | t(58) = 0.22, p = 0.83b | t(59) = −0.15, p = 0.88b |
| Smoking status | |||||
| Not at all | 29 (94%) | 29 (91%) | 26 (90%) | p = 0.67c | p = 1.0c |
| Max. 5 cigarettes a day | 2 (6%) | 3 (9%) | 3 (10%) | ||
| Faculty | |||||
| of Medicine | 22 (71%) | 21 (66%) | 20 (69%) | χ2(1) = 0.03, p = 0.87a | χ2(1) = 0.08, p = 0.78a |
| of Military Medicine | 9 (29%) | 11 (34%) | 9 (31%) | ||
| Perceived health statusd,e | |||||
| From “very bad” (1) to “very good” (5) | 4 (4–5) | 4 (4–5) | 4 (4–4) | Z = 1.81, p = 0.070f | Z = 1.24, p = 0.21f |
| Eating healthy dietd,e | |||||
| From “definitely not” (1) to “definitely yes” (5) | 4 (3–4) | 4 (3–4) | 4 (3–4) | Z = 0.70, p = 0.49f | Z = 0.38, p = 0.70f |
| Consumption of fermented productsd,e | |||||
| From “very rarely” (1) to “very often” (5) | 3 (2–4) | 3 (3–4) | 3 (3–4) | Z = −0.91, p = 0.36f | Z = −0.38, p = 0.71f |
| Perceived economic statusd,e | |||||
| From “very low” (1) to “very high” (5) | 4 (4–5) | 4 (4–4.5) | 4 (4–5) | Z = 0.18, p = 0.85f | Z = 0.09, p = 0.93f |
| Psychometricse | |||||
| EAT-26 | 8 (3–13) | 7.5 (4.5–14) | 9 (4–13) | Z = −0.19, p = 0.85f | Z = 0.06, p = 0.95f |
| BDI | 6 (3–12) | 5 (1.5–9) | 8 (2–11.5)g | Z = −0.09, p = 0.98f | Z = −1,03, p = 0.30f |
| PSS-10 | 16 (10–25) | 14 (11.5–20) | 16 (12–23) | Z = 0.16, p = 0.88f | Z = −0.69, p = 0.49f |
| STAI trait | 42 (36–49) | 40 (33.5–46.5) | 40 (33–47) | Z = 0.72, p = 0.47f | Z = −0.35, p = 0.72f |
| “Basal” outcome measures | |||||
| STAI statee | 34 (30–40) | 33 (27.5–40.5) | 37 (31–43) | Z = −0.71, p = 0.48f | Z = −1.29, p = 0.20f |
| Salivary cortisol (ng/mL)e | 1.71 (1.32–3.40) | 2.58 (1.63–3.71) | 2.36 (1.69–3.68) | Z = −1.24, p = 0.21f | Z = 0.31, p = 0.76f |
| Salivary metanephrine (pg/mL) | 29.6 (15.6) | 35.4 (17.5) | 31.3 (18.2) | t(58) = −0.39, p = 0.70b | t(59) = 0.89, p = 0.38b |
| Pulse rate (min−1) | 68.8 (9.5) | 70.0 (8.9) | 73.8 (11.4) | t(58) = −1.85, p = 0.070b | t(59) = −1.43, p = 0.16b |
| Increase in State Anxiety | Increase in Salivary Cortisol * | Increase in Salivary MN | Increase in Pulse Rate * | |
|---|---|---|---|---|
| Examination score | 0.10 p = 0.47 | 0.24 p = 0.087 | 0.09 p = 0.53 | 0.22 p = 0.12 |
| Increase in state anxiety | 0.14 p = 0.35 | 0.02 p = 0.91 | 0.29 p = 0.040 | |
| Increase in salivary cortisol | 0.24 p = 0.091 | −0.10 p = 0.48 | ||
| Increase in salivary MN | 0.35 p = 0.012 |
| Product Indicated by Participants | Total | ||||
|---|---|---|---|---|---|
| S | L | P | |||
| Product actually taken | S | 6 (24%) | 5 (20%) | 14 (56%) | 25 (100%) |
| L | 4 (15%) | 13 (50%) | 9 (35%) | 26 (100%) | |
| P | 7 (27%) | 8 (31%) | 11 (42%) | 26 (100%) | |
| Total | 17 | 26 | 34 | 77 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbownik, M.S.; Kręczyńska, J.; Kwarta, P.; Cybula, M.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Pietras, T.; Szemraj, J. Effect of Supplementation with Saccharomyces Boulardii on Academic Examination Performance and Related Stress in Healthy Medical Students: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2020, 12, 1469. https://doi.org/10.3390/nu12051469
Karbownik MS, Kręczyńska J, Kwarta P, Cybula M, Wiktorowska-Owczarek A, Kowalczyk E, Pietras T, Szemraj J. Effect of Supplementation with Saccharomyces Boulardii on Academic Examination Performance and Related Stress in Healthy Medical Students: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2020; 12(5):1469. https://doi.org/10.3390/nu12051469
Chicago/Turabian StyleKarbownik, Michał Seweryn, Joanna Kręczyńska, Paulina Kwarta, Magdalena Cybula, Anna Wiktorowska-Owczarek, Edward Kowalczyk, Tadeusz Pietras, and Janusz Szemraj. 2020. "Effect of Supplementation with Saccharomyces Boulardii on Academic Examination Performance and Related Stress in Healthy Medical Students: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 12, no. 5: 1469. https://doi.org/10.3390/nu12051469
APA StyleKarbownik, M. S., Kręczyńska, J., Kwarta, P., Cybula, M., Wiktorowska-Owczarek, A., Kowalczyk, E., Pietras, T., & Szemraj, J. (2020). Effect of Supplementation with Saccharomyces Boulardii on Academic Examination Performance and Related Stress in Healthy Medical Students: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 12(5), 1469. https://doi.org/10.3390/nu12051469






