The Effect of a Family-Based Lifestyle Education Program on Dietary Habits, Hepatic Fat and Adiposity Markers in 8–12-Year-Old Children with Overweight/Obesity
Abstract
:1. Introduction
2. Material and Methods
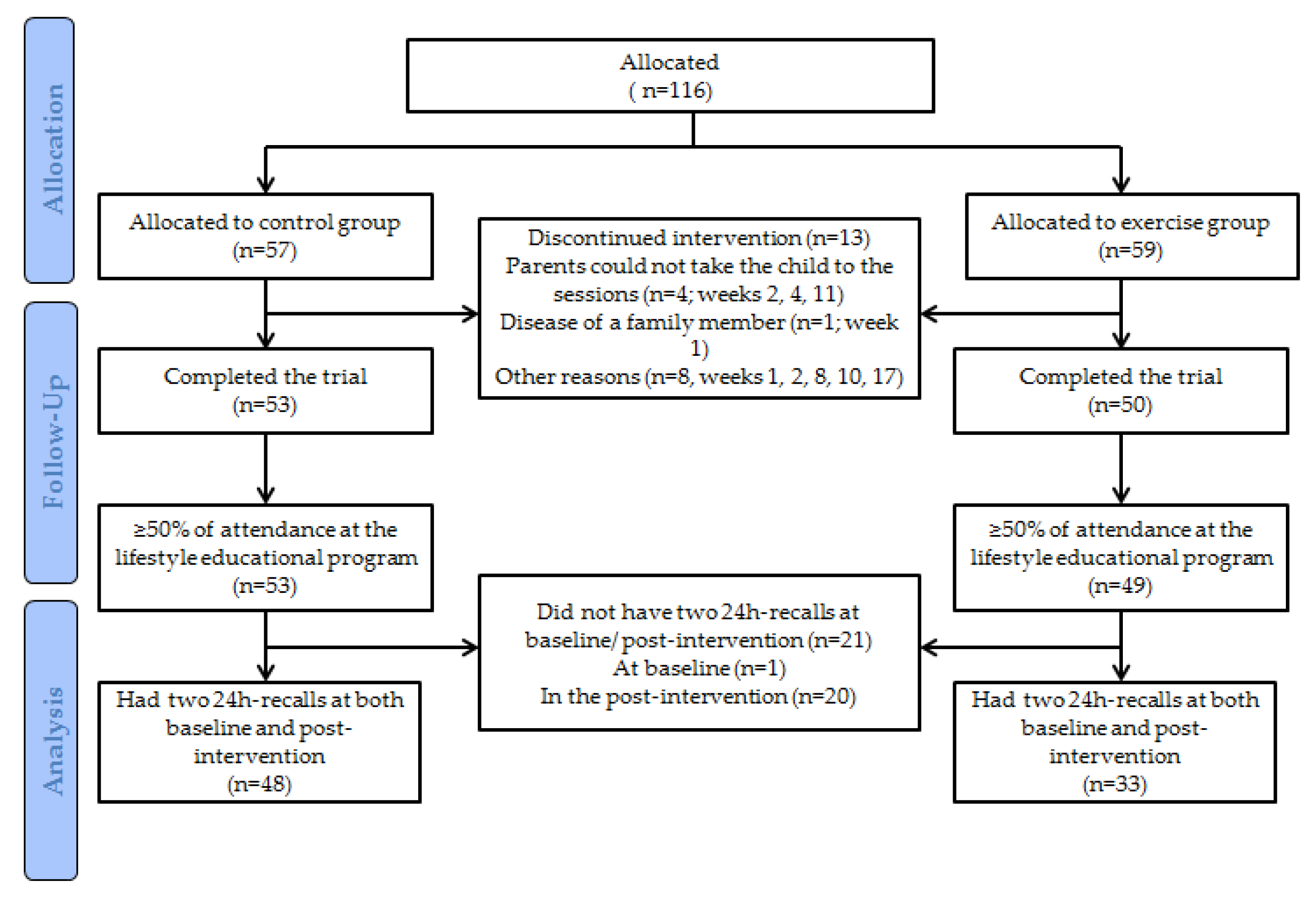
2.1. Study Design and Participants
2.2. Family-based Healthy Lifestyle Program
2.3. Hepatic Fat and Adiposity
2.4. Dietary Habits
2.4.1. Dietary Intake
2.4.2. Adherence to Dietary Patterns
2.4.3. Breakfast Habits
2.4.4. Meal Frequency and Daily Energy Distribution (Morning vs. Evening Energy Intake)
2.5. Statistical Analysis
3. Results
3.1. Dietary Intake
3.2. Association between Dietary Improvements and Changes in Hepatic Fat and Adiposity Markers
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Llewellyn, A.; Simmonds, M.; Owen, C.G.; Woolacott, N. Childhood obesity as a predictor of morbidity in adulthood: A systematic review and meta-analysis. Obes. Rev. 2015, 17, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the Metabolic Syndrome in Children and Adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Cruz, J.-J.; José-Juan, J.-M.; Fernández-Quesada, F.; María-José, S. Prevalencia de obesidad infantil y juvenil en España en 2012. Revista Española de Cardiología 2013, 66, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Aranceta-Bartrina, J.; Pérez-Rodrigo, C.; Serra-Majem, L.; Bellido, D.; De La Torre, M.L.; Formiguera, X.; Moreno, B. Prevention of overweight and obesity: A Spanish approach. Public Health Nutr. 2007, 10, 1187–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.-H.; Wong, T.-C.; Hsu, Y.-Y.; Kuo, K.-L.; Yang, S.-H. The Correlation Between Body Fat, Visceral Fat, and Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat. Disord. 2017, 15, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-L.; Chen, H.; Wang, C.-L.; Liang, L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef]
- Alkhater, S.A. Paediatric non-alcoholic fatty liver disease: An overview. Obes. Rev. 2015, 16, 393–405. [Google Scholar] [CrossRef]
- Veum, V.L.; Laupsa-borge, J.; Eng, Ø.; Rostrup, E.; Larsen, T.H.; Nordrehaug, J.E. Visceral adiposity and metabolic syndrome after very high–fat and low-fat isocaloric diets: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Burchett, H.E.D.; Sutcliffe, K.; Melendez-Torres, G.; Rees, R.; Thomas, J. Lifestyle weight management programmes for children: A systematic review using Qualitative Comparative Analysis to identify critical pathways to effectiveness. Prev. Med. 2018, 106, 1–12. [Google Scholar] [CrossRef]
- Robertson, W.; Fleming, J.; Kamal, A.; Hamborg, T.; Khan, K.A.; Griffiths, F.; Stewart-Brown, S.; Stallard, N.; Petrou, S.; Simkiss, D.; et al. Randomised controlled trial evaluating the effectiveness and cost-effectiveness of ‘Families for Health’, a family-based childhood obesity treatment intervention delivered in a community setting for ages 6 to 11 years. Health Technol. Assess. 2017, 21, 1–180. [Google Scholar] [CrossRef] [PubMed]
- Golan, M. Parents as agents of change in childhood obesity practice. Int. J. Pediatr. Obes. 2006, 1, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Hens, W.; Vissers, D.; Hansen, D.; Peeters, S.; Gielen, J.; Van Gaal, L.; Taeymans, J. The effect of diet or exercise on ectopic adiposity in children and adolescents with obesity: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Bertot, L.C.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Gómez, M.R. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- Hens, W.; Taeymans, J.; Cornelis, J.; Gielen, J.; Van Gaal, L.; Vissers, D. The Effect of Lifestyle Interventions on Excess Ectopic Fat Deposition Measured by Noninvasive Techniques in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. J. Phys. Act. Health 2016, 13, 671–694. [Google Scholar] [CrossRef]
- Medrano, M.; Cadenas-Sanchez, C.; Álvarez-Bueno, C.; Cavero-Redondo, I.; Ruiz, J.R.; Ortega, F.B.; Labayen, I. Evidence-Based Exercise Recommendations to Reduce Hepatic Fat Content in Youth- a Systematic Review and Meta-Analysis. Prog. Cardiovasc. Dis. 2018, 61, 222–231. [Google Scholar] [CrossRef]
- Brown, M.L.; Wilfley, D.E. Overweight and Obesity: Implications for Treatment. Curr. Obes. Rep. 2018, 7, 235–246. [Google Scholar]
- Medrano, M.; Maiz, E.; Maldonado-Martín, S.; Arenaza, L.; Rodríguez-Vigil, B.; Ortega, F.B.; Ruiz, J.; Larrarte, E.; López, I.D.; Sarasúa-Miranda, A.; et al. The effect of a multidisciplinary intervention program on hepatic adiposity in overweight-obese children: Protocol of the EFIGRO study. Contemp. Clin. Trials 2015, 45, 346–355. [Google Scholar] [CrossRef]
- Labayen, I.; Medrano, M.; Arenaza, L.; Maíz, E.; Osés, M.; Martinez-Vizcaino, V.; Ruiz, J.R.; Ortega, F.B. Effects of Exercise in Addition to a Family-Based Lifestyle Intervention Program on Hepatic Fat in Children with Overweight. Diabetes Care 2019, 43, 306–313. [Google Scholar] [CrossRef]
- Cole, T.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinnes, overweight and obesity. Pediatr Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Cabrera, S.G.; Fernández, N.H.; Hernández, C.R.; Nissensohn, M.; Roman-Viñas, B.; Serra-Majem, L. kidmed test; prevalence of low adherence to the mediterranean diet in children and young; a systematic review. Nutrición Hospitalaria 2015, 32, 2390–2399. [Google Scholar]
- Cohen, J.F.; Lehnerd, M.E.; Houser, R.F.; Rimm, E.B. Dietary Approaches to Stop Hypertension Diet, Weight Status, and Blood Pressure among Children and Adolescents: National Health and Nutrition Examination Surveys 2003-2012. J. Acad. Nutr. Diet. 2017, 117, 1437–1444.e2. [Google Scholar] [CrossRef] [PubMed]
- Struijk, E.A.; May, A.M.; Wezenbeek, N.L.; Fransen, H.P.; Soedamah-Muthu, S.S.; Geelen, A.; Boer, J.M.; Van Der Schouw, Y.T.; Bueno-De-Mesquita, H.B.; Beulens, J.W. Adherence to dietary guidelines and cardiovascular disease risk in the EPIC-NL cohort. Int. J. Cardiol. 2014, 176, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Arenaza, L.; Muñoz-Hernández, V.; Medrano, M.; Oses, M.; Amasene, M.; Merchán-Ramírez, E.; Cadenas-Sanchez, C.; Ortega, F.B.; Ruiz, J.R.; Labayen, I. Association of Breakfast Quality and Energy Density with Cardiometabolic Risk Factors in Overweight/Obese Children: Role of Physical Activity. Nutrients 2018, 10, 1066. [Google Scholar] [CrossRef] [Green Version]
- House, B.T.; Shearrer, G.E.; Miller, S.J.; Pasch, K.E.; Goran, M.I.; Davis, J.N. Increased eating frequency linked to decreased obesity and improved metabolic outcomes. Int. J. Obes. 2014, 39, 136–141. [Google Scholar] [CrossRef]
- Aljuraiban, G.S.; Chan, Q.; Griep, L.M.O.; Brown, I.J.; Daviglus, M.L.; Stamler, J.; Van Horn, L.; Elliott, P.; Frost, G.; INTERMAP Research Group. The impact of eating frequency and time of intake on nutrient quality and Body Mass Index: The INTERMAP Study, a Population-Based Study. J. Acad. Nutr. Diet. 2015, 115, 528–536.e1. [Google Scholar] [CrossRef] [Green Version]
- Della Corte, C.; Mosca, A.; Vania, A.; Alterio, A.; Iasevoli, S.; Nobili, V. Good adherence to the Mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: The results of an Italian Study. Nutrients 2017, 39, 8–14. [Google Scholar] [CrossRef]
- Comments to the draft ‘WHO-Guideline: Sugars intake for adults and children’. Sugar Ind. 2014, 48, 311–316. [CrossRef]
- Reinehr, T.; Schaefer, A.; Winkel, K.; Finne, E.; Toschke, A.M.; Kolip, P. An effective lifestyle intervention in overweight children: Findings from a randomized controlled trial on “Obeldicks Light”. Clin. Nutr. 2010, 29, 331–336. [Google Scholar] [CrossRef]
- Mytton, O.T.; Nnoaham, K.; Eyles, H.; Scarborough, P.; Ni Mhurchu, C. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health 2014, 14, 886. [Google Scholar] [CrossRef] [Green Version]
- Puchau, B.; Ochoa, M.C.; Zulet, M.A.; Marti, A.; Martinez, J.A.; Members, G. Dietary total antioxidant capacity and obesity in children and adolescents. Int. J. Food Sci. Nutr. 2010, 61, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd-Jones, D.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. Available online: https://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf;jsessionid=7CF9C1534F0D663F22AB3E602BDBA8F1?sequence=1 (accessed on 28 January 2020).
- Departamento de Salud del Gobierno Vasco. Encuesta de Nutrición 2005: Hábitos Alimentarios y Estado de Salud de la Población Vasca de 4 a 18 años; Servicio Central de Publicaciones del Gobierno Vasco: Vitoria-Gasteiz, Spain, 2006. [Google Scholar]
- Gold, A.; Larson, M.; Tucker, J.M.; Strang, M. Classroom Nutrition Education Combined with Fruit and Vegetable Taste Testing Improves Children’s Dietary Intake. J. Sch. Health 2017, 87, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.W.; Monnat, S.M.; Lohse, B. Middle school-aged child enjoyment of food tastings predicted interest in nutrition education on osteoporosis prevention. J. Sch. Health 2015, 85, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Estrategia NAOS. Cuidar la Cantidad: Las Raciones; Programa Perseo; Estrateg Naos. Agencia Española de Seguridad Alimentaria y Nutrición, Gobierno de España: Madrid, Spain, 2006. [Google Scholar]
- Dietary Reference Values for nutrients Summary report. EFSA Support. Public 2017, 14. [CrossRef] [Green Version]
- Gunther, C.; Rogers, C.; Holloman, C.; Hopkins, L.C.; Anderson, S.E.; Miller, C.K.; Copeland, K.A.; Dollahite, J.S.; Pratt, K.J.; Webster, A.; et al. Child diet and health outcomes of the simple suppers program: A 10-week, 2-group quasi-experimental family meals trial. BMC Public Health 2019, 19, 1657. [Google Scholar] [CrossRef]
- Haerens, L.; De Bourdeaudhuij, I.; Maes, L.; Vereecken, C.; Brug, J.; Deforche, B. The effects of a middle-school healthy eating intervention on adolescents’ fat and fruit intake and soft drinks consumption. Public Health Nutr. 2007, 10, 443–449. [Google Scholar] [CrossRef]
- Van De Gaar, V.M.; Jansen, W.; Van Grieken, A.; Borsboom, G.J.J.M.; Kremers, S.P.J.; Raat, H. Effects of an intervention aimed at reducing the intake of sugar-sweetened beverages in primary school children: A controlled trial. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Ojeda-Rodríguez, A.; Zazpe, I.; Morell-Azanza, L.; Chueca, M.; Campoy, C.; Marti, A. Improved Diet Quality and Nutrient Adequacy in Children and Adolescents with Abdominal Obesity after a Lifestyle Intervention. Nutrients 2018, 10, 1500. [Google Scholar] [CrossRef] [Green Version]
- Manz, K.; Mensink, G.B.M.; Finger, J.D.; Haftenberger, M.; Brettschneider, A.-K.; Barbosa, C.L.; Krug, S.; Schienkiewitz, A. Associations between Physical Activity and Food Intake among Children and Adolescents: Results of KiGGS Wave 2. Nutrients 2019, 11, 1060. [Google Scholar] [CrossRef] [Green Version]
- King, N.; Horner, K.; Hills, A.P.; Byrne, N.M.; Wood, R.; Bryant, E.; Caudwell, P.; Finlayson, G.; Gibbons, C.; Hopkins, M.; et al. Exercise, appetite and weight management: Understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. Br. J. Sports Med. 2011, 46, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Williamson, S.A.; Vazquez, A.I.; Fernandez, J.R.; Bray, M.S. The Influence of 15-week Exercise Training on Dietary Patterns among Young Adults. Int. J. Obes. 2019, 43, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Steinsbekk, S.; Wichstrøm, L.; Ødegård, R.; Mehus, I. Change in Body Fat during a Family-Based Treatment of Obesity in Children: The Relative Importance of Energy Intake and Physical Activity. Obes. Facts 2012, 5, 515–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulton, J.E.; Dai, S.; Steffen, L.M.; Grunbaum, J.A.; Shah, S.M.; Labarthe, D.R. Physical activity, energy intake, sedentary behavior, and adiposity in youth. Am. J. Prev. Med. 2009, 37, S40–S49. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Roberts, S.B.; Bhapkar, M.V.; Villareal, D.T.; Fontana, L.; Martin, C.K.; Racette, S.; Fuss, P.; Kraus, W.E.; Wong, W.; et al. Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: A 2-y randomized controlled trial of calorie restriction in nonobese humans. Am. J. Clin. Nutr. 2017, 105, 913–927. [Google Scholar] [CrossRef]
- Arenaza, L.; Medrano, M.; Oses, M.; Huybrechts, I.; Díez, I.; Henriksson, H. Dietary determinants of hepatic fat content and insulin resistance in overweight / obese children: A cross-sectional analysis of the Prevention of Diabetes in Kids ( PREDIKID ) study. Br. J. Nutr. 2019, 121, 1158–1165. [Google Scholar] [CrossRef]
- Assy, N.N.; Nasser, G.; Kamayse, I.; Nseir, W.; Beniashvili, Z.; Djibre, A.; Grosovski, M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can. J. Gastroenterol. 2008, 22, 811–816. [Google Scholar] [CrossRef]
- Abid, A.; Taha, O.; Nseir, W.; Farah, R.; Grosovski, M.; Assy, N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome q. J. Hepatol. 2009, 51, 918–924. [Google Scholar] [CrossRef]
- Kajons, N.; David, M.; Gowland-Ella, J.; Lewis, P.; Batchelor, S. Thirsty? Choose Water! Behavioural interventions and water stations in secondary schools a two-by-two factorial randomised controlled trial. BMC Public Health 2018, 18, 788. [Google Scholar] [CrossRef]
- Ramon-Krauel, M.; Salsberg, S.L.; Ebbeling, C.B.; Voss, S.D.; Mulkern, R.V.; Apura, M.M.; Cooke, E.A.; Sarao, K.; Jonas, M.M.; Ludwig, D.S. A Low-Glycemic-Load versus Low-Fat Diet in the Treatment of Fatty Liver in Obese Children. Child Obes. 2013, 9, 252–260. [Google Scholar] [CrossRef]
| Control Group | Exercise Group | p | |||
|---|---|---|---|---|---|
| N | N | ||||
| Age (years) | 48 | 10.6 (1.1) | 33 | 10.5 (1.1) | 0.597 |
| Girls (N, %) | 48 | 26, 54.2 | 33 | 17, 51.5 | 0.814 |
| High educational level of the mother (N, %) | 48 | 39, 81.3 | 32 | 21, 63.6 | 0.114 |
| Children with Spanish-origin mother (N, %) | 48 | 43, 89.6 | 33 | 30, 90.9 | 0.844 |
| Body mass index (kg/m2) | 48 | 25.2 (2.8) | 33 | 25.7 (3.6) | 0.536 |
| Children with obesity (N, %) | 48 | 26, 54.2 | 33 | 19, 58 | 0.762 |
| Fat mass index (kg/m2) | 48 | 9.8 (2.2) | 32 | 10.4 (2.6) | 0.270 |
| Abdominal fat (kg) | 48 | 2.4 (1.0) | 32 | 2.6 (1.1) | 0.448 |
| Hepatic fat (%) | 48 | 5.1 (2.8) | 33 | 5.8 (5.2) | 0.471 |
| Control Group | Exercise Group | Baseline Control vs. Exercise | Intervention Effect Control vs. Exercise | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Pre | Post | p * | N | Pre | Post | p * | p ** | p *** | |
| Energy, nutrients and food groups | ||||||||||
| Energy intake (kcal/day) | 48 | 1827 (423) | 1652 (376) | 0.009 | 33 | 1855 (430) | 1622 (326) | 0.003 | 0.774 | 0.566 |
| Carbohydrates intake (g/day) | 48 | 197 (53) | 179 (44) | 0.039 | 33 | 189 (53) | 178 (39) | 0.248 | 0.501 | 0.168 |
| Simple sugar intake (g/day) | 48 | 87 (32) | 78 (24) | 0.067 | 33 | 84 (28) | 73 (25) | 0.044 | 0.622 | 0.970 |
| Added sugar intake (g/day) | 48 | 55 (31) | 43 (19) | 0.024 | 33 | 58 (29) | 40 (19) | 0.006 | 0.955 | 0.517 |
| Fat intake (g/day) | 48 | 81 (28) | 70 (24) | 0.010 | 33 | 88 (27) | 66 (19) | <0.001 | 0.229 | 0.062 |
| Protein intake (g/day) | 48 | 77 (20) | 75 (20) | 0.591 | 33 | 75 (18) | 76 (18) | 0.865 | 0.736 | 0.226 |
| Cholesterol (mg/day) | 48 | 306 (127) | 301 (145) | 0.858 | 33 | 308 (153) | 249 (102) | 0.029 | 0.948 | 0.268 |
| Fiber (g/day) | 48 | 14 (5) | 14 (5) | 0.641 | 33 | 14 (9) | 16 (6) | 0.281 | 0.720 | 0.400 |
| Calcium (mg/day) | 48 | 661 (217) | 665 (201) | 0.920 | 33 | 639 (203) | 659 (229) | 0.610 | 0.948 | 0.898 |
| Magnesium (mg/day) | 48 | 234 (57) | 234 (58) | 0.963 | 33 | 233 (121) | 248 (73) | 0.454 | 0.647 | 0.299 |
| Sodium (mg/day) | 48 | 2227 (1003) | 2022 (756) | 0.199 | 33 | 2191 (764) | 2248 (776) | 0.688 | 0.948 | 0.068 |
| Potassium (mg/day) | 48 | 2352 (480) | 2430 (653) | 0.442 | 33 | 2292 (795) | 2540 (644) | 0.090 | 0.718 | 0.107 |
| Vegetables (g/day) | 48 | 76 (58) | 93 (77) | 0.192 | 33 | 111 (107) | 140 (85) | 0.171 | 0.090 | 0.363 |
| Fruits (g/day) | 48 | 149 (126) | 240 (168) | 0.001 | 33 | 138 (155) | 189 (166) | 0.027 | 0.727 | 0.420 |
| Dairy products (g/day) | 48 | 333 (156) | 336 (134) | 0.910 | 33 | 306 (162) | 339 (152) | 0.239 | 0.452 | 0.658 |
| Low-fat/skimmed dairy (g/day) | 48 | 109 (144) | 179 (157) | 0.001 | 33 | 93 (138) | 174 (174) | 0.014 | 0.622 | 0.672 |
| Cereals (g/day) | 48 | 166 (72) | 160 (61) | 0.577 | 33 | 145 (57) | 175 (71) | 0.070 | 0.144 | 0.028 |
| Whole cereals (g/day) | 48 | 12 (23) | 19 (32) | 0.177 | 33 | 4 (11) | 7 (15) | 0.460 | 0.055 | 0.413 |
| Nuts and legumes (g/day) | 48 | 16 (21) | 14 (21) | 0.743 | 33 | 13 (17) | 16 (19) | 0.493 | 0.605 | 0.742 |
| Fish and seafood (g/day) | 48 | 41 (53) | 41 (47) | 0.958 | 33 | 34 (48) | 30 (28) | 0.739 | 0.535 | 0.976 |
| Meat and meat products (g/day) | 48 | 104 (88) | 81 (64) | 0.162 | 33 | 93 (62) | 89 (57) | 0.916 | 0.519 | 0.084 |
| Sugar-sweetened beverages(g/day) | 48 | 85 (146) | 47 (81) | 0.135 | 33 | 63 (92) | 37 (101) | 0.282 | 0.412 | 0.313 |
| Adherence to dietary patterns | ||||||||||
| KIDMED score (0–12) | 45 | 5.7 (1.9) | 8.1 (1.9) | <0.001 | 25 | 5.4 (2.1) | 7.7 (2.0) | <0.001 | 0.552 | 0.652 |
| DASH score (0–9) | 48 | 1.3 (0.9) | 1.6 (1.3) | 0.182 | 33 | 1.1 (1.0) | 1.9 (1.1) | <0.001 | 0.320 | 0.048 |
| HDI score (0–7) | 48 | 1.6 (1.1) | 1.6 (1.1) | 1.000 | 33 | 1.8 (1.2) | 1.8 (0.9) | 0.891 | 0.531 | 0.949 |
| Breakfast habits | ||||||||||
| Skipping breakfast (N, %) | 48 | 0, 0 | 1, 2.1 | 0.100 | 33 | 1, 3 | 2, 6.1 | 0.100 | 0.407 | 0.652 |
| BQI score (0–10) | 48 | 3.9 (1.0) | 5.2 (1.6) | <0.001 | 33 | 3.7 (0.9) | 4.3 (1.2) | 0.025 | 0.269 | 0.207 |
| Meal frequency and daily energy distribution | ||||||||||
| Having ≥ 4 meals/day (N, %) | 48 | 45, 93.8 | 44, 91.7 | 0.100 | 33 | 28, 84.8 | 32, 97.0 | 0.125 | 0.173 | 0.154 |
| Evening/morning energy intake ratio | 48 | 0.73 (0.29) | 0.61 (0.24) | 0.014 | 33 | 0.71 (0.28) | 0.69 (0.22) | 0.749 | 0.796 | 0.322 |
| Δ FMI (kg/m2) | Δ Abdominal Fat (kg) | Δ Hepatic Fat (%) | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Main nutritional goals * | ||||||
| Δ Energy intake (kcal/day) ** | 0.086 | 0.466 | 0.008 | 0.946 | 0.136 | 0.245 |
| Δ Fat intake (g/day) | 0.056 | 0.665 | 0.064 | 0.619 | 0.233 | 0.066 |
| Δ Simple sugar (g/day) | −0.087 | 0.496 | −0.100 | 0.438 | 0.013 | 0.919 |
| Δ Fruits and vegetables (g/day) | 0.136 | 0.288 | −0.007 | 0.956 | −0.045 | 0.729 |
| Δ SSB consumption (g/day) | −0.017 | 0.897 | −0.083 | 0.516 | 0.266 | 0.035 |
| Δ Meal frequency (times/day) | −0.105 | 0.414 | −0.081 | 0.528 | −0.097 | 0.451 |
| Dietary patterns | ||||||
| Δ KIDMED score | 0.004 | 0.976 | −0.080 | 0.535 | 0.191 | 0.134 |
| Δ DASH score | −0.166 | 0.194 | −0.113 | 0.376 | −0.137 | 0.285 |
| Δ HDI score | −0.096 | 0.453 | −0.043 | 0.740 | 0.157 | 0.220 |
| Δ BQI score | 0.004 | 0.976 | 0.022 | 0.864 | −0.062 | 0.628 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arenaza, L.; Medrano, M.; Oses, M.; Amasene, M.; Díez, I.; Rodríguez-Vigil, B.; Labayen, I. The Effect of a Family-Based Lifestyle Education Program on Dietary Habits, Hepatic Fat and Adiposity Markers in 8–12-Year-Old Children with Overweight/Obesity. Nutrients 2020, 12, 1443. https://doi.org/10.3390/nu12051443
Arenaza L, Medrano M, Oses M, Amasene M, Díez I, Rodríguez-Vigil B, Labayen I. The Effect of a Family-Based Lifestyle Education Program on Dietary Habits, Hepatic Fat and Adiposity Markers in 8–12-Year-Old Children with Overweight/Obesity. Nutrients. 2020; 12(5):1443. https://doi.org/10.3390/nu12051443
Chicago/Turabian StyleArenaza, Lide, María Medrano, Maddi Oses, Maria Amasene, Ignacio Díez, Beatriz Rodríguez-Vigil, and Idoia Labayen. 2020. "The Effect of a Family-Based Lifestyle Education Program on Dietary Habits, Hepatic Fat and Adiposity Markers in 8–12-Year-Old Children with Overweight/Obesity" Nutrients 12, no. 5: 1443. https://doi.org/10.3390/nu12051443





