Dietary Recommendations for Bariatric Patients to Prevent Kidney Stone Formation
Abstract
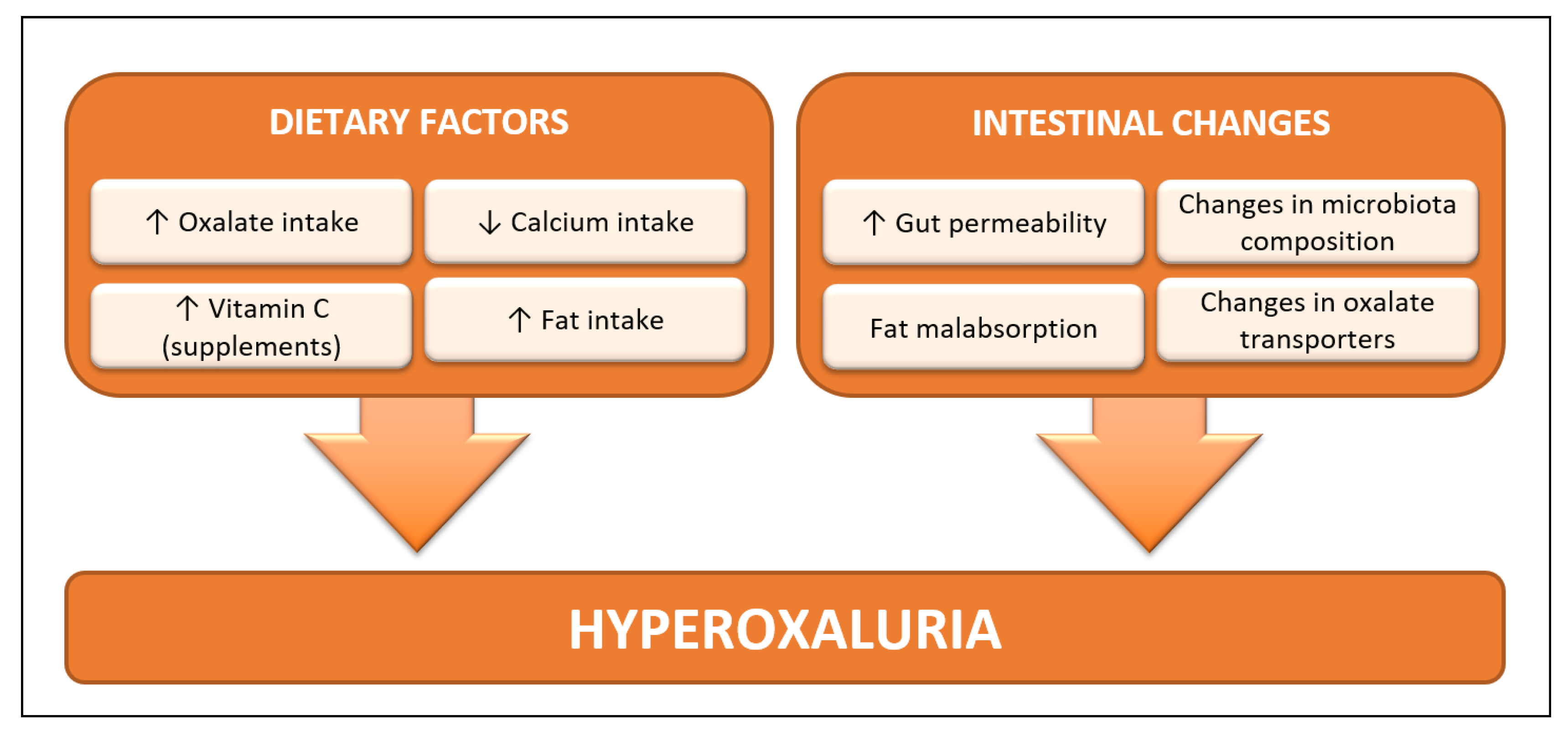
1. Introduction
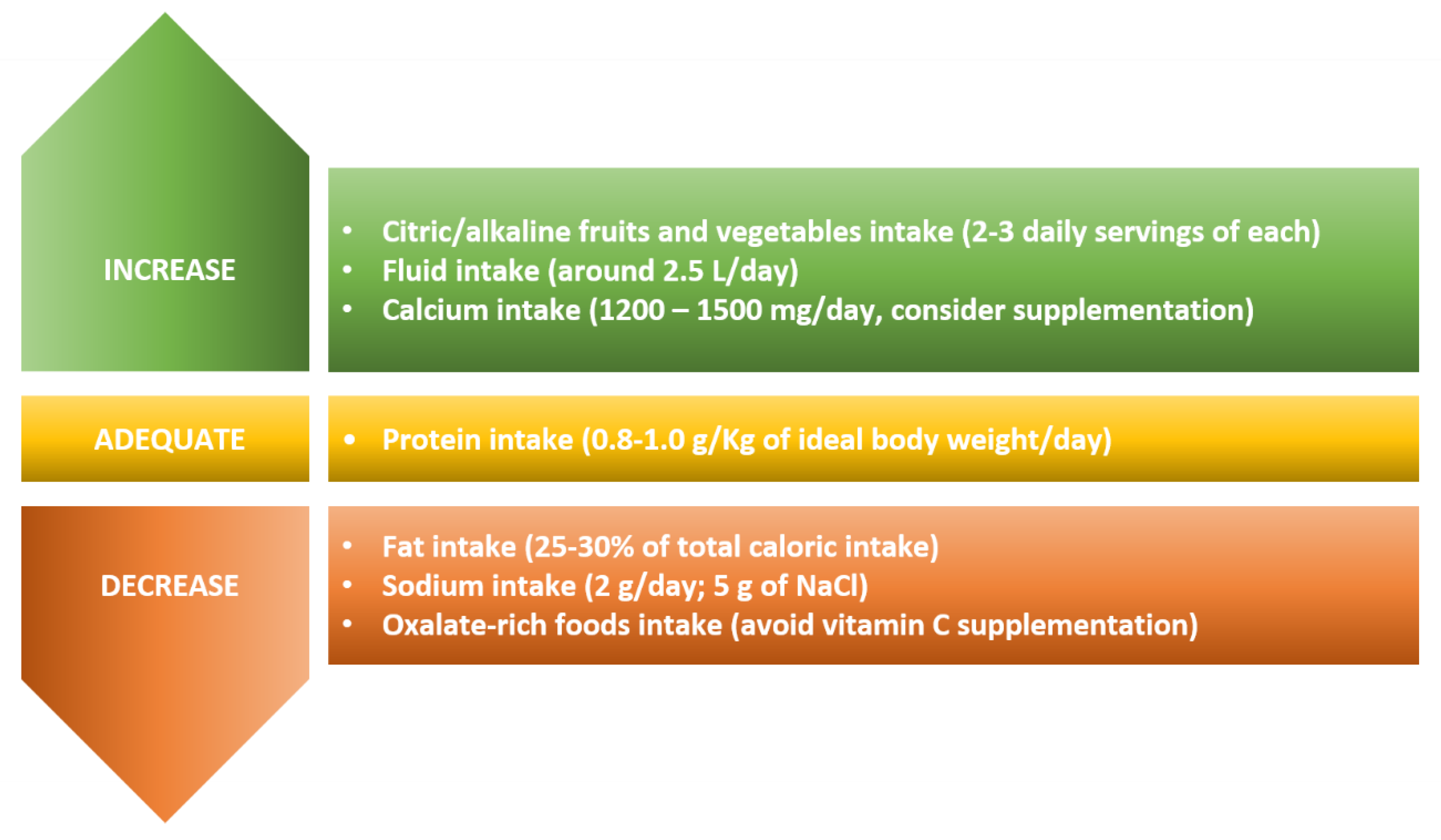
2. Dietary Recommendations
2.1. Oxalate
2.2. Calcium and Vitamin D
2.3. Vitamin B6
2.4. Vitamin C
2.5. Citrate and Potassium
2.6. Probiotics
2.7. Protein and Sodium
2.8. Fluids
2.9. Fat
Author Contributions
Funding
Conflicts of Interest
References
- Chang, A.R.; Grams, M.E.; Navaneethan, S.D. Bariatric Surgery and Kidney-Related Outcomes. Kidney Int. Rep. 2017, 2, 261–270. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric Surgery: A Systematic Review and Meta-analysis. JAMA 2020, 292, 1724–1737. [Google Scholar] [CrossRef]
- Sakhaee, K.; Poindexter, J.; Aguirre, C. The Effects of Bariatric Surgery on Bone and Nephrolithiasis. Bone 2016, 84, 1–8. [Google Scholar] [CrossRef]
- Melo, T.L.; Froeder, L.; Baia, L.D.C.; Heilberg, I.P. Bone turnover after bariatric surgery. Arch. Endocrinol. Metab. 2017, 61, 332–336. [Google Scholar] [CrossRef]
- Mishra, T.; Shapiro, J.B.; Ramirez, L.; Kallies, K.J.; Kothari, S.N.; Londergan, T.A. Nephrolithiasis after bariatric surgery: A comparison of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy. Am. J. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Matlaga, B.R.; Shore, A.D.; Magnuson, T.; Clark, J.M.; Johns, R.; Makary, M.A. Effect of gastric bypass surgery on kidney stone disease. J. Urol. 2009, 181, 2573–2577. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C.; Mehta, R.A.; Milliner, D.S.; Rule, A.D.; Bergstralh, E.J.; Sarr, M.G. Kidney stones are common after bariatric surgery. Kidney Int. 2015, 87, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Duffey, B.G.; Pedro, R.N.; Makhlouf, A.; Kriedberg, C.; Stessman, M.; Hinck, B.; Ikramuddin, S.; Kellogg, T.; Slusarek, B.; Monga, M. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J. Am. Coll. Surg. 2008, 206, 1145–1153. [Google Scholar] [CrossRef]
- Patel, B.N.; Passman, C.M.; Fernandez, A.; Asplin, J.R.; Coe, F.L.; Kim, S.C.; Lingeman, J.E.; Assimos, D.G. Prevalence of hyperoxaluria after bariatric surgery. J. Urol. 2009, 181, 161–166. [Google Scholar] [CrossRef]
- DeFoor, W.R.; Asplin, J.R.; Kollar, L.; Jackson, E.; Jenkins, T.; Schulte, M.; Inge, T. Prospective evaluation of urinary metabolic indices in severely obese adolescents after weight loss surgery. Surg. Obes. Relat. Dis. 2016, 12, 363–367. [Google Scholar] [CrossRef][Green Version]
- Duffey, B.G.; Alanee, S.; Pedro, R.N.; Hinck, B.; Kriedberg, C.; Ikramuddin, S.; Kellogg, T.; Stessman, M.; Moeding, A.; Monga, M. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: A 2-year prospective longitudinal study. J. Am. Coll. Surg. 2010, 211, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Espino-Grosso, P.M.; Canales, B.K. Kidney Stones After Bariatric Surgery: Risk Assessment and Mitigation. Bariatr. Surg. Pract. Patient Care 2017, 12, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, J.L.; Heilberg, I.P. Scientific American Nephrology, Dialysis and Transplantation; Singh, A.K., Riella, L.V., Eds.; Decker Intellectual Properties: Hamilton, ON, Canada, 2017. [Google Scholar]
- Froeder, L.; Arasaki, C.H.; Malheiros, C.A.; Baxmann, A.C.; Heilberg, I.P. Response to dietary oxalate after bariatric surgery. Clin. J. Am. Soc. Nephrol. 2012, 7, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.R.; Tiselius, H.G.; Heilberg, I.P. Fat malabsorption induced by gastrointestinal lipase inhibitor leads to an increase in urinary oxalate excretion. Kidney Int. 2004, 66, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Lieske, J.C.; Collazo-Clavell, M.L.; Sarr, M.G.; Olson, E.R.; Vrtiska, T.J.; Bergstralh, E.J.; Li, X. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery 2011, 149, 654–661. [Google Scholar] [CrossRef]
- Siener, R.; Petzold, J.; Bitterlich, N.; Alteheld, B.; Metzner, C. Determinants of urolithiasis in patients with intestinal fat malabsorption. Urology 2013, 81, 17–24. [Google Scholar] [CrossRef]
- Moreland, A.M.; Santa Ana, C.A.; Asplin, J.R.; Kuhn, J.A.; Holmes, R.P.; Cole, J.A.; Odstrcil, E.A.; Van Dinter, T.G., Jr.; Martinez, J.G.; Fordtran, J.S. Steatorrhea and Hyperoxaluria in Severely Obese Patients Before and After Roux-en-Y Gastric Bypass. Gastroenterology 2017, 152, 1055–1067. [Google Scholar] [CrossRef]
- Wu, J.N.; Craig, J.; Chamie, K.; Asplin, J.; Ali, M.R.; Low, R.K. Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg. Obes. Relat. Dis. 2013, 9, 83–87. [Google Scholar] [CrossRef]
- Siener, R.; Bangen, U.; Sidhu, H.; Hönow, R.; von Unruh, G.; Hesse, A. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 2013, 83, 1144–1149. [Google Scholar] [CrossRef]
- Stewart, C.S.; Duncan, S.H.; Cave, D.R. Oxalobacter formigenes and its role in oxalate metabolism in the human gut. FEMS Microbiol. Lett. 2004, 230, 1–7. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Heilberg, I.P. Oxalobacter Formigenes, Lactic acid Bacteria, and Hyperoxaluria: An Update NephSAP. Disord. Divalent Ions Renal Bone Disease Nephrolithiasis 2012, 11, 231–235. [Google Scholar]
- Canales, B.K.; Hatch, M. Oxalobacter formigenes colonization normalizes oxalate excretion in a gastric bypass model of hyperoxaluria. Surg. Obes. Relat. Dis. 2017, 13, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.W.; Kelly, J.P.; Curhan, G.C.; Anderson, T.E.; Dretler, S.P.; Preminger, G.M.; Cave, D.R. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J. Am. Soc. Nephrol. 2008, 19, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Duffey, B.G.; Miyaoka, R.; Holmes, R.; Assimos, D.; Hinck, B.; Korman, E.; Kieley, F.; Ikramuddin, S.; Kellogg, T.; Moeding, A.; et al. Oxalobacter colonization in the morbidly obese and correlation with urinary stone risk. Urology 2011, 78, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.W.; Choy, D.; Penniston, K.L.; Lange, D. Inhibition of urinary stone disease by a multi-species bacterial network ensures healthy oxalate homeostasis. Kidney Int. 2019, 96, 180–188. [Google Scholar] [CrossRef]
- Lieske, J.C.; Goldfarb, D.S.; Simone, C.D.; Regnier, C. Use of a probioitic to decrease enteric hyperoxaluria. Kidney Int. 2005, 68, 1244–1249. [Google Scholar] [CrossRef]
- Hatch, M.; Canales, B.K. The mechanistic basis of hyperoxaluria following gastric bypass in obese rats. Urolithiasis 2015, 44, 221–230. [Google Scholar] [CrossRef]
- Freel, R.W.; Hatch, M.; Green, M.; Soleimani, M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G719–G728. [Google Scholar] [CrossRef]
- Freel, R.W.; Whittamore, J.M.; Hatch, M. Transcellular oxalate and Cl- absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G520–G527. [Google Scholar] [CrossRef]
- Ormanji, M.S.; Korkes, F.; Meca, R.; Ishiy, C.S.R.A.; Finotti, G.H.C.; Ferraz, R.R.N.; Heilberg, I.P. Hyperoxaluria in a Model of Mini-Gastric Bypass Surgery in Rats. Obes. Surg. 2017, 1–7. [Google Scholar] [CrossRef]
- De Prisco, C.; Levine, S.N. Metabolic bone disease after gastric bypass surgery for obesity. Am. J. Med. Sci. 2005, 329, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, D.; Zhang, J.F.; Xu, D.; Xu, W.F.; Chen, Y.; Liu, B.Y.; Li, P.; Li, L. Changes in Bone Metabolism in Morbidly Obese Patients After Bariatric Surgery: A Meta-Analysis. Obes. Surg. 2016, 26, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Schafer, A.L. Bone Health After Bariatric Surgery. JBMR Plus 2018, 2, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Silverberg, S.J. Bone Loss After Bariatric Surgery: Causes, Consequences and Management. Lancet Diabetes Endocrinol. 2014, 2, 165–174. [Google Scholar] [CrossRef]
- Yu, E.W. Bone metabolism after bariatric surgery. J. Bone Miner. Res. 2014, 29, 1507–1518. [Google Scholar] [CrossRef]
- Yu, E.W.; Bouxsein, M.L.; Putman, M.S.; Monis, E.L.; Roy, A.E.; Pratt, J.S.; Butsch, W.S.; Finkelstein, J.S. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J. Clin. Endocrinol. Metab. 2015, 100, 1452–1459. [Google Scholar] [CrossRef]
- Harvard. Oxalate Content of Foods. Available online: https://regepi.bwh.harvard.edu/health/Oxalate/files (accessed on 10 March 2020).
- Kynast-Gales, S.A.; Massey, L.K. Food oxalate: An international database. J. Am. Diet Assoc. 2007, 107, 1099. [Google Scholar] [CrossRef]
- Holmes, R.P.; Kennedy, M. Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int. 2000, 57, 1662–1667. [Google Scholar] [CrossRef]
- Shimizu, M.H.; Gois, P.H.; Volpini, R.A.; Canale, D.; Luchi, W.M.; Froeder, L.; Heilberg, I.P.; Seguro, A.C. N-acetylcysteine protects against star fruit-induced acute kidney injury. Ren. Fail. 2017, 39, 193–202. [Google Scholar] [CrossRef]
- Chen, C.L.; Fang, H.C.; Chou, K.J.; Wang, J.S.; Chung, H.M. Acute oxalate nephropathy after ingestion of star fruit. Am. J. Kidney Dis. 2001, 37, 418–422. [Google Scholar] [CrossRef]
- Taylor, E.N.; Curhan, G.C. Oxalate intake and the risk for nephrolithiasis. J. Am. Soc. Nephrol. 2007, 18, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- American Dietetic Association. Urolithiasis/Urinary Stones. In ADA Nutrition Care Manual; American Dietetic Association: Chicago, IL, USA, 2005. [Google Scholar]
- Singh, P.P.; Kothari, L.K.; Sharma, D.C.; Saxena, S.N. Nutritional value of foods in relation to their oxalic acid content. Am. J. Clin. Nutr. 1972, 25, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, J.L.; Martini, L.A.; Mendonca, C.O.; Schor, N.; Heilberg, I.P. Effect of calcium intake on urinary oxalate excretion in calcium stone-forming patients. Braz. J. Med. Biol. Res. 2002, 35, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, C.O.G.; Martini, L.A.; Baxmann, A.C.; Nishiura, J.L.; Cuppari, L.; Sigulem, D.M.; Heilberg, I.P. Effects of an oxalate load on urinary oxalate excretion in calcium stone formers. J. Ren. Nutr. 2003, 13, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Massey, L.K. Food oxalate: Factors affecting measurement, biological variation, and bioavailability. J. Am. Diet Assoc. 2007, 107, 1191–1194. [Google Scholar] [CrossRef]
- Holmes, R.P.; Assimos, D.G. The impact of dietary oxalate on kidney stone formation. Urol. Res. 2004, 32, 311–316. [Google Scholar] [CrossRef]
- Brinkley, L.; McGuire, J.; Gregory, J.; Pak, C.Y. Bioavailability of oxalate in foods. Urology 1981, 17, 534–538. [Google Scholar] [CrossRef]
- Chai, W.; Liebman, M. Assessment of oxalate absorption from almonds and black beans with and without the use of an extrinsic label. J. Urol. 2004, 172, 953–957. [Google Scholar] [CrossRef]
- Von Unruh, G.E.; Voss, S.; Sauerbruch, T.; Hesse, A. Dependence of oxalate absorption on the daily calcium intake. J. Am. Soc. Nephrol. 2004, 15, 1567–1573. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N. Engl. J. Med 1993, 328, 833–838. [Google Scholar] [CrossRef]
- Brogren, M.; Savage, G. Bioavailability of soluble oxalate from spinach eaten with and without milk products. Asia Pac. J. Clin. Nutr. 2003, 12, 219. [Google Scholar] [PubMed]
- Albihn, P.B.; Savage, G.P. The bioavailability of oxalate from Oca (Oxalis tuberosa). J. Urol. 2001, 166, 420–422. [Google Scholar] [CrossRef]
- Chai, W.; Liebman, M. Oxalate content of legumes, nuts, and grainbased flours. J. Food Comp. Anal. 2005, 18, 723–729. [Google Scholar] [CrossRef]
- Wilson, C.; Shaw, P.; Knight, R. Analysis of oxalic acid in carambola (Averrhoa carambola L.) and spinach by high-performance liquid chromatography. J. Agric. Food Chem. 1982, 30, 1106–1108. [Google Scholar] [CrossRef]
- Schroder, T.; Vanhanen, L.; Savage, G.P. Oxalate content in commercially produced cocoa and dark chocolate. J. Food Compos. Anal. 2011, 24, 916–922. [Google Scholar] [CrossRef]
- Charrier, M.J.; Savage, G.P.; Vanhanen, L. Oxalate content and calcium binding capacity of tea and herbal teas. Asia Pac. J. Clin. Nutr. 2002, 11, 298–301. [Google Scholar] [CrossRef]
- Duran de Campos, C.; Dalcanale, L.; Pajecki, D.; Garrido, A.B., Jr.; Halpern, A. Calcium intake and metabolic bone disease after eight years of Roux-en-Y gastric bypass. Obes. Surg. 2008, 18, 386–390. [Google Scholar] [CrossRef]
- Schafer, A.L.; Weaver, C.M.; Black, D.M.; Wheeler, A.L.; Chang, H.; Szefc, G.V.; Stewart, L.; Rogers, S.J.; Carter, J.T.; Posselt, A.M.; et al. Intestinal Calcium Absorption Decreases Dramatically After Gastric Bypass Surgery Despite Optimization of Vitamin D Status. J. Bone Miner. Res. 2015, 30, 1377–1385. [Google Scholar] [CrossRef]
- Deitel, M. Bariatric surgery, proton pump inhibitors, and possibility of osteoporosis. Surg. Obes. Relat. Dis. 2010, 6, 461–462. [Google Scholar] [CrossRef]
- Coupaye, M.; Breuil, M.C.; Riviere, P.; Castel, B.; Bogard, C.; Dupre, T.; Msika, S.; Ledoux, S. Serum vitamin D increases with weight loss in obese subjects 6 months after Roux-en-Y gastric bypass. Obes. Surg. 2013, 23, 486–493. [Google Scholar] [CrossRef]
- Mahlay, N.F.; Verka, L.G.; Thomsen, K.; Merugu, S.; Salomone, M. Vitamin D status before Roux-en-Y and efficacy of prophylactic and therapeutic doses of vitamin D in patients after Roux-en-Y gastric bypass surgery. Obes. Surg. 2009, 19, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient—2013 Update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery*. Obesity 2013, 21, S1–S27. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, A.P.; Emous, M.; Laville, M.; Tack, J. Dumping syndrome after esophageal, gastric or bariatric surgery: Pathophysiology, diagnosis, and management. Obes. Rev. 2017, 18, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K.; Baker, S.; Zerwekh, J.; Poindexter, J.; Garcia-Hernandez, P.A.; Pak, C.Y. Limited risk of kidney stone formation during long-term calcium citrate supplementation in nonstone forming subjects. J. Urol. 1994, 152, 324–327. [Google Scholar] [CrossRef]
- Tondapu, P.; Provost, D.; Adams-Huet, B.; Sims, T.; Chang, C.; Sakhaee, K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes. Surg. 2009, 19, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K.; Pak, C. Superior calcium bioavailability of effervescent potassium calcium citrate over tablet formulation of calcium citrate after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2013, 9, 743–748. [Google Scholar] [CrossRef]
- Tarplin, S.; Ganesan, V.; Monga, M. Stone formation and management after bariatric surgery. Nat. Rev. Urol. 2015, 12, 263–270. [Google Scholar] [CrossRef]
- Cruz, S.; de Matos, A.C.; da Cruz, S.P.; Pereira, S.; Saboya, C.; Ramalho, A. Non-pregnant Women Have a Lower Vitamin D than Pregnant Women After Gastric Bypass. Obes. Surg. 2020. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Busetto, L.; Dicker, D.; Azran, C.; Batterham, R.L.; Farpour-Lambert, N.; Fried, M.; Hjelmesaeth, J.; Kinzl, J.; Leitner, D.R.; Makaronidis, J.M.; et al. Practical Recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the Post-Bariatric Surgery Medical Management. Obes. Facts 2017, 10, 597–632. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Vitamin B6 intake and the risk of incident kidney stones. Urolithiasis 2018, 46, 265–270. [Google Scholar] [CrossRef] [PubMed]
- IOM. Dietary Reference Intakes: RDA and AI for Vitamins and Elements; Institute of Medicine: Food and Nutrition Board; IOM: Washington DC, USA, 2006. [Google Scholar]
- U. S. Department of Agriculture, Agricultural Research Service. Available online: https://fdc.nal.usda.gov/ (accessed on 13 March 2020).
- Massey, K.; Shultz, T.D.; Mitchell, M.E. Urinary oxalate is not increased by vitamin B-6 depletion in young women. Nutr. Res. 1997, 17, 1499–1502. [Google Scholar] [CrossRef]
- Rao, T.V.; Choudhary, V.K. Effect of pyridoxine (Vitamin-B(6)) supplementation on calciuria and oxaluria levels of some normal healthy persons and urinary stone patients. Indian J. Clin. Biochem. 2005, 20, 166–169. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J. Urol. 1996, 155, 1847–1851. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Stampfer, M.J. Intake of vitamins B6 and C and the risk of kidney stones in women. J. Am. Soc. Nephrol. 1999, 10, 840–845. [Google Scholar] [PubMed]
- Patel, J.J.; Mundi, M.S.; Hurt, R.T.; Wolfe, B.; Martindale, R.G. Micronutrient Deficiencies After Bariatric Surgery: An Emphasis on Vitamins and Trace Minerals. Nutr. Clin. Pract. 2017, 32, 471–480. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Mandel, E.I.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Dietary Protein and Potassium, Diet-Dependent Net Acid Load, and Risk of Incident Kidney Stones. Clin. J Am. Soc. Nephrol. 2016, 11, 1834–1844. [Google Scholar] [CrossRef]
- Baxmann, A.C.; De, O.G.; Mendonça, C.; Heilberg, I.P. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int. 2003, 63, 1066–1071. [Google Scholar] [CrossRef]
- Traxer, O.; Huet, B.; Poindexter, J.; Pak, C.Y.; Pearle, M.S. Effect of ascorbic acid consumption on urinary stone risk factors. J. Urol. 2003, 170, 397–401. [Google Scholar] [CrossRef]
- Massey, L.K.; Liebman, M.; Kynast-Gales, S.A. Ascorbate increases human oxaluria and kidney stone risk. J. Nutr. 2005, 135, 1673–1677. [Google Scholar] [CrossRef]
- Berger, M.M.; Pantet, O.; Schneider, A.; Ben-Hamouda, N. Micronutrient Deficiencies in Medical and Surgical Inpatients. J. Clin. Med. 2019, 8, 931. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, N.M.; Tondapu, P.; Guth, E.S.; Livingston, E.H.; Sakhaee, K. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J. Urol. 2010, 183, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Storm, D.W.; Fulmer, B.R.; Still, C.D.; Wood, G.C.; Hartle, J.E. A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J. Urol. 2009, 182, 2334–2339. [Google Scholar] [CrossRef] [PubMed]
- Valezi, A.C.; Fuganti, P.E.; Junior, J.M.; Delfino, V.D. Urinary evaluation after RYGBP: A lithogenic profile with early postoperative increase in the incidence of urolithiasis. Obes. Surg. 2013, 23, 1575–1580. [Google Scholar] [CrossRef]
- Maalouf, N.M.; Sakhaee, K.; Parks, J.H.; Coe, F.L.; Adams-Huet, B.; Pak, C.Y. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004, 65, 1422–1425. [Google Scholar] [CrossRef]
- Meschi, T.; Maggiore, U.; Fiaccadori, E.; Schianchi, T.; Bosi, S.; Adorni, G.; Ridolo, E.; Guerra, A.; Allegri, F.; Novarini, A.; et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004, 66, 2402–2410. [Google Scholar] [CrossRef]
- Odvina, C.V. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin. J. Am. Soc. Nephrol. 2006, 1, 1269–1274. [Google Scholar] [CrossRef]
- Baia, L.A.C.; Baxmann, A.C.; Moreira, S.R.; Holmes, R.P.; Heilberg, I.P. Noncitrus alkaline fruit: A dietary alternative for the treatment of hypocitraturic stone formers. J. Endourol. 2012, 26, 1221–1226. [Google Scholar] [CrossRef]
- Tosukhowong, P.; Yachantha, C.; Sasivongsbhakdi, T.; Ratchanon, S.; Chaisawasdi, S.; Boonla, C.; Tungsanga, K. Citraturic, alkalinizing and antioxidative effects of limeade-based regimen in nephrolithiasis patients. Urol. Res. 2008, 36, 149–155. [Google Scholar] [CrossRef]
- Kang, D.E.; Sur, R.L.; Haleblian, G.E.; Fitzsimons, N.J.; Borawski, K.M.; Preminger, G.M. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J. Urol. 2007, 177, 1358–1362. [Google Scholar] [CrossRef]
- Siener, R. Can the manipulation of urinary pH by beverages assist with the prevention of stone recurrence? Urolithiasis 2016, 44, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Heilberg, I.P.; Goldfarb, D.S. Optimum nutrition for kidney stone disease. Adv. Chronic Kidney Dis. 2013, 20, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Leeman, M.; Gadiot, R.P.M.; Wijnand, J.M.A.; Birnie, E.; Apers, J.A.; Biter, L.U.; Dunkelgrun, M. Effects of standard v. very long Roux limb Roux-en-Y gastric bypass on nutrient status: A 1-year follow-up report from the Dutch Common Channel Trial (DUCATI) Study. Br. J. Nutr. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Golzarand, M.; Toolabi, K.; Djafarian, K. Changes in Body Composition, Dietary Intake, and Substrate Oxidation in Patients Underwent Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy: A Comparative Prospective Study. Obes. Surg. 2019, 29, 406–413. [Google Scholar] [CrossRef] [PubMed]
- IOM. Dietary Reference Intakes: Water, Potassium, Sodium, Chloride, and Sulfate; Institute of Medicine: Food and Nutrition Board: Washington, DC, USA, 2004. [Google Scholar]
- Moize, V.L.; Pi-Sunyer, X.; Mochari, H.; Vidal, J. Nutritional pyramid for post-gastric bypass patients. Obes. Surg. 2010, 20, 1133–1141. [Google Scholar] [CrossRef]
- Davies, N.K.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: A systematic review. Surg. Obes. Relat. Dis. 2019, 15, 656–665. [Google Scholar] [CrossRef]
- Peat, C.M.; Kleiman, S.C.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiome in Bariatric Surgery Patients. Eur. Eat Disord. Rev. 2015, 23, 496–503. [Google Scholar] [CrossRef]
- Hatch, M. Gut microbiota and oxalate homeostasis. Ann. Transl. Med. 2017, 5, 36. [Google Scholar] [CrossRef]
- Ferraz, R.R.; Marques, N.C.; Froeder, L.; Menon, V.B.; Siliano, P.R.; Baxmann, A.C.; Heilberg, I.P. Effects of Lactobacillus casei and Bifidobacterium breve on urinary oxalate excretion in nephrolithiasis patients. Urol. Res. 2009, 37, 95–100. [Google Scholar] [CrossRef]
- Jiang, J.; Knight, J.; Easter, L.H.; Neiberg, R.; Holmes, R.P.; Assimos, D.G. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J. Urol. 2011, 186, 135–139. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Modersitzki, F.; Asplin, J.R. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2007, 2, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Bade, D.J.; Hesse, A.; Hoppe, B. Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J. Transl. Med. 2013, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.R.F.; Zaparolli, M.R.; Cruz, M.R.R.; Schieferdecker, M.E.M.; Campos, A.C.L. Postoperative changes in intestinal microbiota and use of probiotics in Roux-en-Y gastric bypass and sleeve gastrectomy: An integrative review. Arq. Bras. Cir. Dig. 2018, 31, e1400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Stahlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fandriks, L.; le Roux, C.W.; Nielsen, J.; Backhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- De Groot, P.; Scheithauer, T.; Bakker, G.J.; Prodan, A.; Levin, E.; Khan, M.T.; Herrema, H.; Ackermans, M.; Serlie, M.J.M.; de Brauw, M.; et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut 2020, 69, 502–512. [Google Scholar] [CrossRef]
- Lieske, J.C. Probiotics for prevention of urinary stones. Ann. Transl. Med. 2017, 5, 29. [Google Scholar] [CrossRef]
- Steenackers, N.; Gesquiere, I.; Matthys, C. The relevance of dietary protein after bariatric surgery: What do we know? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 58–63. [Google Scholar] [CrossRef]
- Sherf Dagan, S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef]
- Park, S.M.; Jee, J.; Joung, J.Y.; Cho, Y.Y.; Sohn, S.Y.; Jin, S.M.; Hur, K.Y.; Kim, J.H.; Kim, S.W.; Chung, J.H.; et al. High Dietary Sodium Intake Assessed by 24-hour Urine Specimen Increase Urinary Calcium Excretion and Bone Resorption Marker. J. Bone Metab. 2014, 21, 189–194. [Google Scholar] [CrossRef]
- Martini, L.A.; Cuppari, L.; Cunha, M.A.; Schor, N.; Heilberg, I.P. Potassium and sodium intake and excretion in calcium stone forming patients. J. Ren. Nutr. 1998, 8, 127–131. [Google Scholar] [CrossRef]
- Martini, L.A.; Cuppari, L.; Colugnati, F.A.; Sigulem, D.M.; Szejnfeld, V.L.; Schor, N.; Heilberg, I.P. High sodium chloride intake is associated with low bone density in calcium stone-forming patients. Clin. Nephrol. 2000, 54, 85–93. [Google Scholar]
- WHO. Guideline: Sodium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 226. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Meschi, T.; Amato, F.; Briganti, A.; Novarini, A.; Giannini, A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J. Urol. 1996, 155, 839–843. [Google Scholar] [CrossRef]
- Mahawar, K.K.; Sharples, A.J. Contribution of Malabsorption to Weight Loss After Roux-en-Y Gastric Bypass: A Systematic Review. Obes. Surg. 2017, 27, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.H.; Ren, C.J.; Siegel, N.; Williams, T.; Barr, D.; Wolfe, B.; Dolan, K.; Fielding, G.A. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J. Gastrointest. Surg. 2004, 8, 48–55. [Google Scholar] [CrossRef]
- Topart, P.; Becouarn, G.; Delarue, J. Weight Loss and Nutritional Outcomes 10 Years after Biliopancreatic Diversion with Duodenal Switch. Obes. Surg. 2017, 27, 1645–1650. [Google Scholar] [CrossRef]
- Borbély, Y.M.; Osterwalder, A.; Kröll, D.; Nett, P.C.; Inglin, R.A. Diarrhea after bariatric procedures: Diagnosis and therapy. World J. Gastroenterol. 2017, 23, 4689–4700. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Moizé, V.; Andreu, A.; Flores, L.; Torres, F.; Ibarzabal, A.; Delgado, S.; Lacy, A.; Rodriguez, L.; Vidal, J. Long-term dietary intake and nutritional deficiencies following sleeve gastrectomy or Roux-En-Y gastric bypass in a mediterranean population. J. Acad. Nutr. Diet. 2013, 113, 400–410. [Google Scholar] [CrossRef]
| Food | Description | Oxalate Content (mg/100g) | References |
|---|---|---|---|
| Spinach | Cooked | 755–957 | [38,50,54] |
| Spinach | Raw | 656–900 | [38,50,54] |
| Rhubarb | Raw | 541 | [38] |
| Beet | Roots | 76 | [38] |
| Okra | Cooked | 45–70 | [38,40] |
| Turnip | Raw | 30 | [38] |
| Oca | Cooked | 373 | [55] |
| Potato | Baked | 24–97 | [38,40] |
| Chips | 75 | [38] | |
| French fries | 20–51 | [38,40] | |
| Sweet potato | Baked | 0.2–86.9 | [38,40] |
| Legumes | Navy Beans | 56–76 | [38,56] |
| Black Beans | 71 | [56] | |
| Fava Beans | 20 | [38] | |
| Red Kidney Beans | 13–26 | [38,56] | |
| Pinto Beans | 25–29 | [56] | |
| Soybeans | 7.0–57 | [38,56] | |
| Lentils | 8.0–39 | [38,56] | |
| Star fruit | Raw | 80–730 | [42,57] |
| Raspberry | Raw | 48 | [38] |
| Orange | Raw | 29 | [38] |
| Avocado | Raw | 19 | [38] |
| Nuts | Almonds | 435–491 | [38,56] |
| Cashews | 175–263 | [38,56] | |
| Walnuts | 77–111 | [38,56] | |
| Peanuts | 96–148 | [38,56] | |
| Peanut Butter | 65 | [38] | |
| Pistachios | 46–51 | [38,56] | |
| Pecans | 12–66 | [38,50,56] | |
| Sunflower seeds | 12 | [38] | |
| Macadamia nuts | 40–43 | [56] | |
| Bran | Rice bran | 281 | [38] |
| Oat bran | 10 | [38] | |
| Wheat bran | 34 | [38] | |
| Whole wheat flour | 29–67 | [38,56] | |
| White flour | 17–41 | [38,56] | |
| Chocolate * | Milk chocolate bar # | 18–140 | [38,56] |
| Dark Chocolate bar # | 155–485 | [58] | |
| Cocoa powder # | 84–783 | [38,58] | |
| Coffee * | Filtered | 1.0 | [38] |
| Decaffeinated, filtered | 2.0 | [38] | |
| Tea * | Black, Brewed | 4.0–16 | [38,59] |
| Green, Brewed | 0.3–2.3 | [59] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ormanji, M.S.; Rodrigues, F.G.; Heilberg, I.P. Dietary Recommendations for Bariatric Patients to Prevent Kidney Stone Formation. Nutrients 2020, 12, 1442. https://doi.org/10.3390/nu12051442
Ormanji MS, Rodrigues FG, Heilberg IP. Dietary Recommendations for Bariatric Patients to Prevent Kidney Stone Formation. Nutrients. 2020; 12(5):1442. https://doi.org/10.3390/nu12051442
Chicago/Turabian StyleOrmanji, Milene S., Fernanda G. Rodrigues, and Ita P. Heilberg. 2020. "Dietary Recommendations for Bariatric Patients to Prevent Kidney Stone Formation" Nutrients 12, no. 5: 1442. https://doi.org/10.3390/nu12051442
APA StyleOrmanji, M. S., Rodrigues, F. G., & Heilberg, I. P. (2020). Dietary Recommendations for Bariatric Patients to Prevent Kidney Stone Formation. Nutrients, 12(5), 1442. https://doi.org/10.3390/nu12051442





