Yeast Beta-Glucan Supplementation Downregulates Markers of Systemic Inflammation after Heated Treadmill Exercise
Abstract
1. Introduction
2. Materials and Methods
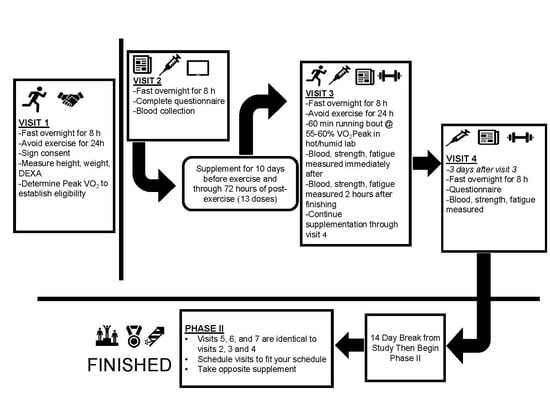
2.1. Experimental Design
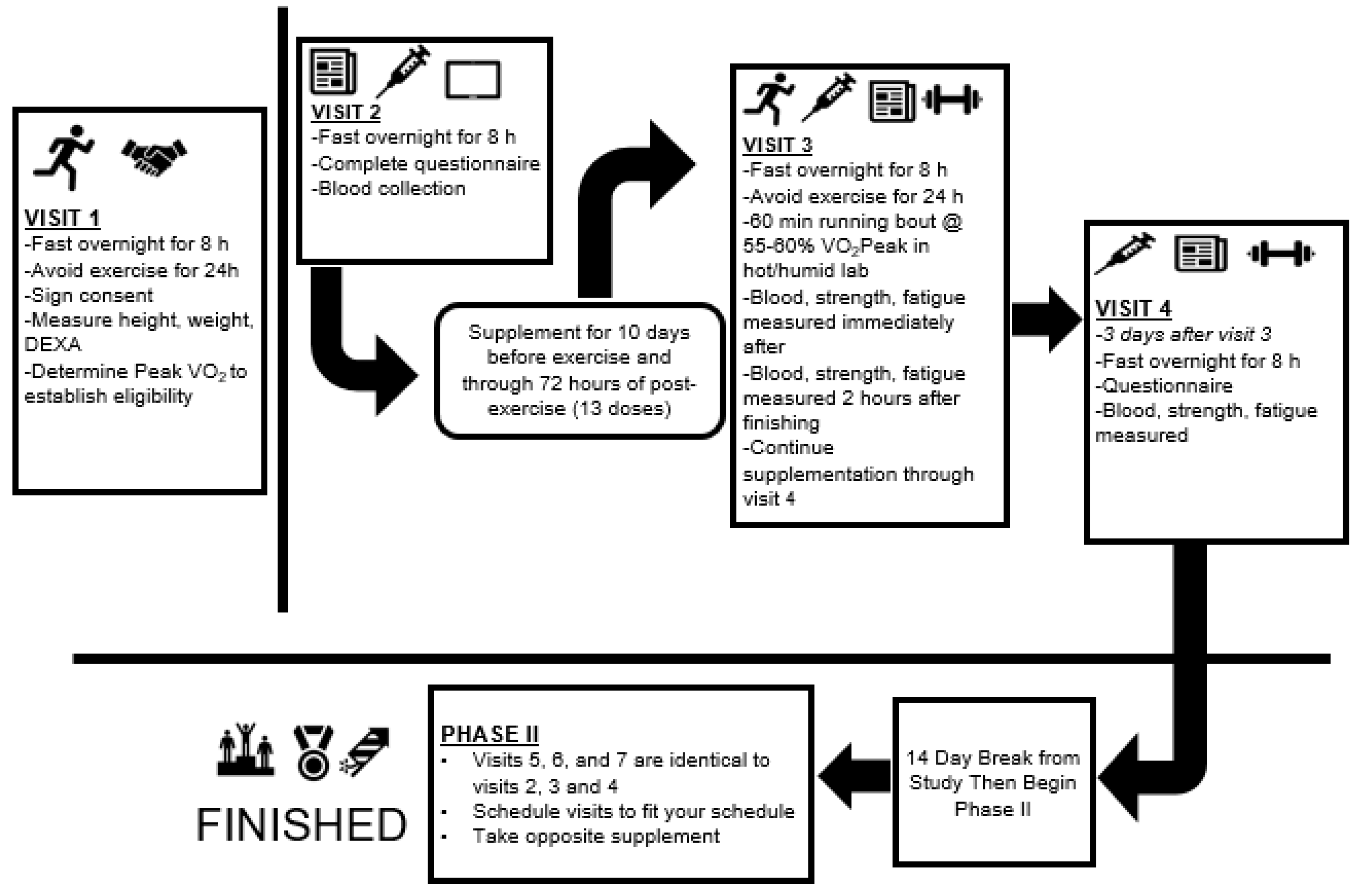
2.2. Participants
2.3. Procedures
2.3.1. Body Composition
2.3.2. VO2Peak Assessment
2.3.3. Blood Collection and Analysis
2.3.4. Biochemical Analysis
2.3.5. Muscular Force and Fatigue Assessments
2.3.6. Assessment of Mood State and Soreness
2.3.7. Supplementation
2.3.8. Exercise Bouts
2.3.9. Safety Measures: Temperature Monitoring and Rehydration
2.3.10. Dietary Control
2.4. Statistical Analysis
3. Results
3.1. Physiological Stress
3.2. Cytokine and Chemokine Expression
3.3. Complete Blood Counts
3.4. Comprehensive Metabolic Panel
3.5. Muscle Damage
3.6. Muscle Function
3.7. Profile of Mood States
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fitness 1990, 30, 316–328. [Google Scholar]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Carpenter, K.C.; Breslin, W.L.; Davidson, T.; Adams, A.; McFarlin, B.K. Baker’s yeast beta-glucan supplementation increases monocytes and cytokines post-exercise: Implications for infection risk? Br. J. Nutr. 2013, 109, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Walberg-Rankin, J.; Shute, M.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J. Appl. Physiol. 2003, 94, 1917–1925. [Google Scholar] [CrossRef]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.; Senden, J.; Saris, W.H.; Wagenmakers, A.J. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. (Lond) 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Tidball, J.G.; Dorshkind, K.; Wehling-Henricks, M. Shared signaling systems in myeloid cell-mediated muscle regeneration. Development 2014, 141, 1184–1196. [Google Scholar] [CrossRef]
- Varga, T.; Mounier, R.; Horvath, A.; Cuvellier, S.; Dumont, F.; Poliska, S.; Ardjoune, H.; Juban, G.; Nagy, L.; Chazaud, B. Highly Dynamic Transcriptional Signature of Distinct Macrophage Subsets during Sterile Inflammation, Resolution, and Tissue Repair. J. Immunol. 2016, 196, 4771–4782. [Google Scholar] [CrossRef]
- Nieman, D.C.; Zwetsloot, K.A.; Meaney, M.P.; Lomiwes, D.D.; Hurst, S.M.; Hurst, R.D. Post-Exercise Skeletal Muscle Glycogen Related to Plasma Cytokines and Muscle IL-6 Protein Content, but not Muscle Cytokine mRNA Expression. Front. Nutr. 2015, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Ostrowski, K.; Rohde, T.; Bruunsgaard, H. Nutrition, exercise and the immune system. Proc. Nutr. Soc. 1998, 57, 43–47. [Google Scholar] [CrossRef]
- Nieman, D.C. Influence of carbohydrate on the immune response to intensive, prolonged exercise. Exerc. Immunol. Rev. 1998, 4, 64–76. [Google Scholar] [PubMed]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, B.K.; Carpenter, K.C.; Davidson, T.; McFarlin, M.A. Baker’s yeast beta glucan supplementation increases salivary IgA and decreases cold/flu symptomatic days after intense exercise. J. Diet. Suppl. 2013, 10, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Davis, J.M.; Brown, A.S.; Carmichael, M.D.; Carson, J.A.; Van Rooijen, N.; Ghaffar, A.; Mayer, E.P. Benefits of oat beta-glucan on respiratory infection following exercise stress: Role of lung macrophages. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1593–R1599. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; McMahon, M.; Wrieden, J.L.; Davis, J.M.; Murphy, E.A.; Gross, S.J.; McAnulty, L.S.; Dumke, C.L. Beta-glucan, immune function, and upper respiratory tract infections in athletes. Med. Sci. Sports Exerc. 2008, 40, 1463–1471. [Google Scholar] [CrossRef]
- Noss, I.; Doekes, G.; Thorne, P.S.; Heederik, D.J.; Wouters, I.M. Comparison of the potency of a variety of beta-glucans to induce cytokine production in human whole blood. Innate Immun. 2013, 19, 10–19. [Google Scholar] [CrossRef]
- Auinger, A.; Riede, L.; Bothe, G.; Busch, R.; Gruenwald, J. Yeast (1,3)-(1,6)-beta-glucan helps to maintain the body’s defence against pathogens: A double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur. J. Nutr. 2013, 52, 1913–1918. [Google Scholar] [CrossRef]
- Dharsono, T.; Rudnicka, K.; Wilhelm, M.; Schoen, C. Effects of Yeast (1,3)-(1,6)-Beta-Glucan on Severity of Upper Respiratory Tract Infections: A Double-Blind, Randomized, Placebo-Controlled Study in Healthy Subjects. J. Am. Coll. Nutr. 2019, 38, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Graubaum, H.-I.; Busch, R.; Stier, H.; Gruenwald. A Double-Blind, Randomized, Placebo-Controlled Nutritional Study Using an Insoluble Yeast Beta-Glucan to Improve the Immune Defense System. Food Nutr. Sci. 2012, 3, 738–746. [Google Scholar] [CrossRef]
- Talbott, S.; Talbott, J. Effect of BETA 1, 3/1, 6 GLUCAN on Upper Respiratory Tract Infection Symptoms and Mood State in Marathon Athletes. J. Sports Sci. Med. 2009, 8, 509–515. [Google Scholar] [PubMed]
- McFarlin, B.K.; Venable, A.S.; Carpenter, K.C.; Henning, A.L.; Ogenstad, S. Oral Supplementation with Baker’s Yeast Beta Glucan Is Associated with Altered Monocytes, T Cells and Cytokines following a Bout of Strenuous Exercise. Front. Physiol. 2017, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Kreider, R.B.; Willoughby, D.S. Intramuscular adaptations to eccentric exercise and antioxidant supplementation. Amino Acids 2010, 39, 219–232. [Google Scholar] [CrossRef] [PubMed]
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Cole, N.; McCormick, J.J.; Kerksick, C.M.; Vaughan, R.A.; Dokladny, K.; et al. Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise. Nutrients 2018, 10, 1389. [Google Scholar] [CrossRef]
- Thompson, W.R.; Gordon, N.F.; Pescatello, L.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Camic, C.L.; Kovacs, A.J.; Enquist, E.A.; VanDusseldorp, T.A.; Hill, E.C.; Calantoni, A.M.; Yemm, A.J. An electromyographic-based test for estimating neuromuscular fatigue during incremental treadmill running. Physiol. Meas. 2014, 35, 2401–2413. [Google Scholar] [CrossRef]
- Thorstensson, A.; Karlsson, J. Fatiguability and fibre composition of human skeletal muscle. Acta Physiol. Scand. 1976, 98, 318–322. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018; 472p. [Google Scholar]
- Stasiule, L.; Capkauskiene, S.; Vizbaraite, D.; Stasiulis, A. Deep mineral water accelerates recovery after dehydrating aerobic exercise: A randomized, double-blind, placebo-controlled crossover study. J. Int. Soc. Sports Nutr. 2014, 11, 34. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Gleeson, M. Immune function in sport and exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef] [PubMed]


| Variable | Gender | Mean ± SD |
|---|---|---|
| Age (years) | Men (n = 16) | 29.6 ± 6.7 |
| Women (n = 15) | 30.1 ± 8.9 | |
| Total (n = 31) | 29.9 ± 7.7 | |
| Height (cm) | Men | 178.1 ± 7.2 |
| Women | 165.6 ± 4.1 | |
| Total | 172.1 ± 8.6 | |
| Body weight (kg) | Men | 83.2 ± 11.2 |
| Women | 66.7 ± 10.0 | |
| Total | 75.2 ± 13.4 | |
| DEXA Body fat (%) | Men | 21.2 ± 4.4 |
| Women | 29.6 ± 5.3 | |
| Total | 25.3 ± 6.4 | |
| DEXA Fat mass (kg) | Men | 18.0 ± 5.1 |
| Women | 20.4 ± 6.2 | |
| Total | 19.1 ± 5.7 | |
| DEXA FFM (kg) | Men | 66.2 ± 8.4 |
| Women | 47.3 ± 5.0 | |
| Total | 57.1 ± 11.8 | |
| VO2Peak (mL/kg/min) | Men | 49.6 ± 5.1 |
| Women | 38.7 ± 5.8 | |
| Total | 44.3 ± 7.7 |
| 0 min | 10 min | 15 min | 20 min | 30 min | 40 min | 45 min | 50 min | Final* | |
|---|---|---|---|---|---|---|---|---|---|
| Heart Rate (beats/min) | |||||||||
| PLA | 93 ± 13 | --- | 143 ±16 | --- | 153 ± 18 | --- | 161 ± 17 | --- | 167 ± 15 |
| YBG | 92 ± 16 | --- | 143 ± 15 | --- | 154 ± 19 | --- | 160 ± 18 | --- | 167 ± 19 |
| Exercise VO2 (mL/kg/min) | |||||||||
| PLA | 4.7 ± 1.5 | --- | 23.4 ± 4.5 | --- | 24.2 ± 4.5 | --- | 23.5 ± 6.3 | --- | 22.3 ± 6.6 |
| YBG | 4.9 ± 1.2 | --- | 22.9 ± 6.0 | --- | 24.5 ± 4.8 | --- | 24.3 ± 4.6 | --- | 25.2 ± 6.3 |
| Rating of Perceived Exertion (RPE) | |||||||||
| PLA | 7 ± 1 | 10 ± 2 | --- | 11 ± 2 | 13 ± 2 | 15 ± 5 | --- | 15 ± 3 | 15 ± 3 |
| YBG | 6 ± 1 | 9 ± 2 | --- | 11 ± 2 | 13 ± 2 | 13 ± 2 | --- | 15 ± 3 | 15 ± 3 |
| Core Temperature (°C) | |||||||||
| PLA | 36.5 ± 3.7 | 36.7 ± 3.7 | --- | 36.9 ± 3.8 | 37.3 ± 3.8 | 37.5 ± 4.1 | --- | 37.6 ± 4.3 | 37.9 ± 3.9 |
| YBG | 37.2 ± 0.3 | 37.4 ± 0.4 | --- | 37.7 ± 0.4 | 38.1 ± 0.4 | 38.2 ± 0.5 | --- | 38.5 ± 0.5 | 38.7 ± 0.6 |
| Variable | Group | Pre-Exercise | 0 h Post-Exercise | 2 h Post-Exercise | 72 h Post-Exercise | Within (p) | Time (p) | G × T (p) |
|---|---|---|---|---|---|---|---|---|
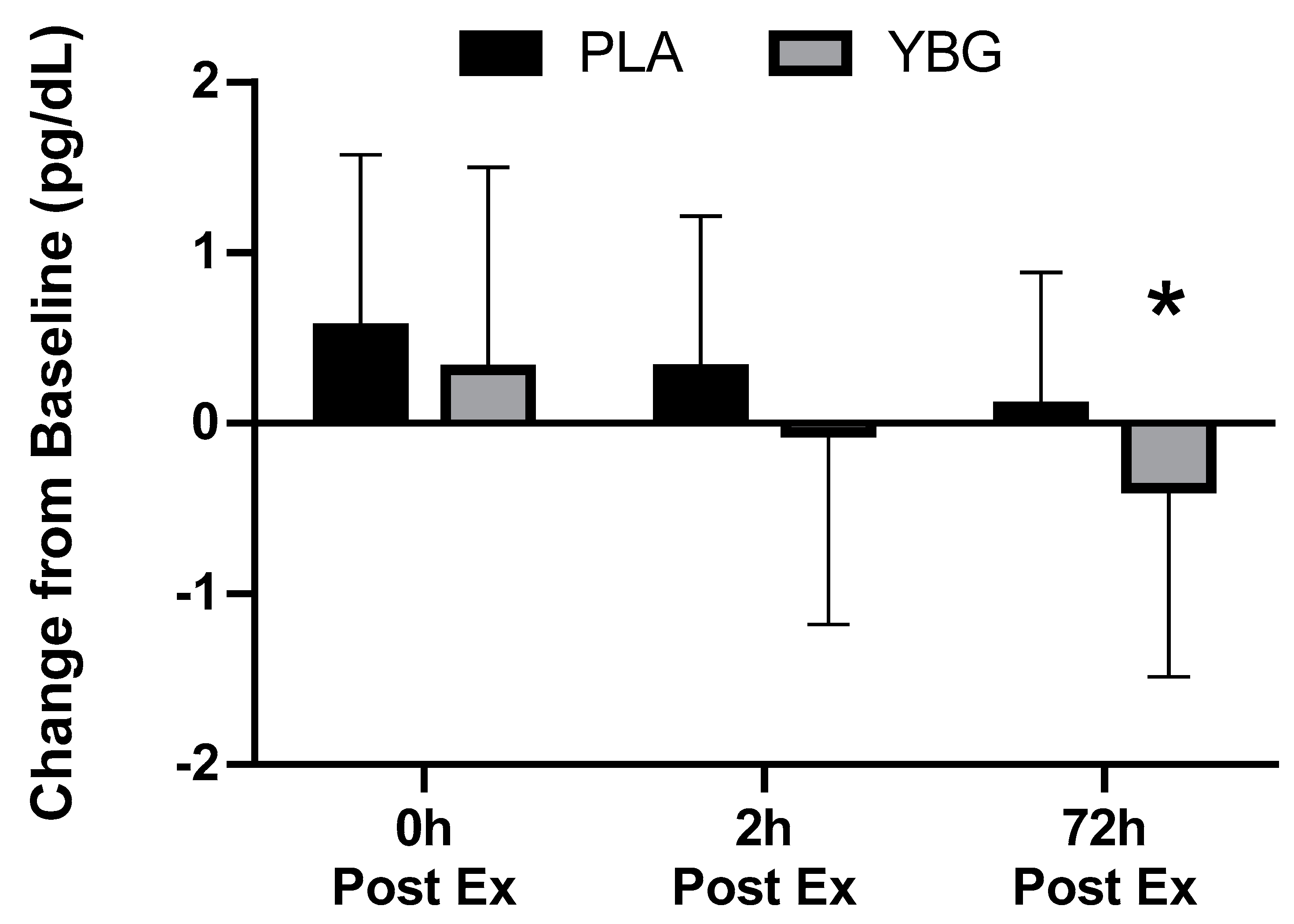
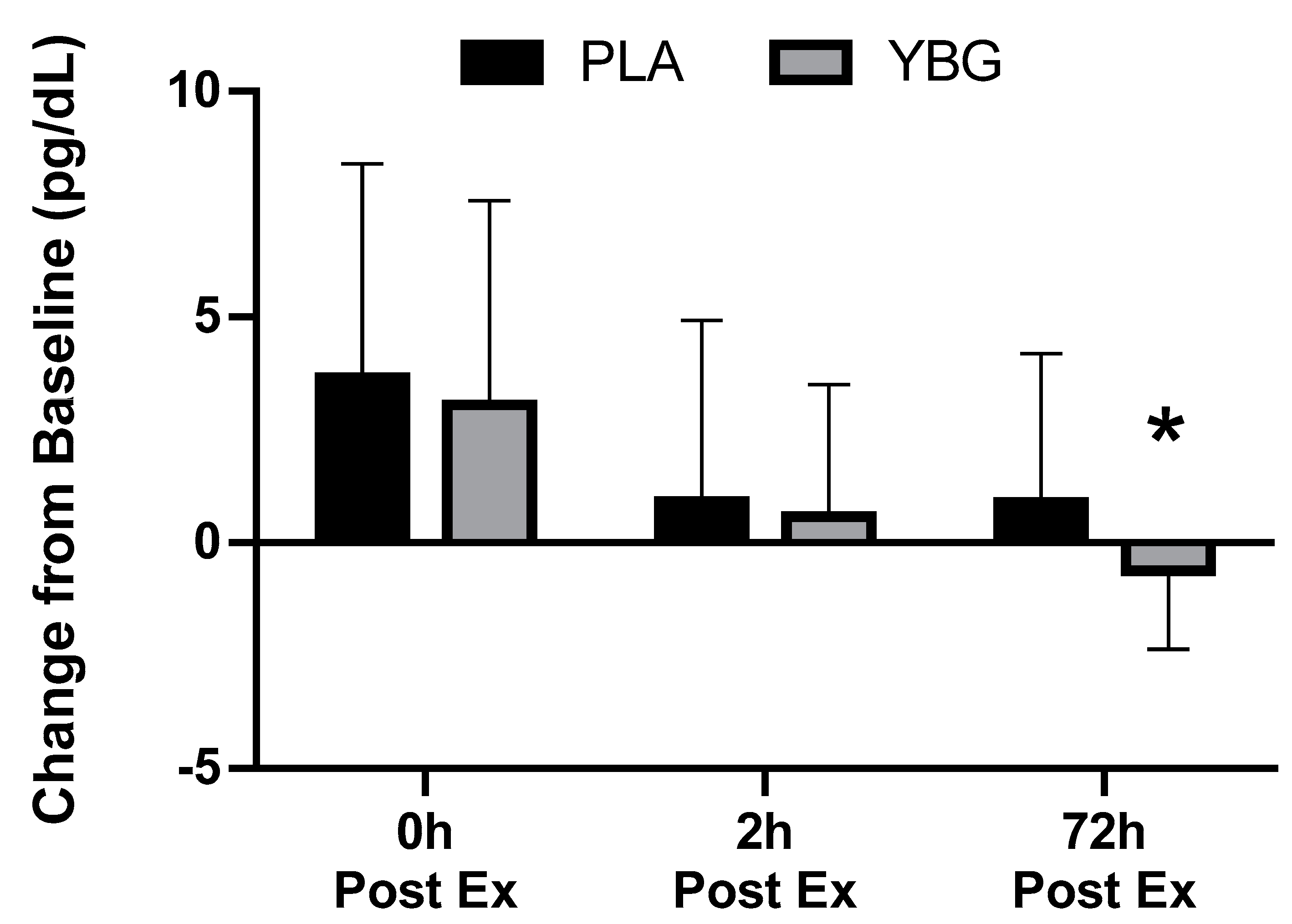
| Interferon-γ (pg/mL) | PLA | 1.36 ± 1.45 | 1.91 ± 3.15 +40.4% | 1.31 ± 1.27 −3.7% | 1.46 ± 1.44 +7.4% | 0.177 | 0.021 | 0.451 |
| YBG | 1.60 ± 1.90 | 2.11 ± 2.94 +36.5% | 1.68 ± 1.63 +5.0% | 1.31 ± 1.35 −18.1% | 0.017 | |||
| IL-6 (pg/mL) | PLA | 0.95 ± 1.73 | 1.53 ± 1.97 a +61.0% | 1.06 ± 1.76 a,b +11.6% | 0.66 ± 0.62 b −30.5% | <0.001 | <0.001 | 0.697 |
| YBG | 0.81 ± 0.75 | 1.46 ± 1.07 a +80.2% | 0.99 ± 0.77 b +22.2% | 0.61 ± 0.51 b,c −24.7% | <0.001 | |||
| IL-8 (pg/mL) | PLA | 1.74 ± 1.43 | 2.30 ± 1.95 +32.2% | 2.06 ± 1.75 +18.4% | 1.84 ± 1.15 +5.8% | 0.003 | <0.001 | 0.079 |
| YBG | 2.06 ± 1.66 | 2.37 ± 1.83 +15.0% | 2.00 ± 1.36 −2.6% | 1.68 ± 1.28 −18.4% | 0.001 | |||
| MCP-1 (pg/mL) | PLA | 8.52 ± 5.15 | 12.18 ± 7.60 +43.0% | 9.43 ± 5.56 +10.7% | 9.41 ± 5.42 +10.5% | <0.001 | <0.001 | 0.095 |
| YBG | 9.17 ± 4.92 | 12.22 ± 7.80 +33.0% | 9.75 ± 5.49 +6.3% | 8.55 ± 4.81 −6.8% | 0.001 | |||
| MIP-1β (pg/mL) | PLA | 1.68 ± 1.24 | 1.92 ± 1.60 +14.1% | 2.11 ± 1.56 +25.7% | 1.98 ± 1.37 +17.6% | 0.043 | 0.039 | 0.044 |
| YBG | 2.13 ± 1.59 | 2.21 ± 2.03 +3.7% | 2.41 ± 1.94 +12.9% | 1.94 ± 1.68 −9.1% | 0.039 | |||
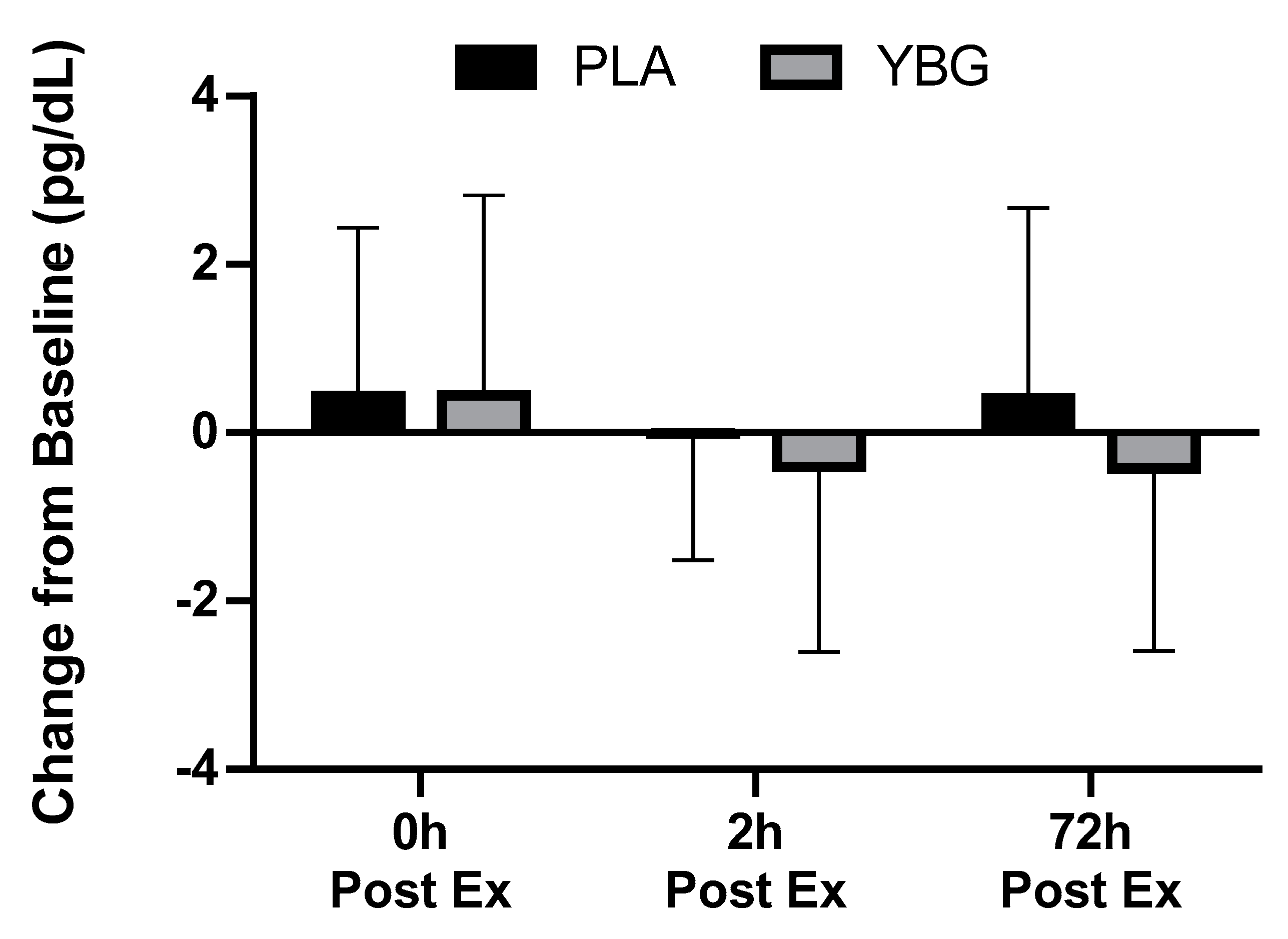
| TNF-α (pg/mL) | PLA | 5.94 ± 4.31 | 6.38 ± 5.23 +7.4% | 5.93 ± 3.87 −0.2% | 6.35 ± 4.00 +6.9% | 0.245 | 0.168 | 0.085 |
| YBG | 6.56 ± 4.61 | 7.00 ± 5.43 +6.8% | 6.16 ± 4.12 −6.1% | 6.13 ± 4.25 −6.5% | 0.043 |
| Variable | Group | Pre-Exercise | 0 h Post-Exercise | 2 h Post-Exercise | 72 h Post-Exercise | Within (p) | Time (p) | G × T (p) |
|---|---|---|---|---|---|---|---|---|
| Perceived Soreness (mm) | PLA | 12.9 ± 11.9 | 20.4 ± 17.8 a,b | 18.9 ± 16.2 a,b | 8.8 ± 6.5 | <0.001 | <0.001 | 0.78 |
| YBG | 14.4 ± 16.0 | 19.7 ± 20.9 b | 19.3 ± 18.5 b | 9.8 ± 7.1 | 0.004 | |||
| Creatine Kinase (U/L) | PLA | 108.3 ± 55.4 | 125.3 ± 64.5 a | 125.9 ± 64.5 a | 87.9 ± 44.6 b,c | <0.001 | <0.001 | 0.72 |
| YBG | 106.8 ± 64.3 | 123.1 ± 74.9 a | 125.8 ± 69.8 a | 87.2 ± 42.4 b,c | <0.001 | |||
| Myoglobin (mg/dL) | PLA | 28.1 ± 32.4 | 36.1 ± 34.9 | 35.7 ± 37.1 a | 31.5 ± 39.4 | 0.07 | <0.001 | 0.62 |
| YBG | 26.8 ± 30.7 | 33.4 ± 34.6 a | 31.0 ± 24.1 | 25.6 ± 26.4 b,c | 0.001 |
| Variable | Group | Pre-Exercise | 0 h Post-Exercise | 2 h Post-Exercise | 72 h Post-Exercise | Within (p) | Time (p) | G × T (p) |
|---|---|---|---|---|---|---|---|---|
| Isometric Peak Torque (N·m) | PLA | 171.5 ± 48.9 | 158.1 ± 47.7 a | 159.0 ± 52.0 a | 167.9 ± 50.1 | <0.001 | <0.001 | 0.79 |
| YBG | 165.9 ± 56.8 | 151.2 ± 46.0 | 152.8 ± 46.3 b | 165.0 ± 49.5 b, c | 0.003 | |||
| Isometric Average Peak Torque (N·m) | PLA | 164.7 ± 47.8 | 148.1 ± 43.6 a | 150.8 ± 48.8 a | 160.1 ± 49.5 b, c | <0.001 | <0.001 | 0.92 |
| YBG | 160.6 ± 46.6 | 142.9 ± 45.6 a | 146.7 ± 45.6 a | 157.1 ± 47.7 b, c | <0.001 | |||
| Isokinetic Peak Torque (N·m) | PLA | 136.9 ± 44.6 | 126.2 ± 40.7 a | 126.7 ± 43.0 a | 129.1 ± 44.5 a | <0.001 | 0.08 | 0.35 |
| YBG | 132.5 ± 48.3 | 128.7 ± 44.8 | 125.5 ± 44.3 a | 123.6 ± 50.5 | 0.19 | |||
| Isokinetic Average Peak Torque (N·m) | PLA | 87.4 ± 29.6 | 84.5 ± 29.9 | 86.3 ± 31.9 | 86.5 ± 30.9 | 0.07 | 0.04 | 0.94 |
| YBG | 86.5 ± 31.1 | 84.5 ± 31.5 | 85.3 ± 32.1 | 85.9 ± 31.5 | 0.20 | |||
| Fatigue Index (%) | PLA | 48.9 ± 10.9 | 45.7 ± 11.2 | 45.5 ± 13.1 | 46.9 ± 15.0 | 0.69 | 0.46 | 0.45 |
| YBG | 48.5 ± 12.7 | 46.5 ± 14.2 | 44.2 ± 14.5 | 47.2 ± 11.5 | 0.07 | |||
| Total Work Completed (J) | PLA | 5176 ± 1371 | 4749 ± 1363 a | 5009 ± 1491 | 5172 ± 1689 b | 0.05 | 0.001 | 0.69 |
| YBG | 5257 ± 1665 | 4912 ± 1496 a | 5039 ± 1566 | 5176 ± 1607 b | <0.001 |
| Variable | Group | Pre-Exercise | 0 h Post-Exercise | 2 h Post-Exercise | 72 h Post-Exercise | Within Group | Time (p) | G × T (p) |
|---|---|---|---|---|---|---|---|---|
| Tension | PLA | 14.2 ± 4.2 | 13.8 ± 3.1 | 12.9 ± 3.3 b | 13.3 ± 4.0 | 0.07 | <0.001 | 0.24 |
| YBG | 15.4 ± 4.9 | 15.4 ± 5.1 | 14.2 ± 4.8 a,b | 13.6 ± 4.4 a,b | <0.001 | |||
| Depression | PLA | 18.4 ± 7.0 | 17.9 ± 6.3 | 17.3 ± 5.4 a,b | 17.7 ± 5.8 | 0.009 | 0.01 | 0.17 |
| YBG | 18.4 ± 5.1 | 18.5 ± 5.6 | 18.1 ± 5.7b | 17.6 ± 4.6 | 0.15 | |||
| Anger | PLA | 14.8 ± 3.6 | 13.6 ± 2.6 a | 13.7 ± 3.1 | 13.5 ± 3.0 a | 0.001 | <0.001 | 0.04 |
| YBG | 16.0 ± 4.2 | 15.5 ± 4.4 | 14.4 ± 3.8 a | 14.2 ± 3.1 a | <0.001 | |||
| Vigor | PLA | 25.6 ± 7.5 | 24.8 ± 7.0 | 24.8 ± 7.1 | 23.1 ± 7.9 a | 0.003 | 0.09 | 0.04 |
| YBG | 24.7 ± 5.8 | 23.9 ± 6.6 | 24.5 ± 6.8 | 24.6 ± 6.1 | 0.65 | |||
| Fatigue | PLA | 12.4 ± 5.3 | 14.4 ± 5.9 | 12.7 ± 4.7 | 12.1 ± 5.0 b | 0.01 | 0.001 | 0.12 |
| YBG | 13.1 ± 5.1 | 15.3 ± 5.1 | 13.1 ± 4.0 b | 11.2 ± 3.4 b | <0.001 | |||
| Confusion | PLA | 11.4 ± 3.4 | 10.7 ± 3.2 | 10.5 ± 2.7 a | 10.5 ± 2.8 | 0.07 | 0.004 | 0.30 |
| YBG | 11.4 ± 2.8 | 11.4 ± 3.1 | 10.6 ± 2.6 | 10.4 ± 2.7 a,b | 0.001 | |||
| Total Mood Disturbance | PLA | 45.6 ± 23.3 | 45.6 ± 20.1 | 42.2 ± 19.2 | 44.0 ± 20.9 a,b | 0.001 | <0.001 | 0.007 |
| YBG | 49.5 ± 21.5 | 52.2 ± 21.5 | 45.9 ± 21.3 b | 42.4 ± 18.6 | 0.015 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabriskie, H.A.; Blumkaitis, J.C.; Moon, J.M.; Currier, B.S.; Stefan, R.; Ratliff, K.; Harty, P.S.; Stecker, R.A.; Rudnicka, K.; Jäger, R.; et al. Yeast Beta-Glucan Supplementation Downregulates Markers of Systemic Inflammation after Heated Treadmill Exercise. Nutrients 2020, 12, 1144. https://doi.org/10.3390/nu12041144
Zabriskie HA, Blumkaitis JC, Moon JM, Currier BS, Stefan R, Ratliff K, Harty PS, Stecker RA, Rudnicka K, Jäger R, et al. Yeast Beta-Glucan Supplementation Downregulates Markers of Systemic Inflammation after Heated Treadmill Exercise. Nutrients. 2020; 12(4):1144. https://doi.org/10.3390/nu12041144
Chicago/Turabian StyleZabriskie, Hannah A., Julia C. Blumkaitis, Jessica M. Moon, Brad S. Currier, Riley Stefan, Kayla Ratliff, Patrick S. Harty, Richard A. Stecker, Karolina Rudnicka, Ralf Jäger, and et al. 2020. "Yeast Beta-Glucan Supplementation Downregulates Markers of Systemic Inflammation after Heated Treadmill Exercise" Nutrients 12, no. 4: 1144. https://doi.org/10.3390/nu12041144
APA StyleZabriskie, H. A., Blumkaitis, J. C., Moon, J. M., Currier, B. S., Stefan, R., Ratliff, K., Harty, P. S., Stecker, R. A., Rudnicka, K., Jäger, R., Roberts, M. D., Young, K., Jagim, A. R., & Kerksick, C. M. (2020). Yeast Beta-Glucan Supplementation Downregulates Markers of Systemic Inflammation after Heated Treadmill Exercise. Nutrients, 12(4), 1144. https://doi.org/10.3390/nu12041144