A Randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study
Abstract
1. Introduction
2. Materials and Methods
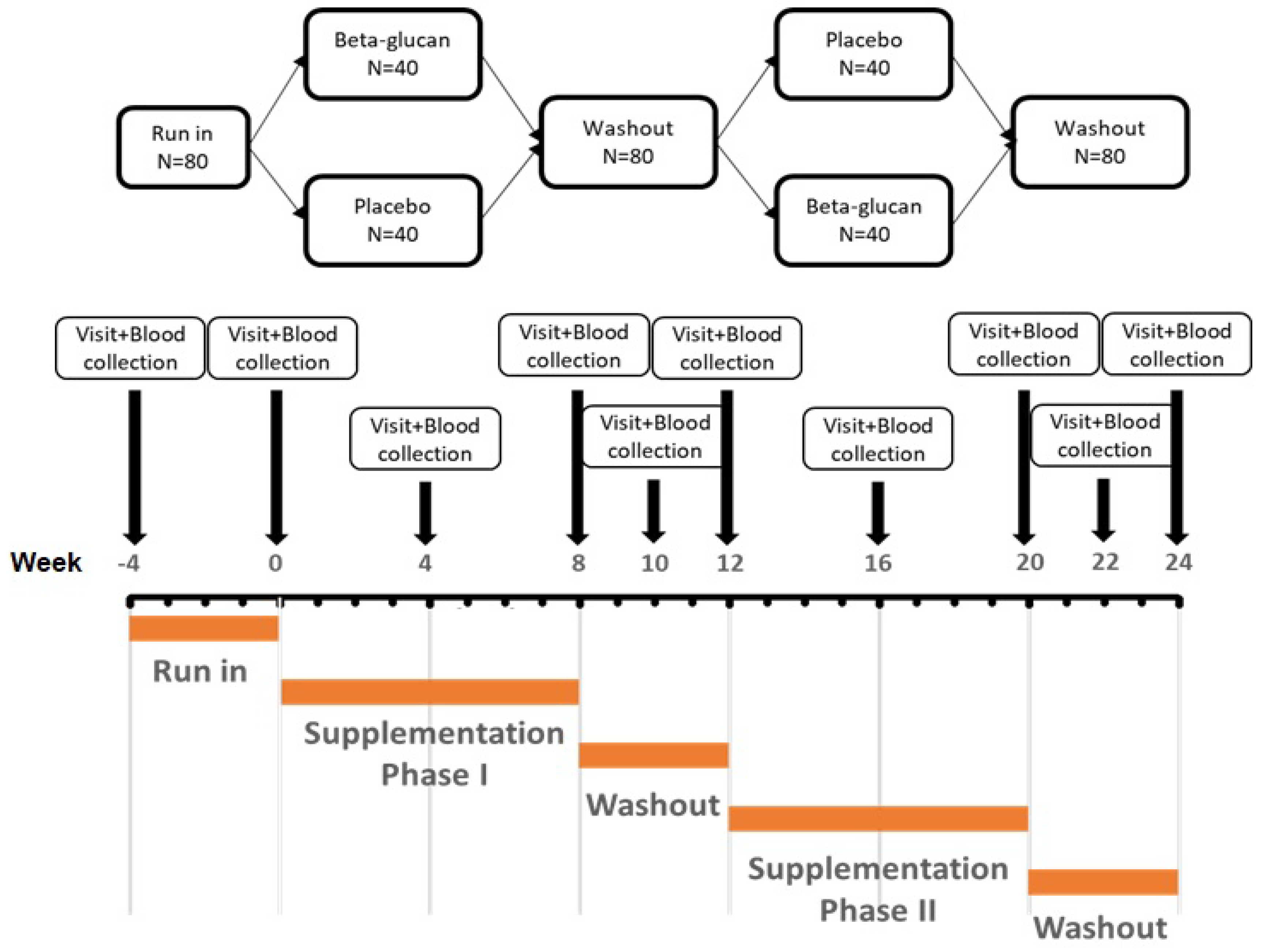
2.1. Study Design and Participants
2.2. Treatment
2.3. Assessments
2.3.1. Clinical Data and Anthropometric Measurements
2.3.2. Blood Pressure Measurements
2.3.3. Laboratory Data
2.3.4. Safety and Tolerability
2.3.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Ho, H.V.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.G.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef]
- Sima, P.; Vannucci, L.; Vetvicka, V. β-glucans and cholesterol (Review). Int J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Axelsen, M.; Augustin, L.S.; Vuksan, V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr. Opin. Lipidol. 2000, 11, 49–56. [Google Scholar] [CrossRef]
- Gee, J.M.; Blackburn, N.A.; Johnson, I.T. The influence of guar gum on intestinal cholesterol transport in the rat. Br. J. Nutr. 1983, 50, 215–224. [Google Scholar] [CrossRef]
- Kinner, M.; Nitschko, S.; Sommeregger, J.; Petrasch, A.; Linsberger-Martin, G.; Grausgruber, H.; Berghofer, E.; Siebenhandl-Ehn, S. Naked barley-Optimized recipe for pure barley bread with sufficient beta-glucan according to the EFSA health claims. J. Cereal Sci. 2011, 53, 225–230. [Google Scholar] [CrossRef]
- Karmally, W.; Montez, M.G.; Palmas, W.; Martinez, W.; Branstetter, A.; Ramakrishnan, R.; Holleran, S.F.; Haffner, S.M.; Ginsberg, H.N. Cholesterol-lowering benefits of oat-containing cereal in Hispanic americans. J. Am. Diet. Assoc. 2005, 105, 967–970. [Google Scholar] [CrossRef]
- Leadbetter, J.; Ball, M.J.; Mann, J.I. Effects of increasing quantities of oat bran in hypercholesterolemic people. Am. J. Clin. Nutr. 1991, 54, 841–845. [Google Scholar] [CrossRef]
- Fornari, C.; Donfrancesco, C.; Riva, M.A.; Palmieri, L.; Panico, S.; Vanuzzo, D.; Ferrario, M.M.; Pilotto, L.; Giampaoli, S.; Cesana, G. Social status and cardiovasculardisease: A Mediterranean case. Results from the Italian Progetto CUORE cohort study. BMC Public Health 2010, 10, 574. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Tosh, S.M.; Gibbs, A.L.; Brand-Miller, J.; Duncan, A.M.; Hart, V.; Lamarche, B.; Thomson, B.A.; Duss, R.; Wood, P.J. Physicochemical properties of oat b-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomied clinical trial. Am. J. Clin. Nutr. 2010, 92, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Rosticci, M.; Parini, A.; Giovannini, M.; Veronesi, M.; D’Addato, S.; Borghi, C. Effect of a short-term dietary supplementation with phytosterols, red yeast rice or both on lipid pattern in moderately hypercholesterolemic subjects: A three-arm, double-blind, randomized clinical trial. Nutr. Metab. 2017, 14, 61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cicero, A.F.G.; Caliceti, C.; Fogacci, F.; Giovannini, M.; Calabria, D.; Colletti, A.; Veronesi, M.; Roda, A.; Borghi, C. Effect of apple polyphenols on vascular oxidative stress and endothelium function: A translational study. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. Authors/Task Force Members. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Grandi, E.; Rosticci, M.; D’Addato, S.; Borghi, C. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci. Rep. 2018, 8, 11529. [Google Scholar] [CrossRef]
- Frank, L.; Kleinman, L.; Farup, C.; Taylor, L.; Miner, P., Jr. Psychometric validation of a constipation symptom assessment questionnaire. Scand. J. Gastroenterol. 1999, 34, 870–877. [Google Scholar] [CrossRef]
- Theuwissen, E.; Plat, J.; Mensink, R.P. Consumption of oat beta-glucan with or without plant stanols did not influence inflammatory markers in hypercholesterolemic subjects. Mol. Nutr. Food Res. 2009, 53, 370–376. [Google Scholar] [CrossRef]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-lowering effects of oat β-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef]
- Grundy, M.M.; Fardet, A.; Tosh, S.M.; Rich, G.T.; Wilde, P.J. Processing of oat: The impact on oat’s cholesterol lowering effect. Food Funct. 2018, 9, 1328–1343. [Google Scholar] [CrossRef]
- Wang, Y.; Harding, S.V.; Eck, P.; Thandapilly, S.J.; Gamel, T.H.; Abdel-Aal, E.S.M.; Crow, G.H.; Tosh, S.M.; Jones, P.J.; Ames, N.P. High-Molecular-Weight β-Glucan Decreases Serum Cholesterol Differentially Based on the CYP7A1 rs3808607 Polymorphism in Mildly Hypercholesterolemic Adults. J. Nutr. 2016, 146, 720–727. [Google Scholar] [CrossRef]
- He, L.X.; Zhao, J.; Huang, Y.S.; Li, Y. The difference between oats and beta-glucan extract intake in the management of HbA1c, fasting glucose and insulin sensitivity: A meta-analysis of randomized controlled trials. Food Funct. 2016, 7, 1413–1428. [Google Scholar] [CrossRef]
- Patti, A.M.; Al-Rasadi, K.; Giglio, R.V.; Nikolic, D.; Mannina, C.; Castellino, G.; Chianetta, R.; Banach, M.; Cicero, A.F.G.; Lippi, G.; et al. Natural approaches in metabolic syndrome management. Arch. Med. Sci. 2018, 14, 422–441. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef]
- Lia, A.; Hallmans, G.; Sandberg, A.S.; Sundberg, B.; Aman, P.; Andersson, H. Oat beta-glucan increases bile acid excretion and a fiber-rich barley fraction increases cholesterol excretion in ileostomy subjects. Am. J. Clin. Nutr. 1995, 62, 1245–1251. [Google Scholar] [CrossRef]
- Ellegård, L.; Andersson, H. Oat bran rapidly increases bile acid excretion and bile acid synthesis: An ileostomy study. Eur J. Clin. Nutr. 2007, 61, 938–945. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.M.; Tian, L.; van den Berg, M.; Bruggeman, G.; Bruininx, E.; Schols, H.A.; Faas, M.M.; de Vos, P. Endo-glucanase digestion of oat beta-Glucan enhances Dectin-1 activation in human dendritic cells. J. Funct. Foods. 2016, 21, 104–112. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, X.; Wang, M.; Zhang, C.; Cao, Y.; Mo, G.; Liang, J.; Zhu, S. Quantitative assessment of the effects of beta-glucan consumption on serum lipid profile and glucose level in hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 714–723. [Google Scholar] [CrossRef]
| Total dietary fiber | 44 g |
| - Glucan soluble fiber | 22 g |
| Carbohydrate | 22 g |
| Protein | 20 g |
| Total lipids | 5 g |
| - Saturated FA | 1 g |
| - Polyunsaturated FA | 2 g |
| - Monounsaturated FA | 2 g |
| Water | 5 g |
| Minerals | |
| - Sodium (Na) | 3 mg |
| - Magnesium (Mg) | 250 mg |
| - Calcium (Ca) | 120 mg |
| - Potassium (K) | 700 mg |
| - Iron (Fe) | 9 mg |
| - Zinc (Zn) | 6 mg |
| Parameters | Pre-Diet Standardization (N = 83) |
|---|---|
| Age (years) | 52.3 ± 4.4 |
| Weight (kg) | 74.5 ± 17.4 |
| Waist (cm) | 91.3 ± 14.6 |
| Systolic blood pressure (mmHg) | 128.3 ± 15.3 |
| Diastolic blood pressure (mmHg) | 81 ± 9.6 |
| Total cholesterol (mmol/L) | 5.75 ± 0.49 |
| Triglycerides (mmol/L) | 1.51 ± 0.80 |
| HDL-cholesterol (mmol/L) | 1.28 ± 0.29 |
| Non-HDL-cholesterol (mmol/L) | 4.48 ± 0.49 |
| LDL-cholesterol (mmol/L) | 3.78 ± 0.42 |
| VLDL-cholesterol (mmol/L) | 0.33 (0.32) |
| Apolipoprotein-A1 (mg/dL) | 149.3 ± 22.9 |
| Apolipoprotein -B (mg/dL) | 100.8 ± 18.5 |
| Fasting plasma glucose (mmol/L) | 4.97 ± 0.73 |
| Aspartate aminotransferase (μkat/L) | 0.38 ± 0.12 |
| Alanine aminotransferase (μkat/L) | 0.39 ± 0.24 |
| Parameters | Diet Composition |
|---|---|
| Total Energy (kcal/day) | 1388.7 ± 245.7 |
| Alcohol (% of total energy) | 4.4 (0.1–10.4) |
| Lipids (% of total energy) | 32.9 ± 4.9 |
| Saturated Fatty Acids (% of total energy) | 7.9 ± 2.4 |
| Monounsaturated Fatty Acids (% of total energy) | 12.4 ± 3.1 |
| Poliunsaturated Fatty Acids (% of total energy) | 4.4 ± 1.1 |
| Proteins (% of total energy) | 18.3 ± 5 |
| Animal Proteins (% of total energy) | 10.2 ± 6 |
| Vegetal Proteins (% of total energy) | 5.1 ± 2.1 |
| Carbohydrates (% of total energy) | 50.4 ± 5.9 |
| Soluble Charbohydrates (% of total energy) | 15.9 ± 3.7 |
| Starch (% of total energy) | 25.3 ± 4.5 |
| Total dietary fibers (% of total energy) | 2.2 ± 0.9 |
| Cholesterol (mg/day) | 148.4 ± 22.3 |
| Parameters | Baseline | Treatment Period | Wash-Out Period | ||
|---|---|---|---|---|---|
| 4 Weeks | 8 Weeks | 2 Weeks | 4 Weeks | ||
| Weight (kg) | 73.8 ± 17.2 | 73.8 ± 17.1 | 73.7 ± 17.1 | 73.8 ± 17.9 | 74 ± 17.2 |
| Waist (cm) | 90.9 ± 14.4 | 90.8 ± 14.3 | 90.6 ± 14 | 90.6 ± 14.9 | 90.7 ± 14.1 |
| Systolic blood pressure (mmHg) | 120.9 ± 15.8 | 120.9 ± 15.2 | 120.3 ± 15.8 | 120.2 ± 16.7 | 121.1 ± 15.1 |
| Diastolic blood pressure (mmHg) | 78.5 ± 8.6 | 78.8 ± 8.6 | 79.2 ± 9.9 | 78.1 ± 8.4 | 78 ± 9.1 |
| Heart rate (bpm) | 71.9 ± 10.6 | 66.3 ± 4.7 | 70.78 ± 12.9 | 72.6 ± 10.2 | 70.2 ± 9.6 |
| Total cholesterol (mmol/L) | 5.75 ± 0.77 | 5.84 ± 0.73 | 5.75 ± 0.75 | 5.79 ± 0.81 | 5.85 ± 0.87 |
| Triglycerides (mmol/L) | 1.48 ± 0.70 | 1.46 ± 0.88 | 1.4 ± 0.78 | 1.59 ± 0.81 | 1.5 ± 0.76 |
| HDL-cholesterol (mmol/L) | 1.31 ± 0.29 | 1.33 ± 0.29 | 1.31 ± 0.3 | 1.26 ± 0.3 | 1.34 ± 0.28 |
| LDL-cholesterol (mmol/L) | 3.76 ± 0.75 | 3.84 ± 0.65 | 3.8 ± 0.65 | 3.81 ± 0.8 | 3.83 ± 0.80 |
| Non HDL-cholesterol (mmol/L) | 4.44 ± 0.71 | 4.51 ± 0.73 | 4.44 ± 0.72 | 4.53 ± 0.75 | 4.51 ± 0.83 |
| Apolipoprotein A1 (mg/dL) | 157.3 ± 32.5 | 151.2 ± 25.7 | 151.6 ± 26. 5 | 148.4 ± 25.7 | 161 ± 29.2 |
| Apolipoprotein B (mg/dL) | 101.4 ± 22.7 | 101.6 ± 15.3 | 99.8 ± 17.6 | 102.9 ± 14.4 | 100.3 ± 17.1 |
| VLDL-cholesterol (mmol/L) | 0.68 ± 0.32 | 0.71 ± 0.65 | 0.68 ± 0.62 | 0.73 ± 0.37 | 0.69 ± 0.35 |
| Fasting plasma glucose (mmol/L) | 5.01 ± 0.56 | 5.06 ± 0.50 | 5.02 ± 0.49 | 4.99 ± 0.51 | 5.09 ± 0.59 |
| Aspartate aminotransferase (μkat/L) | 0.36 ± 0.09 | 0.37 ± 0.11 | 0.37 ± 0.12 | 0.36 ± 0.10 | 0.35 ± 0.08 |
| Alanine aminotransferase (μkat/L) | 0.36 ± 0.17 | 0.4 ± 0.27 | 0.44 ± 0.37 | 0.4 ± 0.18 | 0.41 ± 0.20 |
| Parameters | Baseline | Treatment Period | Wash-Out Period | ||
|---|---|---|---|---|---|
| 4 Weeks | 8 Weeks | 2 Weeks | 4 Weeks | ||
| Weight (kg) | 73.9 ± 17.6 | 73.8 ± 17.7 | 73.4 ± 17.6 | 73.9 ± 14.6 | 73.6 ± 17.7 |
| Waist (cm) | 90.3 ± 14.2 | 90.2 ± 14.0 | 89.9 ± 13.7 | 88.5 ± 13.7 | 90.3 ± 13.8 |
| Systolic blood pressure (mmHg) | 124.3 ± 16.0 | 121.1 ± 14.1 | 119.5 ± 15.2 | 122.2 ± 16.5 | 120.7 ± 14.2 |
| Diastolic blood pressure (mmHg) | 80.4 ± 9.6 | 78.6 ± 8.8 | 79.4 ± 9 | 80.4 ± 8.9 | 78.4 ± 9.4 |
| Heart rate (bpm) | 72.1 ± 11.5 | 68 ± 1.4 | 72.3 ± 12.9 | 72.1 ± 11.8 | 72 ± 8.2 |
| Total cholesterol (mmol/L) | 5.77 ± 0.68 | 5.38 ± 0.58 *,° | 5.24 ± 0.57 *,° | 5.76 ± 0.71 | 5.81 ± 0.76 |
| Triglycerides (mmol/L) | 1.48 ± 0.81 | 1.6 ± 0.89 | 1.62 ± 1.10 | 1.46 ± 0.80 | 1.52 ± 0.72 |
| HDL-cholesterol (mmol/L) | 1.29 ± 0.33 | 1.32 ± 0.29 | 1.3 ± 0.29 | 1.28 ± 0.32 | 1.33 ± 0.29 |
| LDL-cholesterol (mmol/L) | 3.8 ± 0.64 | 3.33 ± 0.60 *,§ | 3.21 ± 0.65 *,§ | 3.82 ± 0.64 | 3.78 ± 0.74 |
| Non HDL-cholesterol (mmol/L) | 4.48 ± 0.67 | 4.07 ± 0.60 *,§ | 3.95 ± 0.62 *,§ | 4.49 ± 0.66 | 4.48 ± 0.73 |
| Apolipoprotein A1 (mg/dL) | 151.7 ± 27.2 | 149.1 ± 27.5 | 147.33 ± 26.9 | 150.1 ± 25.7 | 166.8 ± 27.6 |
| Apolipoprotein B (mg/dL) | 99.2 ± 16.7 | 101.5 ± 16 | 100.1 ± 15.4 | 96.9 ± 14.6 | 102.8 ± 14.4 |
| VLDL-cholesterol (mmol/L) | 0.66 ± 0.32 | 0.73 ± 0.41 | 0.81 ± 0.83 | 0.67 ± 0.36 | 0.7 ± 0.33 |
| Fasting plasma glucose (mmol/L) | 4.78 ± 0.55 | 5.05 ± 0.48 | 5.02 ± 0.52 | 4.97 ± 0.51 | 5.07 ± 0.52 |
| Aspartate aminotransferase (μkat/L) | 0.38 ± 0.10 | 0.38 ± 0.11 | 0.37 ± 0.1 | 0.37 ± 0.10 | 0.36 ± 0.10 |
| Alanine aminotransferase (μkat/L) | 0.37 ± 0.18 | 0.42 ± 0.21 | 0.42 ± 0.22 | 0.36 ± 0.18 | 0.42 ± 0.20 |
| Parameters | Placebo | Beta-Glucan | ||
|---|---|---|---|---|
| Mean | p | Mean | p | |
| Number of defecations per week | 7 | 0.264 | 7 | 0.730 |
| Stool consistency | 3 | 0.482 | 3 | 0.438 |
| Easiness of stool expulsion | 4 | 0.610 | 3 | 0.699 |
| Perception of total expulsion | 3 | 0.691 | 4 | 0.583 |
| Abdominal discomfort intensity | 2 | 0.744 | 2 | 0.923 |
| Abdominal swelling perception | 3 | 0.749 | 3 | 0.760 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicero, A.F.G.; Fogacci, F.; Veronesi, M.; Strocchi, E.; Grandi, E.; Rizzoli, E.; Poli, A.; Marangoni, F.; Borghi, C. A Randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients 2020, 12, 686. https://doi.org/10.3390/nu12030686
Cicero AFG, Fogacci F, Veronesi M, Strocchi E, Grandi E, Rizzoli E, Poli A, Marangoni F, Borghi C. A Randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients. 2020; 12(3):686. https://doi.org/10.3390/nu12030686
Chicago/Turabian StyleCicero, Arrigo F.G., Federica Fogacci, Maddalena Veronesi, Enrico Strocchi, Elisa Grandi, Elisabetta Rizzoli, Andrea Poli, Franca Marangoni, and Claudio Borghi. 2020. "A Randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study" Nutrients 12, no. 3: 686. https://doi.org/10.3390/nu12030686
APA StyleCicero, A. F. G., Fogacci, F., Veronesi, M., Strocchi, E., Grandi, E., Rizzoli, E., Poli, A., Marangoni, F., & Borghi, C. (2020). A Randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients, 12(3), 686. https://doi.org/10.3390/nu12030686








