To Eat or Not to eat: A Review of the Relationship between Chocolate and Migraines
Abstract
:1. Introduction
1.1. Chocolate Composition
1.2. Chocolate and Health
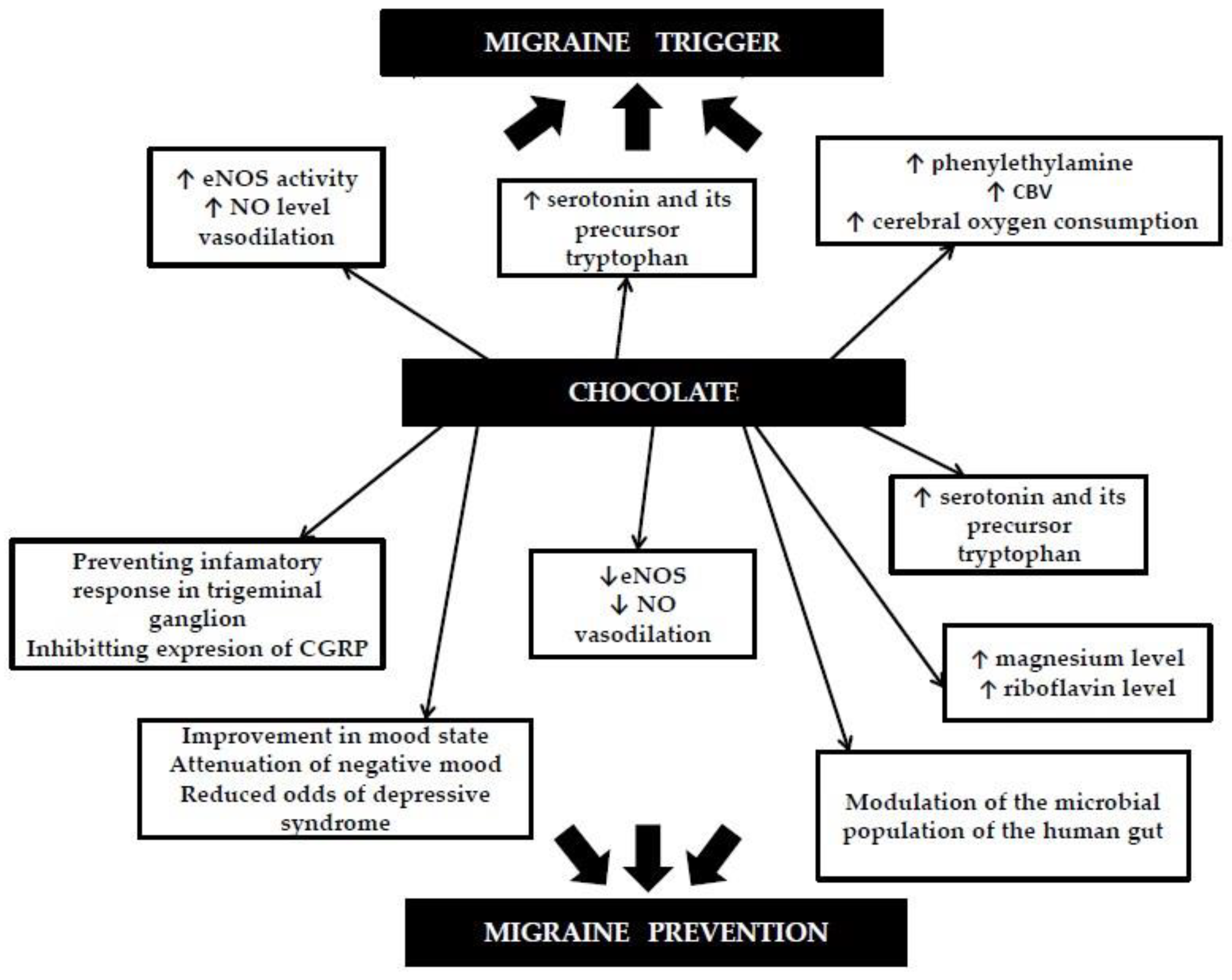
1.3. Chocolate and Migraines as a Potential Mechanism of Action
1.4. Migraine Triggers
2. Methods
3. Results and Discussion
3.1. The Prevalence of Chocolate as a Migraine Trigger Factor
3.2. Double Blind Provocative Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Collaborators, G.H. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Finocchi, C.; Sivori, G. Food as trigger and aggravating factor of migraine. Neurol. Sci. 2012, 33 (Suppl. 1), S77–S80. [Google Scholar] [CrossRef]
- Chakravarty, A.; Mukherjee, A.; Roy, D. Trigger factors in childhood migraine: A clinic-based study from eastern India. J. Headache Pain 2009, 10, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, P.T.; Gonçalves, T.R.; Strabelli, C.G.; Lucchino, N.M.; Matos, F.C.; Santos, J.P.; Zukerman, E.; Zukerman-Guendler, V.; Mercante, J.P.; Masruha, M.R.; et al. Trigger factors in migraine patients. Arq. Neuropsiquiatr. 2008, 66, 494–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007, 27, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Mollaoğlu, M. Trigger factors in migraine patients. J. Health Psychol. 2013, 18, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Pavlovic, J.M.; Haut, S.R.; Grosberg, B.M.; Buse, D.C. Methodological issues in studying trigger factors and premonitory features of migraine. Headache 2014, 54, 1661–1669. [Google Scholar] [CrossRef]
- Peroutka, S.J. What turns on a migraine? A systematic review of migraine precipitating factors. Curr. Pain Headache Rep. 2014, 18, 454. [Google Scholar] [CrossRef]
- Hoffmann, J.; Recober, A. Migraine and triggers: Post hoc ergo propter hoc? Curr. Pain Headache Rep. 2013, 17, 370. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Mattiuzzi, C.; Cervellin, G. Chocolate and migraine: The history of an ambiguous association. Acta Biomed. 2014, 85, 216–221. [Google Scholar]
- Geoff, T. Chocolate and Cocoa Butter—Structure and Composition, in Cocoa Butter and Related Compound; Garti, N., Widlak, N.R., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 1–33. [Google Scholar]
- Ellam, S.; Williamson, G. Cocoa and human health. Annu. Rev. Nutr. 2013, 33, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Zugravu, C.; Otelea, M.R. Dark Chocolate: To Eat or Not to Eat? A Review. J. AOAC Int. 2019, 102, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Casla, V.; Rosales-Conrado, N.; León-González, M.E.; Pérez-Arribas, L.V.; Polo-Díez, L.M. Determination of serotonin and its precursors in chocolate samples by capillary liquid chromatography with mass spectrometry detection. J. Chromatogr. A 2012, 1232, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cova, I.; Leta, V.; Mariani, C.; Pantoni, L.; Pomati, S. Exploring cocoa properties: Is theobromine a cognitive modulator? Psychopharmacology 2019, 236, 561–572. [Google Scholar] [CrossRef]
- Irsfeld, M.; Spadafore, M.; Prüß, B.M. β-phenylethylamine, a small molecule with a large impact. Webmedcentral 2013, 4, 4409. [Google Scholar]
- Cinquanta, L.; Di Cesare, C.; Manoni, R.; Piano, A.; Roberti, P.; Salvatori, G. Mineral essential elements for nutrition in different chocolate products. Int. J. Food Sci. Nutr. 2016, 67, 773–778. [Google Scholar] [CrossRef]
- Montagna, M.T.; Diella, G.; Triggiano, F.; Caponio, G.R.; De Giglio, O.; Caggiano, G.; Di Ciaula, A.; Portincasa, P. Chocolate, “Food of the Gods”: History, Science, and Human Health. Int. J. Environ. Res. Public Health 2019, 16, 4960. [Google Scholar] [CrossRef] [Green Version]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol 2017, 8, 677. [Google Scholar] [CrossRef] [Green Version]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef] [Green Version]
- Veronese, N.; Demurtas, J.; Celotto, S.; Caruso, M.G.; Maggi, S.; Bolzetta, F.; Firth, J.; Smith, L.; Schofield, P.; Koyanagi, A.; et al. Is chocolate consumption associated with health outcomes? An umbrella review of systematic reviews and meta-analyses. Clin. Nutr. 2019, 38, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Morze, J.; Schwedhelm, C.; Bencic, A.; Hoffmann, G.; Boeing, H.; Przybylowicz, K.; Schwingshackl, L. Chocolate and risk of chronic disease: A systematic review and dose-response meta-analysis. Eur. J. Nutr. 2020, 59, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, J.P.; Santana, A.; Baruqui, D.L.; Suraci, N. The Cardiovascular effects of chocolate. Rev. Cardiovasc. Med. 2018, 19, 123–127. [Google Scholar] [PubMed]
- Latham, L.S.; Hensen, Z.K.; Minor, D.S. Chocolate--guilty pleasure or healthy supplement? J. Clin. Hypertens (Greenwich) 2014, 16, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Faria, A.; Pestana, D.; Teixeira, D.; Couraud, P.O.; Romero, I.; Weksler, B.; de Freitas, V.; Mateus, N.; Calhau, C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011, 2, 39–44. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Massot-Cladera, M.; Abril-Gil, M.; Torres, S.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Impact of cocoa polyphenol extracts on the immune system and microbiota in two strains of young rats. Br. J. Nutr. 2014, 112, 1944–1954. [Google Scholar] [CrossRef] [Green Version]
- Boadas-Vaello, P.; Vela, J.M.; Verdu, E. New Pharmacological Approaches Using Polyphenols on the Physiopathology of Neuropathic Pain. Curr. Drug Targets 2017, 18, 160–173. [Google Scholar] [CrossRef]
- Costa de Miranda, R.; Paiva, E.S.; Suter Correia Cadena, S.M.; Brandt, A.P.; Vilela, R.M. Polyphenol-Rich Foods Alleviate Pain and Ameliorate Quality of Life in Fibromyalgic Women. Int. J. Vitam Nutr. Res. 2017, 87, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.S.; Penn, T.M.; Cable, S.T.; Aroke, E.N.; Goodin, B.R. Higher habitual dietary caffeine consumption is related to lower experimental pain sensitivity in a community-based sample. Psychopharmacology 2018, 235, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Bastian, B.; Jetten, J.; Hornsey, M.J. Gustatory pleasure and pain. The offset of acute physical pain enhances responsiveness to taste. Appetite 2014, 72, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kord-Varkaneh, H.; Ghaedi, E.; Nazary-Vanani, A.; Mohammadi, H.; Shab-Bidar, S. Does cocoa/dark chocolate supplementation have favorable effect on body weight, body mass index and waist circumference? A systematic review, meta-analysis and dose-response of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Andress-Rothrock, D.; King, W.; Rothrock, J. An analysis of migraine triggers in a clinic-based population. Headache 2010, 50, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Camboim Rockett, F.; Castro, K.; Rossoni de Oliveira, V.; da Silveira Perla, A.; Fagundes Chaves, M.L.; Schweigert Perry, I.D. Perceived migraine triggers: Do dietary factors play a role? Nutr. Hosp. 2012, 27, 483–489. [Google Scholar]
- Constantinides, V.; Anagnostou, E.; Bougea, A.; Paraskevas, G.; Kapaki, E.; Evdokimidis, I.; Kararizou, E. Migraine and tension-type headache triggers in a Greek population. Arq. Neuropsiquiatr. 2015, 73, 665–669. [Google Scholar] [CrossRef] [Green Version]
- Dalton, K. Food intake prior to a migraine attack—Study of 2,313 spontaneous attacks. Headache 1975, 15, 188–193. [Google Scholar] [CrossRef]
- Martin, V.T.; Vij, B. Diet and Headache: Part 1. Headache 2016, 56, 1543–1552. [Google Scholar] [CrossRef]
- Takeshima, T.; Ishizaki, K.; Fukuhara, Y.; Ijiri, T.; Kusumi, M.; Wakutani, Y.; Mori, M.; Kawashima, M.; Kowa, H.; Adachi, Y.; et al. Population-based door-to-door survey of migraine in Japan: The Daisen study. Headache 2004, 44, 8–19. [Google Scholar] [CrossRef]
- Yadav, R.K.; Kalita, J.; Misra, U.K. A study of triggers of migraine in India. Pain Med. 2010, 11, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, B.; Dussor, G. Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience 2016, 338, 130–144. [Google Scholar] [CrossRef] [Green Version]
- Abbey, M.J.; Patil, V.V.; Vause, C.V.; Durham, P.L. Repression of calcitonin gene-related peptide expression in trigeminal neurons by a Theobroma cacao extract. J. Ethnopharmacol. 2008, 115, 238–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cady, R.J.; Durham, P.L. Cocoa-enriched diets enhance expression of phosphatases and decrease expression of inflammatory molecules in trigeminal ganglion neurons. Brain Res. 2010, 1323, 18–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, Blood Pressure, and Vascular Function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razeghi Jahromi, S.; Togha, M.; Ghorbani, Z.; Hekmatdoost, A.; Khorsha, F.; Rafiee, P.; Shirani, P.; Nourmohammadi, M.; Ansari, H. The association between dietary tryptophan intake and migraine. Neurol. Sci. 2019, 40, 2349–2355. [Google Scholar] [CrossRef]
- McCulloch, J.; Harper, A.M. Phenylethylamine and cerebral blood flow. Possible involvement of phenylethylamine in migraine. Neurology 1977, 27, 817–821. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Sani, M.A.; Dahri, M.; Ghalichi, F.; Ghavami, A.; Arjang, P.; Tarighat-Esfanjani, A. The role of nutrients in the pathogenesis and treatment of migraine headaches: Review. Biomed. Pharmacother. 2018, 102, 317–325. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef] [Green Version]
- Gallelli, L.; Avenoso, T.; Falcone, D.; Palleria, C.; Peltrone, F.; Esposito, M.; De Sarro, G.; Carotenuto, M.; Guidetti, V. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache 2014, 54, 313–324. [Google Scholar] [CrossRef]
- Thompson, D.F.; Saluja, H.S. Prophylaxis of migraine headaches with riboflavin: A systematic review. J. Clin. Pharm. Ther. 2017, 42, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Goadsby, P.J.; Silberstein, S.D. Migraine triggers: Harnessing the messages of clinical practice. Neurology 2013, 80, 424–425. [Google Scholar] [CrossRef]
- Messlinger, K.; Lennerz, J.K.; Eberhardt, M.; Fischer, M.J. CGRP and NO in the trigeminal system: Mechanisms and role in headache generation. Headache 2012, 52, 1411–1427. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache 2019, 59, 659–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borkum, J.M. CGRP and Brain Functioning: Cautions for Migraine Treatment. Headache 2019, 59, 1339–1357. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Smith, L.; Firth, J.; Grabovac, I.; Soysal, P.; Koyanagi, A.; Hu, L.; Stubbs, B.; Demurtas, J.; Veronese, N.; et al. Is there a relationship between chocolate consumption and symptoms of depression? A cross-sectional survey of 13,626 US adults. Depress. Anxiety 2019, 36, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Owen, L. Effects of chocolate on cognitive function and mood: A systematic review. Nutr. Rev. 2013, 71, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Martami, F.; Togha, M.; Seifishahpar, M.; Ghorbani, Z.; Ansari, H.; Karimi, T.; Jahromi, S.R. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: A randomized double-blind controlled trial. Cephalalgia 2019, 39, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Marmura, M.J. Triggers, Protectors, and Predictors in Episodic Migraine. Curr. Pain Headache Rep. 2018, 22, 81. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, J.M.; Buse, D.C.; Sollars, C.M.; Haut, S.; Lipton, R.B. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache 2014, 54, 1670–1679. [Google Scholar] [CrossRef]
- Blau, J.N. Migraine triggers: Practice and theory. Pathol. Biol. 1992, 40, 367–372. [Google Scholar]
- Tai, M.S.; Yap, J.F.; Goh, C.B. Dietary trigger factors of migraine and tension-type headache in a South East Asian country. J. Pain Res. 2018, 11, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Millichap, J.G.; Yee, M.M. The diet factor in pediatric and adolescent migraine. Pediatr. Neurol. 2003, 28, 9–15. [Google Scholar] [CrossRef]
- Park, J.W.; Chu, M.K.; Kim, J.M.; Park, S.G.; Cho, S.J. Analysis of Trigger Factors in Episodic Migraineurs Using a Smartphone Headache Diary Applications. PLoS ONE 2016, 11, e0149577. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, F.; Vedovello, M.; Ulivi, M.; Vergallo, A.; Poletti, M.; Borelli, P.; Nuti, A.; Bonuccelli, U. How aware are migraineurs of their triggers? Headache 2013, 53, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, A.; Amin, F.M.; Amin, F.; Hauge, A.W.; Ashina, M.; Olesen, J. Provocation of migraine with aura using natural trigger factors. Neurology 2013, 80, 428–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, T. Editorial: Triggers and premonitory features in migraine. Headache 2014, 54, 1680. [Google Scholar] [CrossRef]
- System, M.C.H. 6 Tips for Headache Relief. Available online: https://www.mayoclinichealthsystem.org/hometown-health/speaking-of-health/6-tips-for-headache-relief (accessed on 15 January 2020).
- Foundation, A.M. Top 10 Migraine Triggers and How to Deal with Them. Available online: https://americanmigrainefoundation.org/resource-library/top-10-migraine-triggers-and-how-to-deal-with-them/ (accessed on 15 January 2020).
- Martin, P.R. Managing headache triggers: Think ‘coping’ not ‘avoidance’. Cephalalgia 2010, 30, 634–637. [Google Scholar] [CrossRef]
- Beh, S.C.; Masrour, S.; Smith, S.V.; Friedman, D.I. The Spectrum of Vestibular Migraine: Clinical Features, Triggers, and Examination Findings. Headache 2019, 59, 727–740. [Google Scholar] [CrossRef]
- Taheri, S. Effect of exclusion of frequently consumed dietary triggers in a cohort of children with chronic primary headache. Nutr. Health 2017, 23, 47–50. [Google Scholar] [CrossRef]
- Peris, F.; Donoghue, S.; Torres, F.; Mian, A.; Wöber, C. Towards improved migraine management: Determining potential trigger factors in individual patients. Cephalalgia 2017, 37, 452–463. [Google Scholar] [CrossRef]
- Rist, P.M.; Buring, J.E.; Kurth, T. Dietary patterns according to headache and migraine status: A cross-sectional study. Cephalalgia 2015, 35, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Neut, D.; Fily, A.; Cuvellier, J.C.; Vallée, L. The prevalence of triggers in paediatric migraine: A questionnaire study in 102 children and adolescents. J. Headache Pain 2012, 13, 61–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schürks, M.; Buring, J.E.; Kurth, T. Migraine features, associated symptoms and triggers: A principal component analysis in the Women’s Health Study. Cephalalgia 2011, 31, 861–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wöber, C.; Holzhammer, J.; Zeitlhofer, J.; Wessely, P.; Wöber-Bingöl, C. Trigger factors of migraine and tension-type headache: Experience and knowledge of the patients. J. Headache Pain 2006, 7, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bánk, J.; Márton, S. Hungarian migraine epidemiology. Headache 2000, 40, 164–169. [Google Scholar] [CrossRef]
- Marcus, D.A.; Scharff, L.; Turk, D.; Gourley, L.M. A double-blind provocative study of chocolate as a trigger of headache. Cephalalgia 1997, 17, 855–862. [Google Scholar] [CrossRef]
- Ulrich, V.; Russell, M.B.; Jensen, R.; Olesen, J. A comparison of tension-type headache in migraineurs and in non-migraineurs: A population-based study. Pain 1996, 67, 501–506. [Google Scholar] [CrossRef]
- Van den Bergh, V.; Amery, W.K.; Waelkens, J. Trigger factors in migraine: A study conducted by the Belgian Migraine Society. Headache 1987, 27, 191–196. [Google Scholar] [CrossRef]
- Peatfield, R.C.; Glover, V.; Littlewood, J.T.; Sandler, M.; Clifford Rose, F. The prevalence of diet-induced migraine. Cephalalgia 1984, 4, 179–183. [Google Scholar] [CrossRef]
- Moffett, A.M.; Swash, M.; Scott, D.F. Effect of chocolate in migraine: A double-blind study. J. Neurol. Neurosurg. Psychiatry 1974, 37, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Gibb, C.M.; Davies, P.T.; Glover, V.; Steiner, T.J.; Clifford Rose, F.; Sandler, M. Chocolate is a migraine-provoking agent. Cephalalgia 1991, 11, 93–95. [Google Scholar] [CrossRef]
| Author (Year) | Study Design | Study Design (Method of Identifying Trigger Factors) | Study Group: Type of Headache (Number of Participants) | Study Population Age (Years) | Chocolate/Cocoa Reported as a Trigger Factor (%) | Additional Information |
|---|---|---|---|---|---|---|
| Beh, S.C., 2019 [70] | Retrospective cross-sectional | Retrospective chart review | Vestibular migraine (n = 131) | No data | 3.8 | |
| Tai, M. S., 2018 [61] | Prospective cross-sectional | Comprehensive dietary check list | Migraine (n = 319) TTH (n = 365) MWA (n = 188) MA (n = 128), CM (n = 91) | Migraine 37.1 ± 14.3 TTH 46.5 ± 18.1 | Migraine 11.6% TTH 3.8% | Chocolate was significantly associated with migraines compared to TTH. |
| Taheri, S., 2017 [71] | Prospective observational case series | Food diary | Migraine (n = 65) TTH (n = 50) | Range 10–15 Mean 10.5 | 22 | 87% of patients achieved complete resolution of headaches by the exclusion of 1–3 triggers |
| Park, J.W., 2016 [63] | Prospective cross-sectional | Smartphone headache diary application | Episodic Migraine (n = 62) MWA (n = 60) MA (n = 2) | Mean 37.7 ± 8.6 | Cheese/ Chocolate 1.5 | |
| Peris, F., 2016 [72] | Prospective cross-sectional | Detailed 90-day paper diary database from the PAMINA migraine study | Migraine (n = 326) | No data | 2.5 | |
| Constantinides, V., 2015 [36] | Prospective cross-sectional | Interview | Migraine (n = 21) MWA (n = 39) MA (n = 12) TTH (n = 12) | Migraine 41.4 ± 12.9 TTH 37.5 ± 15.5 | Migraine 11.4 TTH 0 | There was a tendency toward more frequent reports of chocolate as a trigger in migraine patients. |
| Rist, P., 2014 [73] | Cross-sectional study among participants in the Women’s Health Study | Semi-quantitative food frequency questionnaire | Non-migraine headache (n = 5573) Migraine (n = 7042) MWA (n = 2972) MA (n = 1974) | Mean 53.6Mean 53.6 | Not applicable | Migraine patients with an aura were more likely to have a low intake of chocolate. Patients with non-migraine headaches were less likely to have a low intake of chocolate. |
| Mollaoglu, 2013 [7] | Prospective cross-sectional | Interview TF checklist | Migraine (n = 146) MWA (n = 73) MA (n = 53) | Mean 36.32 | 18.3 | |
| Camboim Rockett, F., 2012 [35] | Cross-sectional study | Predetermined list of 22 dietary factors | Migraine (n = 123) MWA (n = 84) MA (n = 39) | Mean 43.2 ± 13.9 | <20 | |
| Neut, D., 2012 [74] | Retrospective | Predetermined list of TF | Migraine (n = 102) MWA (n = 71) MA (n = 22) | Mean 12 Range 7–16 | 11.8 | |
| Finocchi, C. 2012 [3] | Prospective cross-sectional | No data | Migraine without aura (n = 100) | Mean 41.7 ± 14.2 | 20% of migraine attacks were triggered by food, among them 45% from chocolate | |
| Schürks, M., 2011 [75] | Cross-sectional study | Mailed migraine-specific questionnaire | Women’s Health Study (n = 1675) | No data | 24.7 | |
| Yadav, R., 2010 [40] | Prospective cross-sectional | Questionnaire | Migraine without aura (n = 182) | Mean 30.7 Range 14–58 | None | None of the subjects reported chocolate as a trigger. |
| Andress-Rothrock, D., 2000 [34] | Prospective cross-sectional | Headache trigger checklist | Migraine (n = 200) EM (n = 56) CM (n = 144) | Mean 41.1 Range 16–75 | 3 | |
| Chakravarty, A., 2009 [4] | Prospective and retrospective cross-sectional | Migraine trigger checklist | Migraine (n = 200) MWA (n = 197) MA (n = 3) | Range 7–15 | 1.5 retrospective study 0.3 prospective study | |
| Fukui, P., 2008 [5] | Prospective cross-sectional | Predetermined list of TGG | Migraine (n = 200) | Mean 37.7 | 20.5 (22.84% females, 10.53% males) | |
| Wöber, C., 2006 [76] | Cross-sectional study | Two predetermined TF checklists (patients’ personal experience and theoretical knowledge) | Migraine (n = 71) TTH (n = 49) | Range 18–65 Migraine 36.8 ± 11.4 TTH 39.5 ± 12.7 | Theoretical knowledge 61.7 Personal experience 14.3 | The difference between theoretical knowledge and personal experience was statistically significant and the largest for chocolate. |
| Takeschima, T., 2004 [39] | Door to door survey | Structured questionnaires | headache (n = 1628) migraine (n = 342) MWA (n = 301) MA (n = 41) | No data | None | |
| Bank, J., 2000 [77] | Population-based epidemiological survey | Self-administered headache questionnaire | Migraine (n = 62) | Mean Women 41 Men 43 | 1.4 | |
| Marcus, D., 1997 [78] | Double-blind study | Headache (n = 63), 50% migraine, 37.5% TTH, 12.5 migraine + TTH | Mean 28.3 | 17.5 | No significant difference of migraine attacks between chocolate and placebo. | |
| Ulrich, 1996 [79] | A cross-sectional study | Mailed questionnaire | Migraine (n = 484) MWA (n = 342) MA (n = 163) | No data | 1.7 | Only migraineurs experienced chocolate as a precipitant of tension-type headaches |
| Van Den Bergh, 1987 [80] | Retrospective | Unstructured recall/free self-report | Migraine (n = 217) | Mean 40 | 22.5 | |
| Peatfield, R., 1984 [81] | Retrospective cross sectional | Interview | Migraine (n = 490) | No data | 19 | |
| Dalton, 1975 [37] | Prospective cross- sectional | Self-administered postal questionnaire | Migraine in women (n = 1883) | No data | 33 | |
| Moffet, A.M., 1974 [82] | Retrospective study | Questionnaire | Migraine (n = 332) | No data | 26.5 |
| Author (Year) | Study Design | Placebo | Chocolate/Placebo Amount | Study Group: Type of Headache (Number of Participants) | Study Population Age (Years) | Chocolate/Cocoa Reported as a Trigger Factor (%) before the Study | Conclusion |
|---|---|---|---|---|---|---|---|
| Marcus, D., 1997 [78] | Double-blind study | Carob | 60 g | Headache (n = 63), 50% migraine, 37.5% TTH, 12.5 migraine + TTH | 28,3 | 17.5 | No significant difference of migraine attacks between chocolate and placebo. |
| Gibb, C., 1991 [83] | Double-blind, placebo controlled trial | Carob powder, coberine (non-cocoa vegetable fat) | 40 g | Migraine (n = 20) | Chocolate 37 Placebo 42 | 100 | 41.7% developed headaches after chocolate ingestion, none after placebo. |
| Moffet, A.M., 1974 [82] | Double-blind, placebo controlled trial | Synthetic fat made from non-cocoa containing vegetable oils with added sugar, coloring, and flavoring | 44 g | Migraine (n = 25) | Mean 49 Range 22–62 | 100 | No significant difference of migraine attacks between chocolate and placebo. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowaczewska, M.; Wiciński, M.; Kaźmierczak, W.; Kaźmierczak, H. To Eat or Not to eat: A Review of the Relationship between Chocolate and Migraines. Nutrients 2020, 12, 608. https://doi.org/10.3390/nu12030608
Nowaczewska M, Wiciński M, Kaźmierczak W, Kaźmierczak H. To Eat or Not to eat: A Review of the Relationship between Chocolate and Migraines. Nutrients. 2020; 12(3):608. https://doi.org/10.3390/nu12030608
Chicago/Turabian StyleNowaczewska, Magdalena, Michał Wiciński, Wojciech Kaźmierczak, and Henryk Kaźmierczak. 2020. "To Eat or Not to eat: A Review of the Relationship between Chocolate and Migraines" Nutrients 12, no. 3: 608. https://doi.org/10.3390/nu12030608
APA StyleNowaczewska, M., Wiciński, M., Kaźmierczak, W., & Kaźmierczak, H. (2020). To Eat or Not to eat: A Review of the Relationship between Chocolate and Migraines. Nutrients, 12(3), 608. https://doi.org/10.3390/nu12030608






