Short-Term Effects of Dark Chocolate on Retinal and Choriocapillaris Perfusion in Young, Healthy Subjects Using Optical Coherence Tomography Angiography
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Examination
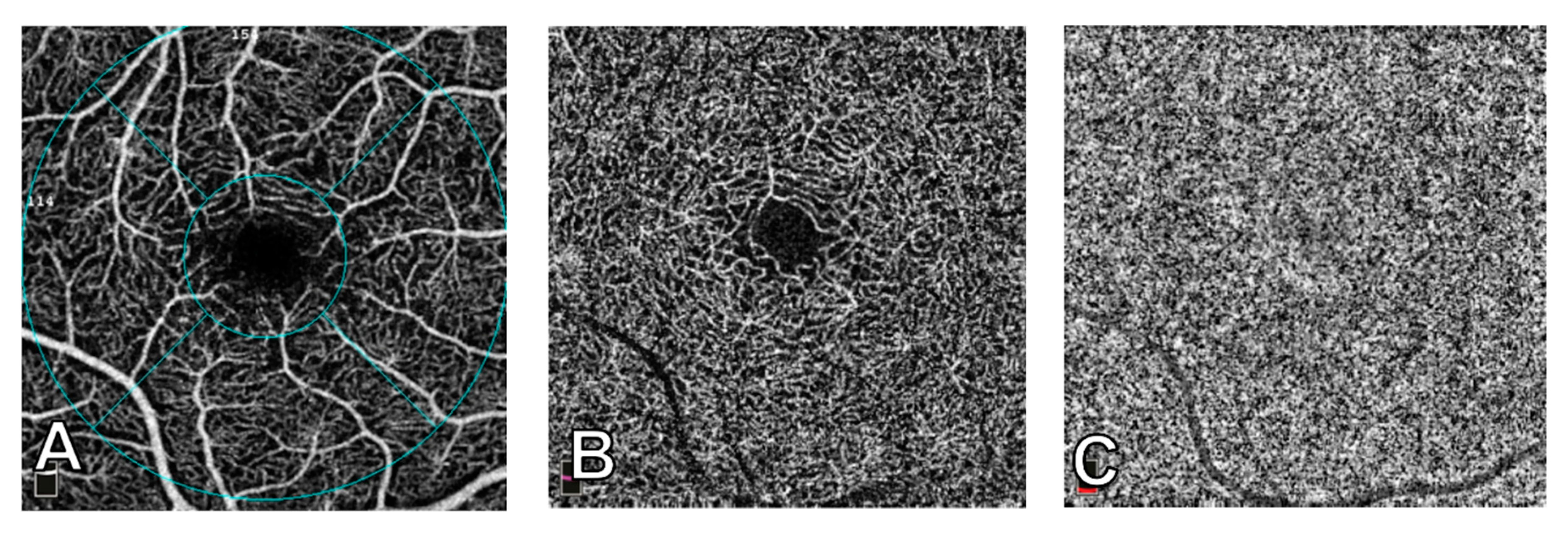
2.3. OCT-Angiography
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zamora-Ros, R.; Forouhi, N.G.; Sharp, S.J.; González, C.A.; Buijsse, B.; Guevara, M.; Schouw, Y.T.; Amiano, P.; Boeing, H.; Bredsdorff, L.; et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J. Nutr. 2013, 144, 335–343. [Google Scholar] [CrossRef]
- D’el-Rei, J.; Cunha, A.R.; Burlá, A.; Burlá, M.; Oigman, W.; Neves, M.F.; Virdis, A.; Medeiros, F. Characterisation of hypertensive patients with improved endothelial function after dark chocolate consumption. Int. J. Hypertens. 2013. [Google Scholar] [CrossRef]
- Faridi, Z.; Nijike, V.Y.; Dutta, S.; Ali, A.; Katz, D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008, 88, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Maldonado, J.; Laranjeiro, M.; Coutinho, R.; Cardoso, E.; Andrade, I.; Conde, J. Central arterial hemodynamic effects of dark chocolate ingestion in young healthy people: A randomized and controlled trial. Cardiol. Res. Pract. 2014. [CrossRef] [PubMed]
- Flammer, A.J.; Hermann, F.; Sudano, I.; Spieker, L.; Hermann, M.; Cooper, K.; Serafini, M.; Lüscher, T.; Ruschitzka, F.; Noll, G.; et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. J. Emerg. Med. 2008, 34, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang-Polagruto, J.F.; Villablanca, A.C.; Polagruto, J.A.; Lee, L.; Holt, R.; Schrader, H.R.; Ensunsa, J.L.; Steinberg, F.M.; Schmitz, H.H.; Keen, C.L. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J. Cardiovasc. Pharmacol. 2006, 47. [Google Scholar] [CrossRef]
- Steinhaus, D.A.; Mostofsky, E.; Levitan, E.B.; Dorans, K.S.; Håkansson, N.; Wolk, A.; Mittleman, M.A. Chocolate intake and incidence of heart failure: Findings from the cohort of Swedish men. Am. Heart J. 2017, 183. [Google Scholar] [CrossRef]
- Fisher, N.D.; Sorond, F.A.; Hollenberg, N.K. Cocoa flavanols and brain perfusion. J. Cardiovasc. Pharmacol. 2006, 47. [Google Scholar] [CrossRef]
- Nogueira, L.D.P.; Knibel, M.P.; Torres, M.R.S.; Neto, J.F.; Sanjuliani, A.F. Consumption of high-polyphenol dark chocolate improves endothelial function in individuals with stage 1 hypertension and excess body weight. Int. J. Hypertens. 2012. [Google Scholar] [CrossRef]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schömig, E. Effects of Low Habitual Cocoa Intake on Blood Pressure and Bioactive Nitric Oxide. JAMA 2007, 298, 49. [Google Scholar] [CrossRef]
- Patel, R.K.; Brouner, J.; Spendiff, O. Dark Chocolate Supplementation Reduces the Oxygen Cost of Moderate Intensity Cycling. J. Int. Soc. Sports Nutr. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: A randomized double-blind, placebo-controlled, crossover trial. J. Nutr. 2015, 145, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- West, S.G.; Mcintyre, M.D.; Piotrowski, M.J.; Poupin, N.; Miller, D.L.; Preston, A.G.; Wagner, P.; Groves, L.F.; Skulas-Ray, A.C. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br. J. Nutr. 2013, 111, 653–661. [Google Scholar] [CrossRef]
- Ghosh, D.; Scheepens, A. Vascular action of polyphenols. Mol. Nutr. Food Res. 2009, 53, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Bayard, V.; Chamorro, F.; Motta, J.; Hollenberg, N.K. Does flavanol intake influence mortality from nitric oxide-dependent processes? Ischemic heart disease, stroke, diabetes mellitus, and cancer in Panama. Int. J. Med. Sci. 2007, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Stoclet, J.; Chataigneau, T.; Ndiaye, M.; Oak, M.; Bedoui, J.; Chataigneau, M.; Schini-Kerth, V.B. Vascular Protection by Dietary Polyphenols. Eur. J. Pharmacol. 2004, 500, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Terai, N.; Gedenk, A.; Spoerl, E.; Pillunat, L.E.; Stodtmeister, R. The Short-Term Effect of Flavonoid-Rich Dark Chocolate on Retinal Vessel Diameter in Glaucoma Patients and Age-Matched Controls. Acta Ophthalmol. 2014, 92, 5. [Google Scholar] [CrossRef]
- Field, D.T.; Williams, C.M.; Butler, L.T. Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol. Behav. 2011. [Google Scholar] [CrossRef]
- Rabin, J.C.; Karunathilake, N.; Patrizi, K. Effects of milk vs. dark chocolate consumption on visual acuity and contrast sensitiviy within 2 h. JAMA Opthalmol. 2018, 136, 678. [Google Scholar] [CrossRef]
- Siedlecki, J.; Mohr, N.; Luft, N.; Schworm, B.; Keidel, L.; Priglinger, S.G. Effects of flavanol-rich dark chocolate on visual function and retinal perfusion measured with optical coherence tomography angiography. JAMA Ophthalmol. 2019, 137, 12. [Google Scholar] [CrossRef]
- Huang, D.; Swason, E.; Lin, C.; Schuman, J.; Stinson, W.; Chang, W.; Hee, M.; Flotte, T.; Gregory, K.; Puliafito, C. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Koizumi, H.; Pozzoni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Flores-Moreno, I.; Ruiz-Medrano, J.; Duker, J.S.; Ruiz-Moreno, J.M. The Relationship between Retinal and Choroidal Thickness and Visual Acuity in Highly Myopic Eyes. Br. J. Ophthalmol. 2013, 97, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Fujiwara, T.; Imamura, Y.; Lima, L.H.; Kurosaka, D.; Spaide, R.F. Choroidal thickness and visual acuity in highly myopic eyes. Retina 2012, 32, 1229–1236. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Felli, L.; Plateroti, R.; Plateroti, A.M.; Giustini, S.; Calvieri, S.; Recupero, S.M. Morphologic and vasculature features of the choroid and associated choroid-retinal thickness alterations in neurofibromatosis type 1. Br. J. Ophthalmol. 2014, 99, 789–793. [Google Scholar] [CrossRef]
- Di Staso, F.; Ciancaglini, M.; Abdolrahimzadeh, S.; D’Apolito, F.; Scuderi, G. Optical Coherence Tomography of Choroid in Common Neurological Diseases. Vivo 2019, 33, 1403–1409. [Google Scholar] [CrossRef]
- Dixon, G.A.; Pérez, C.A. Multiple Sclerosis and the Choroid Plexus: Emerging Concepts of Disease Immunopathophysiology. Pediatric Neurol. 2019, 103, 65–75. [Google Scholar] [CrossRef]
- Abadia, B.; Bartol-Puyal, F.D.A.; Calvo, P.; Verdes, G.; Isanta, C.; Pablo, E.L. Mapping Choroidal Thickness in Patients with Type 2 Diabetes. Can. J. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Parisi, F.; Scavella, V.; Recupero, S.M. Optical coherence tomography evidence on the correlation of choroidal thickness and age with vascularized retinal layers in normal eyes. Retina 2016, 2329–2338. [Google Scholar] [CrossRef]
- Liu, R.; Lu, J.; Liu, Q.; Wang, Y.; Cao, D.; Wang, J.; Wang, X.; Pan, J.; Ma, L.; Jin, C.; et al. Effect of Choroidal Vessel Density on the Ellipsoid Zone and Visual Function in Retinitis Pigmentosa Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4328. [Google Scholar] [CrossRef]
- Linsenmeier, R.A.; Goldstick, T.K.; Blum, R.S.; Enroth-Cugell, C. Estimation of Retinal Oxygen Transients from Measurements Made in the Vitreous Humor. Exp. Eye Res. 1981, 32, 369–379. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Alm, A.; Anders, B. Ocular and Optic Nerve Blood Flow at Normal and Increased Intraocular Pressures in Monkeys (Macaca Irus): A Study with Radioactively Labelled Microspheres Including Flow Determinations in Brain and Some Other Tissues. Exp. Eye Res. 1973, 15, 15–29. [Google Scholar] [CrossRef]
- Riva, C.E.; Alm, A.; Pournaras, C.J. Ocular Circulation. Adler’s Physiol. Eye 2011, 243–273. [Google Scholar] [CrossRef]
- Odabas, Ö.Y.; Demirel, S.; Özmert, E.; Batioglu, F. Repeatability of automated vessel density and superficial and deep foveal avascular zone area measurements using optical coherence tomography angiography: Diurnal findings. Retina 2018, 1238–1245. [Google Scholar] [CrossRef]
- Scuderi, G.L.; Cascone, N.C.; Regine, F.; Perdicchi, A.; Cerulli, A.; Recupero, S.M. Validity and limits of the rebound tonometer (IcareR: Clinical study). Eur. J. Ophthalmol. 2011, 251–257. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; Ruggieri, F.; Blumberg, J.B.; Stornello, M.; Ferri, C. Protective effects of flavanol-rich dark chocolate on endothelial function and wave reflection during acute hyperglycemia. Hypertension 2012, 827–832. [Google Scholar] [CrossRef]
- Chalam, K.V.; Sambhav, K. Optical Coherence Tomography Angiography in Retinal Diseases. J. Ophthalmic Vis. Res. 2016, 11, 84. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image Artifacts In Optical Coherence Tomography Angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef]
- Karti, O.; Zengin, M.O.; Kerci, S.G.; Ayhan, Z.; Kusbeci, T. Acute effect of caffeine on macular microcirculation in healthy subjects. Retina 2019, 964–971. [Google Scholar] [CrossRef]
- Choi, W.; Mohler, K.J.; Potsaid, B.; Lu, C.D.; Liu, J.J.; Jayaraman, V.; Cable, A.E.; Duker, J.S.; Huber, R.; Fujimoto, J.G. Choriocapillaris and choroidal microvascular imaging with ultrahigh speed OCT angiography. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.-F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Kim, H.; Kwon, O.; Chang, N. Associations between Fruits, Vegetables, Vitamin A, β-Carotene and Flavonol Dietary Intake, and Age-Related Macular Degeneration in Elderly Women in Korea: The Fifth Korea National Health and Nutrition Examination Survey. Eur. J. Clin. Nutr. 2017, 1, 161–167. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the Modification of the Authorisation of a Health Claim Related to Cocoa Flavanols and Maintenance of Normal Endothelium-Dependent Vasodilation Pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 5. [Google Scholar] [CrossRef]

| Characteristic | Mean (SD) |
|---|---|
| Age (years) | 26.3 (1.53) |
| IOP (mmHg) | 13.9 (0.96) |
| BCVA | 61.0 (2.07) |
| Sex, N (%) | |
| Male | 10 (56) |
| Female | 8 (44) |
| Spherical equivalent | N (%) |
| +3.00 | 3 (8.3) |
| +2.50 | 2 (5.5) |
| +2.00 | 3 (8.3) |
| +1.50 | 1 (2.8) |
| +0.75 | 1 (2.8) |
| +0.25 | 1 (2.8) |
| No correction | 20 (55.5) |
| −0.25 | 1 (2.8) |
| −1.00 | 1 (2.8) |
| −1.25 | 1 (2.8) |
| −1.50 | 1 (2.8) |
| −2.00 | 1 (2.8) |
| White Chocolate | Dark Chocolate | ||||
|---|---|---|---|---|---|
| Time | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| CC flow area (mm2) | Baseline | 2.2 (0.1) | 2.2 (2.2–2.3) | 2.2 (0.1) | 2.2 (2.2–2.3) |
| 1 h | 2.2 (0.1) | 2.2 (2.2–2.3) | 2.2 (0.1) | 2.2 (2.2–2.3) | |
| 2 h | 2.2 (0.1) | 2.2 (2.2–2.2) | 2.2 (0.1) | 2.2 (2.2–2.3) | |
| 3 h | 2.2 (0.1) | 2.2 (2.2–2.3) | 2.2 (0.1) | 2.2 (2.2–2.3) | |
| DCP foveal density (%) | Baseline | 40.4 (8.9) | 40.7 (35.8–45.1) | 40.5 (8.7) | 40.7 (34.7–44.9) |
| 1 h | 40.3 (8.6) | 40.4 (35.5–45) | 40.3 (8.7) | 40.9 (34.4–46) | |
| 2 h | 39.9 (8.3) | 39.9 (35.1–45) | 40.3 (8.6) | 40.5 (34.1–44.8) | |
| 3 h | 40 (8.5) | 40.8 (34.8–44.5) | 40.3 (8.3) | 40.7 (35.6–44.5) | |
| SCP foveal density (%) | Baseline | 24 (8.0) | 25.1 (18.1–27.6) | 23.8 (8.3) | 25.1 (17.8–27.9) |
| 1 h | 23.42 (8.0) | 23.4 (18.3–27.4) | 23.95 (8.3) | 24.1 (17.5–28.4) | |
| 2 h | 23.28 (7.8) | 23 (17.1–27.3) | 23.6 (8.0) | 23.5 (16.8–28.9) | |
| 3 h | 23.20 (8) | 24.2 (16.2–27.4) | 24.44 (9.5) | 23.3 (17.3–28.1) | |
| DCP whole density (%) | Baseline | 54.12 (2.82) | 54.6 (53.5–55.5) | 54.01 (2.21) | 54.4 (52.2–55.5) |
| 1 h | 53.53 (2.39) | 53.4 (52.7–54.9) | 53.57 (2.44) | 54.1 (52.4–55.4) | |
| 2 h | 53.04 (3.44) | 53.8 (51.4–55) | 52.94 (2.35) | 53.4 (51.1–54.3) | |
| 3 h | 53.19 (3.27) | 53.7 (52.1–55.2) | 52.78 (2.82) | 53.2 (51–54.7) | |
| SCP whole density (%) | Baseline | 48.9 (2.2) | 49.4 (47.6–50.3) | 49.0 (2.1) | 49.2 (47.9–50.6) |
| 1 h | 48.6 (2.3) | 48.7 (47.2–50.3) | 48.7 (2.6) | 48.3 (46.3–50.5) | |
| 2 h | 48.3 (3.1) | 48.8 (47.2–50.2) | 48.3 (2.3) | 48.1 (47.2–50.3) | |
| 3 h | 48.3 (3.0) | 48.7 (47.4–50.2) | 48.0 (2.6) | 48.8 (46.5–50) | |
| BCVA (EDTRS) | Baseline | 85.3 (0.50) | 85 (85–85) | 85.1 (0.49) | 85 (85–85) |
| 1 h | 85 (0.89) | 85 (85–85) | 85 (0.81) | 85 (84.5–85) | |
| 2 h | 85.1 (0.67) | 85 (85–85) | 85 (0.72) | 85 (85–85) | |
| 3 h | 85.2 (0.74) | 85 (85–85) | 85.2 (0.68) | 85 (85–85) | |
| White Chocolate | Dark Chocolate | p Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| After 1 h | |||||
| Choriocapillaris flow area (mm2) | −0.003 | 0.064 | 0.002 | 0.055 | 0.736 |
| DCP foveal density (%) | −0.169 | 1.479 | −0.158 | 1.505 | 0.818 |
| SCP foveal density (%) | −0.589 | 2.727 | 0.147 | 1.712 | 0.298 |
| DCP whole density (%) | −0.589 | 2.359 | −0.436 | 2.726 | 0.880 |
| SCP whole density (%) | −0.389 | 1.517 | −0.328 | 2.088 | 0.915 |
| BCVA (EDTRS) | −0.083 | 2.091 | −0.444 | 1.930 | 0.936 |
| After 2 h | |||||
| Choriocapillaris flow area (mm2) | −0.024 | 0.072 | 0.006 | 0.069 | 0.118 |
| DCP foveal density (%) | −0.592 | 1.701 | −0.122 | 1.947 | 0.328 |
| SCP foveal density (%) | −0.725 | 2.739 | −0.203 | 1.656 | 0.383 |
| DCP whole density (%) | −1.081 | 3.450 | −1.069 | 2.587 | 0.771 |
| SCP whole density (%) | −0.586 | 2.670 | −0.739 | 1.816 | 0.317 |
| BCVA (EDTRS) | 0.194 | 2.190 | −0.167 | 1.978 | 0.834 |
| After 3 h | |||||
| Choriocapillaris flow area (mm2) | 0.724 | 0.078 | 0.724 | 0.058 | 0.724 |
| DCP foveal density (%) | −0.444 | 2.434 | −0.194 | 1.786 | 0.371 |
| SCP foveal density (%) | −0.806 | 2.855 | 0.642 | 7.213 | 0.779 |
| DCP whole density (%) | −0.933 | 4.144 | −1.233 | 3.224 | 0.538 |
| SCP whole density (%) | −0.625 | 2.261 | −1.036 | 1.997 | 0.583 |
| BCVA (EDTRS) | 0.222 | 2.203 | −0.194 | 2.203 | 0.941 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuderi, G.; Ciancimino, C.; D’Apolito, F.; Maurizi Enrici, M.; Guglielmelli, F.; Scuderi, L.; Abdolrahimzadeh, S. Short-Term Effects of Dark Chocolate on Retinal and Choriocapillaris Perfusion in Young, Healthy Subjects Using Optical Coherence Tomography Angiography. Nutrients 2020, 12, 664. https://doi.org/10.3390/nu12030664
Scuderi G, Ciancimino C, D’Apolito F, Maurizi Enrici M, Guglielmelli F, Scuderi L, Abdolrahimzadeh S. Short-Term Effects of Dark Chocolate on Retinal and Choriocapillaris Perfusion in Young, Healthy Subjects Using Optical Coherence Tomography Angiography. Nutrients. 2020; 12(3):664. https://doi.org/10.3390/nu12030664
Chicago/Turabian StyleScuderi, Gianluca, Chiara Ciancimino, Fabian D’Apolito, Maurizio Maurizi Enrici, Fabio Guglielmelli, Luca Scuderi, and Solmaz Abdolrahimzadeh. 2020. "Short-Term Effects of Dark Chocolate on Retinal and Choriocapillaris Perfusion in Young, Healthy Subjects Using Optical Coherence Tomography Angiography" Nutrients 12, no. 3: 664. https://doi.org/10.3390/nu12030664
APA StyleScuderi, G., Ciancimino, C., D’Apolito, F., Maurizi Enrici, M., Guglielmelli, F., Scuderi, L., & Abdolrahimzadeh, S. (2020). Short-Term Effects of Dark Chocolate on Retinal and Choriocapillaris Perfusion in Young, Healthy Subjects Using Optical Coherence Tomography Angiography. Nutrients, 12(3), 664. https://doi.org/10.3390/nu12030664





