The Effect of Orally Dosed Levagen+™ (palmitoylethanolamide) on Exercise Recovery in Healthy Males—A Double-Blind, Randomized, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
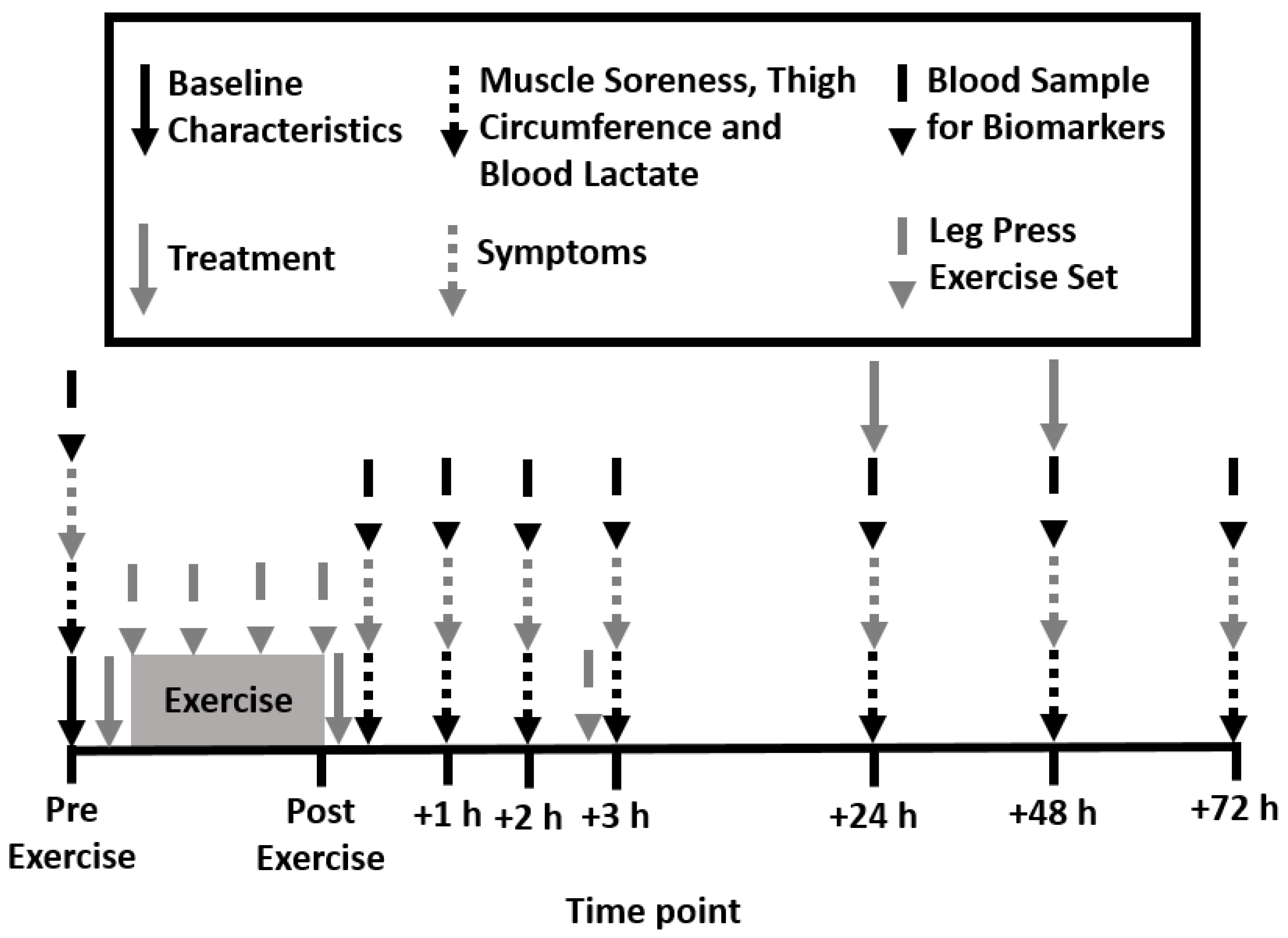
2.2. Experimental Design
2.3. Treatments
2.4. Determination of One Repetition Maximum Load
2.5. Leg Press Exercise
2.6. Muscle Soreness, Thigh Circumference, and Blood Lactate Concentration
2.7. Biomarkers of Muscle Damage and Inflammation and Phosphoprotein Signaling Pathways
2.8. Data Analyses
3. Results
3.1. Leg Press Exercise
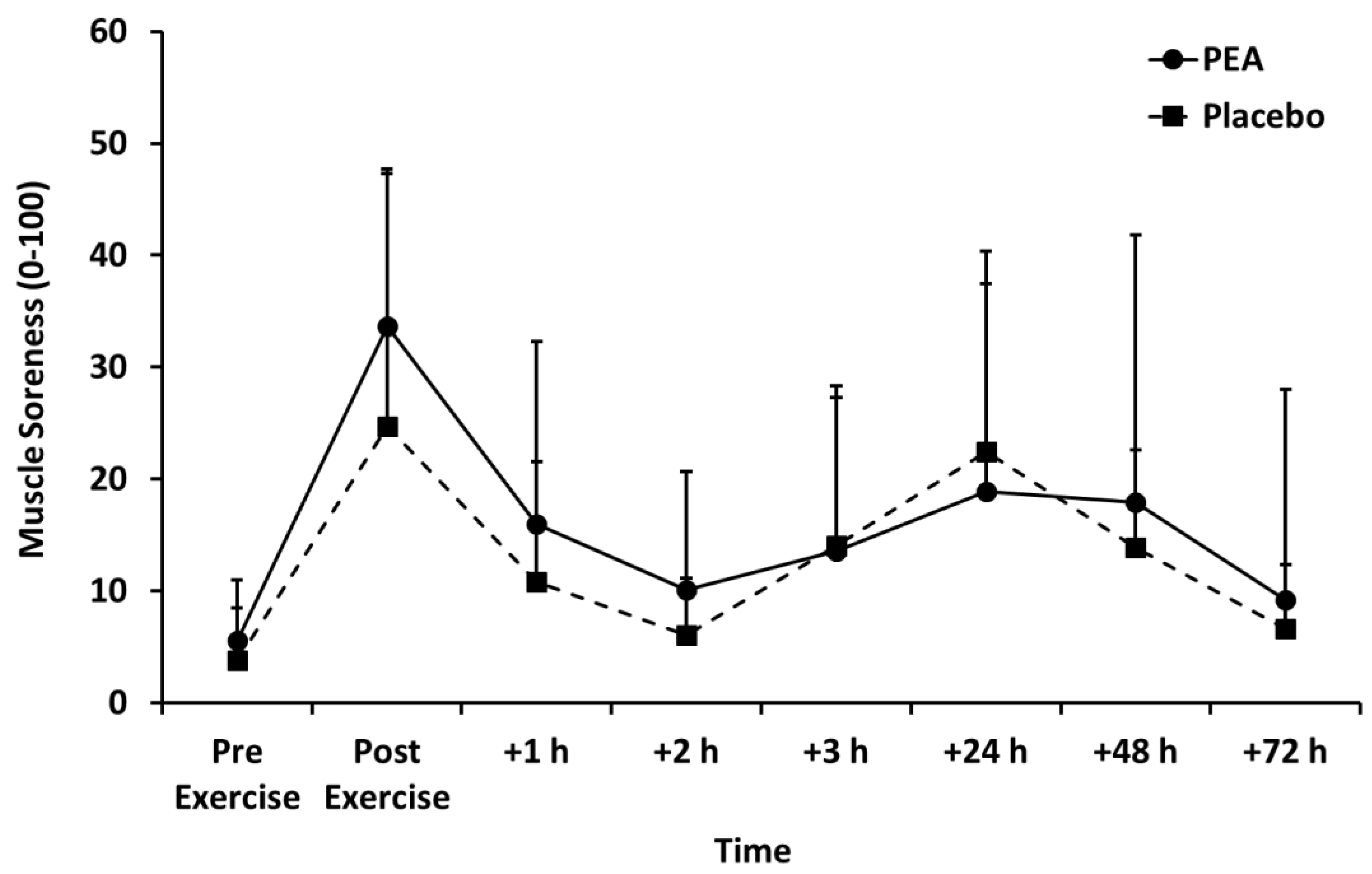
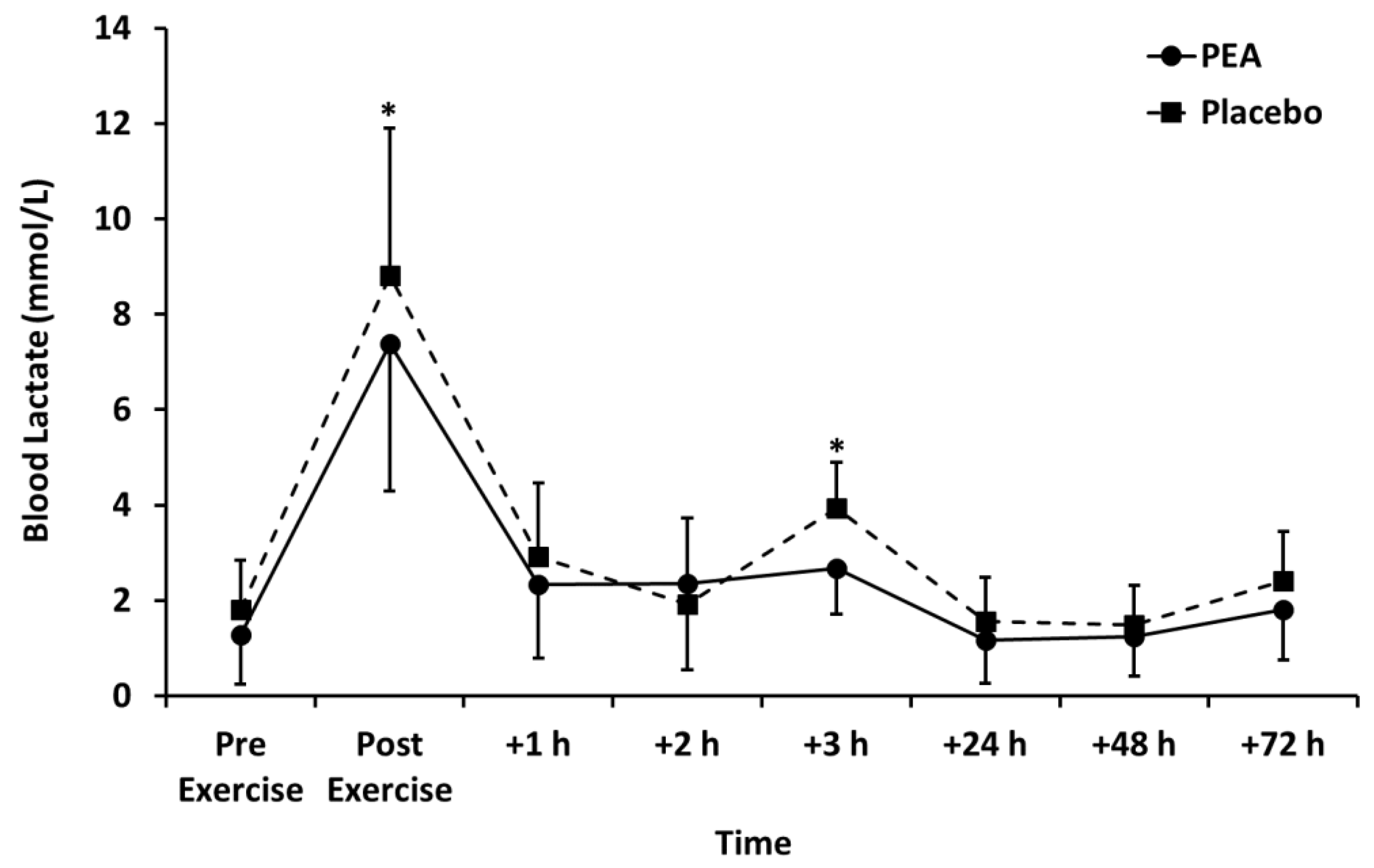
3.2. Muscle Soreness, Thigh Circumference, and Blood Lactate Concentration
3.3. Biomarkers of Muscle Damage
3.4. Biomarkers of Inflammation
3.5. Phosphoprotein Signaling Pathways
4. Discussion
4.1. Leg Press Exercise
4.2. Effects of PEA Supplementation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. (1985) 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Hubal, M.J. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 2014, 49, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Dankel, S.J.; Mattocks, K.T.; Jessee, M.B.; Buckner, S.L.; Mouser, J.G.; Counts, B.R.; Laurentino, G.C.; Loenneke, J.P. Frequency: The overlooked resistance training variable for inducing muscle hypertrophy? Sports Med. 2017, 47, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. The Use of Nonsteroidal anti-inflammatory drugs for exercise-induced muscle damage: Implications for skeletal muscle development. Sports Med. 2012, 42, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and supplementation strategies to prevent and attenuate exercise-induced muscle damage: A brief review. Sports Med. Open 2019, 5, 1. [Google Scholar] [CrossRef]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br. J. Clin. Pharmacol. 2016, 82, 932–942. [Google Scholar] [CrossRef] [Green Version]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Bloch, M.H. Efficacy of palmitoylethanolamide for pain: A meta-analysis. Pain Physician 2017, 20, 353–362. [Google Scholar]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Costa, B.; Conti, S.; Giagnoni, G.; Colleoni, M. Therapeutic effect of the endogenous fatty acid amide, palmitoylethanolamide, in rat acute inflammation: Inhibition of nitric oxide and cyclo-oxygenase systems. Br. J. Pharmacol. 2002, 137, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Mazzari, S.; Canella, R.; Petrelli, L.; Marcolongo, G.; Leon, A. N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996, 300, 227–236. [Google Scholar] [CrossRef]
- Calignano, A.; La Rana, G.; Piomelli, D. Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur. J. Pharmacol. 2001, 419, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Gorostiaga, E.M.; Navarro-Amezqueta, I.; Calbet, J.A.; Sanchez-Medina, L.; Cusso, R.; Guerrero, M.; Granados, C.; Gonzalez-Izal, M.; Ibanez, J.; Izquierdo, M. Blood ammonia and lactate as markers of muscle metabolites during leg press exercise. J. Strength. Cond. Res. 2014, 28, 2775–2785. [Google Scholar] [CrossRef]
- Arroyo, E.; Wells, A.J.; Gordon, J.A., 3rd; Varanoske, A.N.; Gepner, Y.; Coker, N.A.; Church, D.D.; Fukuda, D.H.; Stout, J.R.; Hoffman, J.R. Tumor necrosis factor-alpha and soluble TNF-alpha receptor responses in young vs. middle-aged males following eccentric exercise. Exp. Gerontol. 2017, 100, 28–35. [Google Scholar] [CrossRef]
- Buford, T.W.; Cooke, M.B.; Willoughby, D.S. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur. J. Appl. Physiol. 2009, 107, 463–471. [Google Scholar] [CrossRef]
- Smith, L.L.; Anwar, A.; Fragen, M.; Rananto, C.; Johnson, R.; Holbert, D. Cytokines and cell adhesion molecules associated with high-intensity eccentric exercise. Eur. J. Appl. Physiol. 2000, 82, 61–67. [Google Scholar] [CrossRef]
- Isik, O.; Dogan, I. Effects of bilateral or unilateral lower body resistance exercises on markers of skeletal muscle damage. Biomed. J. 2018, 41, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Callegari, G.A.; Novaes, J.S.; Neto, G.R.; Dias, I.; Garrido, N.D.; Dani, C. Creatine Kinase and Lactate Dehydrogenase Responses after Different Resistance and Aerobic Exercise Protocols. J. Hum. Kinet. 2017, 58, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, W.M.; Davison, G.W.; McClean, C.M.; Murphy, M.H. A Systematic Review of the Acute Effects of Exercise on Immune and Inflammatory Indices in Untrained Adults. Sports Med. Open 2015, 1, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral-Santos, C.; de Lima Junior, E.A.; Fernandes, I.; Pinto, R.Z.; Rosa-Neto, J.C.; Bishop, N.C.; Lira, F.S. Interleukin-10 responses from acute exercise in healthy subjects: A systematic review. J. Cell. Physiol. 2019, 234, 9956–9965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, H.F.; Goodyear, L.J. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J. Appl. Physiol. (1985) 2007, 103, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Wackerhage, H.; Woods, N.M. Exercise-induced signal transduction and gene regulation in skeletal muscle. J. Sports Sci. Med. 2002, 1, 103–114. [Google Scholar] [PubMed]
- D’Agostino, G.; La Rana, G.; Russo, R.; Sasso, O.; Iacono, A.; Esposito, E.; Mattace Raso, G.; Cuzzocrea, S.; Loverme, J.; Piomelli, D.; et al. Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-kappaB nuclear signalling in dorsal root ganglia. Eur. J. Pharmacol. 2009, 613, 54–59. [Google Scholar] [CrossRef]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta. Medica. 1991, 57, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
- Isaacs, A.W.; Macaluso, F.; Smith, C.; Myburgh, K.H. C-Reactive Protein Is Elevated Only in High Creatine Kinase Responders to Muscle Damaging Exercise. Front. Physiol. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| PEA | Placebo | |
|---|---|---|
| Age (years) | 27 ± 4 | 26 ± 4 |
| Height (m) | 1.79 ± 0.10 | 1.80 ± 0.10 |
| Weight (kg) | 84 ± 12 | 83 ± 15 |
| Body mass index (kg/m2) | 25 ± 2.6 | 26 ± 2.8 |
| Systolic blood pressure (mmHg) | 123 ± 9 | 124 ± 10 |
| Diastolic blood pressure (mmHg) | 76 ± 4 | 75 ± 11 |
| Waist circumference (cm) | 87 ± 7 | 87 ± 9 |
| Hip circumference (cm) | 102 ± 7 | 102 ± 7 |
| Waist to hip ratio | 0.86 ± 0.04 | 0.85 ± 0.05 |
| Pre-exercise | Post-exercise | +1 h | +2 h | +3 h | +24 h | +48 h | +72 h | ||
|---|---|---|---|---|---|---|---|---|---|
| PKB (MFI) | PEA | 4682 ± 2933 | 9362 ± 4185# | 6146 ± 4147 | 5355 ± 3694 | 5972 ± 3313 | 6263 ± 4015 | 6163 ± 3667 | 4461 ± 3772 |
| Placebo | 4444 ± 1749 | 4996 ± 4642 | 5405 ± 2574 | 4911 ± 2625 | 4798 ± 2634 | 4832 ± 2515 | 4494 ± 2053 | 5298 ± 2226 | |
| CREB (MFI) | PEA | 98.5 ± 43.7 | 88.4 ± 61.7 | 92.7 ± 63.3 | 87.3 ± 51.0 | 122 ± 123 | 115 ± 105 | 73.0 ± 28.1 | 165 ± 222 |
| Placebo | 66.4 ± 29.7 | 58.3 ± 27.5 | 94.8 ± 99.6 | 65.7 ± 20.7 | 74.5 ± 24.6 | 68.4 ± 38.3 | 62.1 ± 22.1 | 66.8 ± 29.6 | |
| ERK 1/2 (MFI) | PEA | 39.6 ± 13.7 | 37.9 ± 15.8 | 40.3 ± 14.0 | 43.6 ± 13.7 | 40.9 ± 12.2 | 43.3 ± 15.0 | 40.2 ± 16.7 | 42.9 ± 13.0 |
| Placebo | 38.2 ± 12.1 | 41.5 ± 13.2 | 44.5 ± 14.6 | 44.0 ± 13.8 | 42.0 ± 12.1 | 40.5 ± 12.7 | 41.9 ± 14.7 | 38.9 ± 14.9 | |
| JNK (MFI) | PEA | 115 ± 92 | 106 ± 102 | 135 ± 101 | 120 ± 140 | 162 ± 133 | 105 ± 120 | 117 ± 119 | 120 ± 97 |
| Placebo | 103 ± 98 | 78 ± 64 | 138 ± 114 | 107 ± 93 | 126 ± 107 | 101 ± 93 | 87 ± 70 | 99 ± 93 | |
| NF-κB (MFI) | PEA | 17.9 ± 4.1 | 21.6 ± 8.1 | 17.8 ± 5.6 | 19.5 ± 9.1 | 21.0 ± 11.4 | 23.9 ± 17.9 | 17.1 ± 4.5 | 19.0 ± 7.2 |
| Placebo | 16.4 ± 5.7 | 18.0 ± 5.6 | 20.4 ± 7.0 | 17.4 ± 4.1 | 20.3 ± 7.1 | 19.0 ± 4.9 | 20.9 ±4.1 | 22.3 ± 8.5 | |
| p38MAPK (MFI) | PEA | 6188 ± 1791 | 6762 ± 1245 | 6184 ± 1545 | 5605 ± 2759 | 6879 ± 1706 | 6790 ± 2830 | 5772 ± 2896 | 5283 ± 2705 |
| Placebo | 5926 ± 2047 | 6080 ± 1933 | 6415 ± 1698 | 6504 ± 1923 | 6556 ± 1455 | 6448 ± 2629 | 6782 ± 2061 | 5643 ± 2524 | |
| RPS6KB1 (MFI) | PEA | 68.3 ± 70.9 | 47.5 ± 26.6 | 46.5 ± 22.6 | 49.6 ± 34.1 | 51.0 ± 31.3 | 42.6 ± 12.0 | 49.4 ± 36.4 | 55.5 ± 23.3 |
| Placebo | 39.7 ± 13.0 | 30.3 ± 11.8 | 45.0 ± 29.0 | 37.7 ± 10.2 | 38.1 ± 11.1 | 32.9 ± 15.2 | 46.0 ± 42.9 | 44.1 ± 17.5 | |
| STAT3 (MFI) | PEA | 51.9 ± 26.8 | 49.7 ± 19.8 | 55.6 ± 26.5 | 48.9 ± 22.7 | 48.6 ± 19.3 | 51.1 ± 19.3 | 43.4 ± 15.5 | 52.4 ± 27.0 |
| Placebo | 42.5 ± 18.3 | 42.0 ± 16.2 | 44.0 ± 24.6 | 46.2 ± 24.0 | 48.4 ± 28.9 | 45.5 ± 26.5 | 43.2 ± 26.0 | 47.4 ± 19.9 | |
| STAT5 (MFI) | PEA | 47.2 ± 32.4 | 46.3 ± 18.9 | 48.8 ± 20.7 | 50.8 ± 30.5 | 53.4 ± 25.9 | 42.8 ± 14.6 | 45.6 ± 22.8 | 41.1 ± 11.9 |
| Placebo | 38.5 ±10.1 | 40.4 ± 12.4 | 37.7 ± 6.5 | 39.3 ± 13.4 | 39.9 ±9.9 | 38.3 ± 6.5 | 40.6 ± 9.4 | 38.9 ± 12.3 |
| Pre-exercise | Post-exercise | +1 h | +2 h | +3 h | +24 h | +48 h | +72 h | ||
|---|---|---|---|---|---|---|---|---|---|
| Thigh Circumference (cm) | PEA | 57.3 ± 3.93 | 58.5 ± 3.72 | 57.6 ± 3.78 | 57.6 ± 3.69 | 57.7 ± 3.71 | 57.5 ± 3.83 | 56.9 ± 3.82 | 57.5 ± 3.81 |
| Placebo | 56.2 ± 4.81 | 57.4 ± 5.08 | 56.4 ± 4.60 | 56.3 ± 4.78 | 56.5 ± 4.92 | 56.5 ± 4.51 | 56.7 ± 4.40 | 56.6 ± 4.85 | |
| Creatine Kinase (IU/L) | PEA | 213 ± 119 | 228 ± 130 | 256 ± 223 | 257 ± 213 | 261 ± 210 | 310 ± 220 | 298 ± 233 | 233 ± 225 |
| Placebo | 180 ± 91 | 204 ± 106 | 196 ± 101 | 204 ± 97 | 232 ± 107 | 261 ± 100 | 226 ± 88 | 211 ± 138 | |
| Lactate Dehydrogenase (IU/L) | PEA | 217 ± 99 | 183 ± 42 | 193 ± 44 | 204 ± 48 | 176 ± 54 | 164 ± 55 | 170 ± 27 | 197 ± 51 |
| Placebo | 182 ± 30 | 211 ± 44 | 184 ± 61 | 202 ± 86 | 201 ± 84 | 162 ± 24 | 178 ± 31 | 173 ± 22 | |
| HS C-reactive Protein (mg/L) | PEA | 1.52 ± 2.12 | 1.60 ± 2.16 | 1.39 ± 1.88 | 1.49 ± 2.07 | 1.36 ± 1.83 | 1.24 ± 1.49 | 1.41 ± 1.49 | 1.09 ± 1.45 |
| Placebo | 1.26 ± 0.85 | 1.28 ± 0.87 | 1.17 ± 0.81 | 1.14 ± 0.79 | 1.15 ± 0.78 | 1.17 ± 1.04 | 1.17 ± 0.84 | 1.20 ± 0.95 | |
| Interleukin-10 (pg/mL) | PEA | 12.1 ± 10.1 | 20.1 ± 31.6 | 17.0 ± 17.2 | 15.9 ± 17.4 | 15.9 ± 16.8 | 13.5 ± 10.7 | 14.4 ± 12.0 | 12.2 ± 11.6 |
| Placebo | 16.8 ± 22.0 | 17.5 ± 24.0 | 16.0 ± 21.8 | 15.4 ± 17.2 | 14.4 ± 17.6 | 11.6 ± 11.1 | 12.5 ± 11.6 | 12.6 ± 10.0 | |
| Interleukin-6 (pg/mL) | PEA | 12.0 ± 9.43 | 12.2 ± 9.12 | 10.4 ± 7.23 | 11.6 ± 8.09 | 11.7 ± 7.56 | 11.4 ± 7.17 | 10.8 ± 7.01 | 10.6 ± 7.52 |
| Placebo | 6.23 ± 4.93 | 6.59 ± 4.29 | 5.13 ± 3.69 | 5.44 ± 3.71 | 5.92 ± 3.79 | 5.54 ± 3.86 | 5.50 ± 3.73 | 5.20 ± 3.38 | |
| Tumor necrosis factor-α (pg/mL) | PEA | 8.03 ± 5.51 | 9.17 ± 7.22 | 7.25 ± 4.48 | 7.69 ± 4.61 | 7.45 ± 3.40 | 8.38 ± 4.13 | 7.16 ± 3.53 | 7.41 ± 3.51 |
| Placebo | 8.58 ± 3.72 | 9.36 ± 3.11 | 7.80 ± 2.41 | 7.76 ± 3.50 | 7.97 ± 3.14 | 8.07 ± 2.57 | 7.52 ± 1.88 | 7.75 ± 2.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallard, A.; Briskey, D.; Richards, A.; Mills, D.; Rao, A. The Effect of Orally Dosed Levagen+™ (palmitoylethanolamide) on Exercise Recovery in Healthy Males—A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients 2020, 12, 596. https://doi.org/10.3390/nu12030596
Mallard A, Briskey D, Richards A, Mills D, Rao A. The Effect of Orally Dosed Levagen+™ (palmitoylethanolamide) on Exercise Recovery in Healthy Males—A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients. 2020; 12(3):596. https://doi.org/10.3390/nu12030596
Chicago/Turabian StyleMallard, Alistair, David Briskey, Andrew Richards, Dean Mills, and Amanda Rao. 2020. "The Effect of Orally Dosed Levagen+™ (palmitoylethanolamide) on Exercise Recovery in Healthy Males—A Double-Blind, Randomized, Placebo-Controlled Study" Nutrients 12, no. 3: 596. https://doi.org/10.3390/nu12030596





