Quantification of Nervonic Acid in Human Milk in the First 30 Days of Lactation: Influence of Lactation Stages and Comparison with Infant Formulae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Chemicals
2.2. Human Milk Samples and Infant Formulae
2.3. Fatty Acid Methyl Esters Preparation
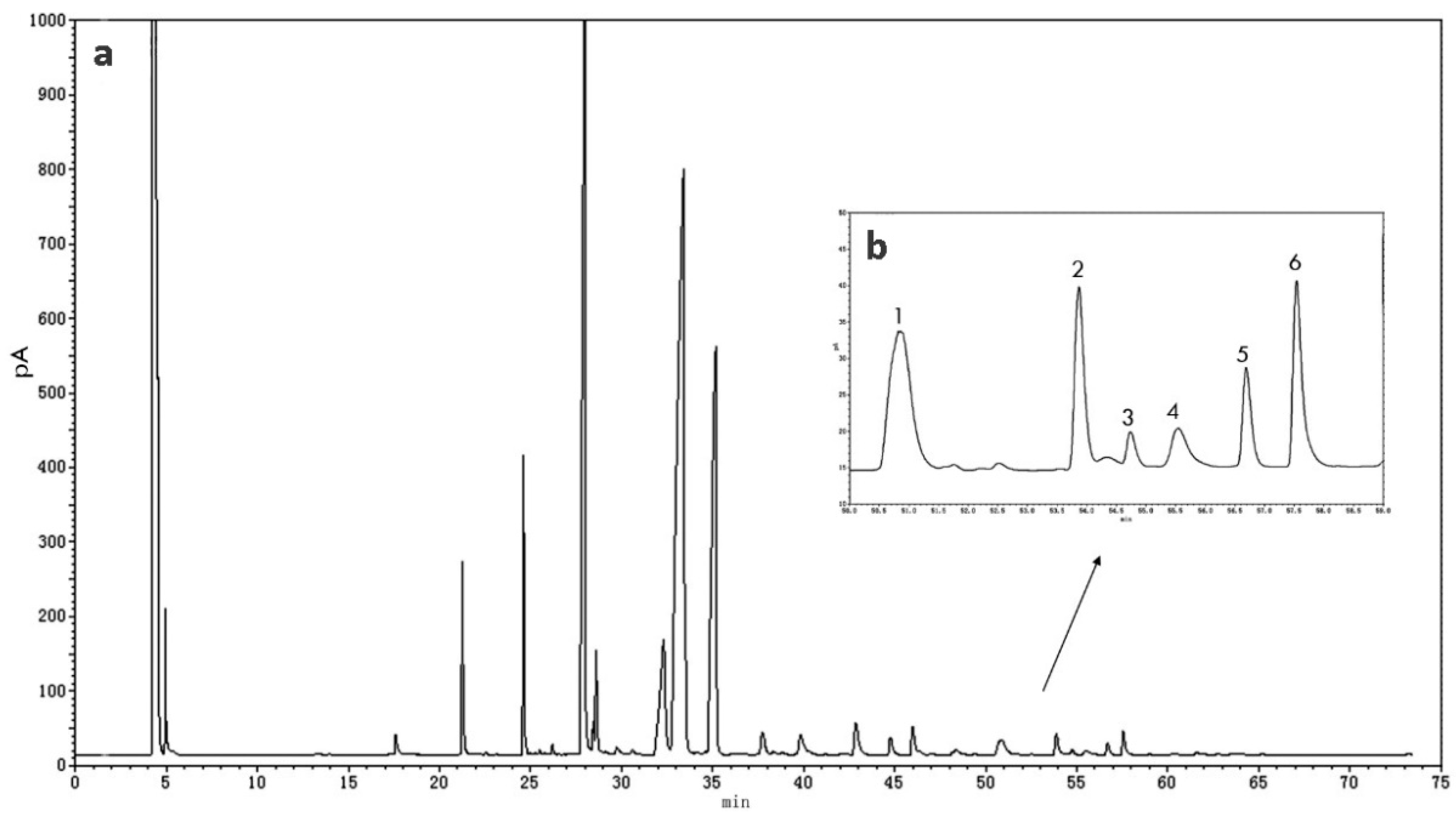
2.4. GC Analysis
2.5. Quantitation of Nervonic Acid
2.6. Validation of the Method
2.7. Statistical Analysis
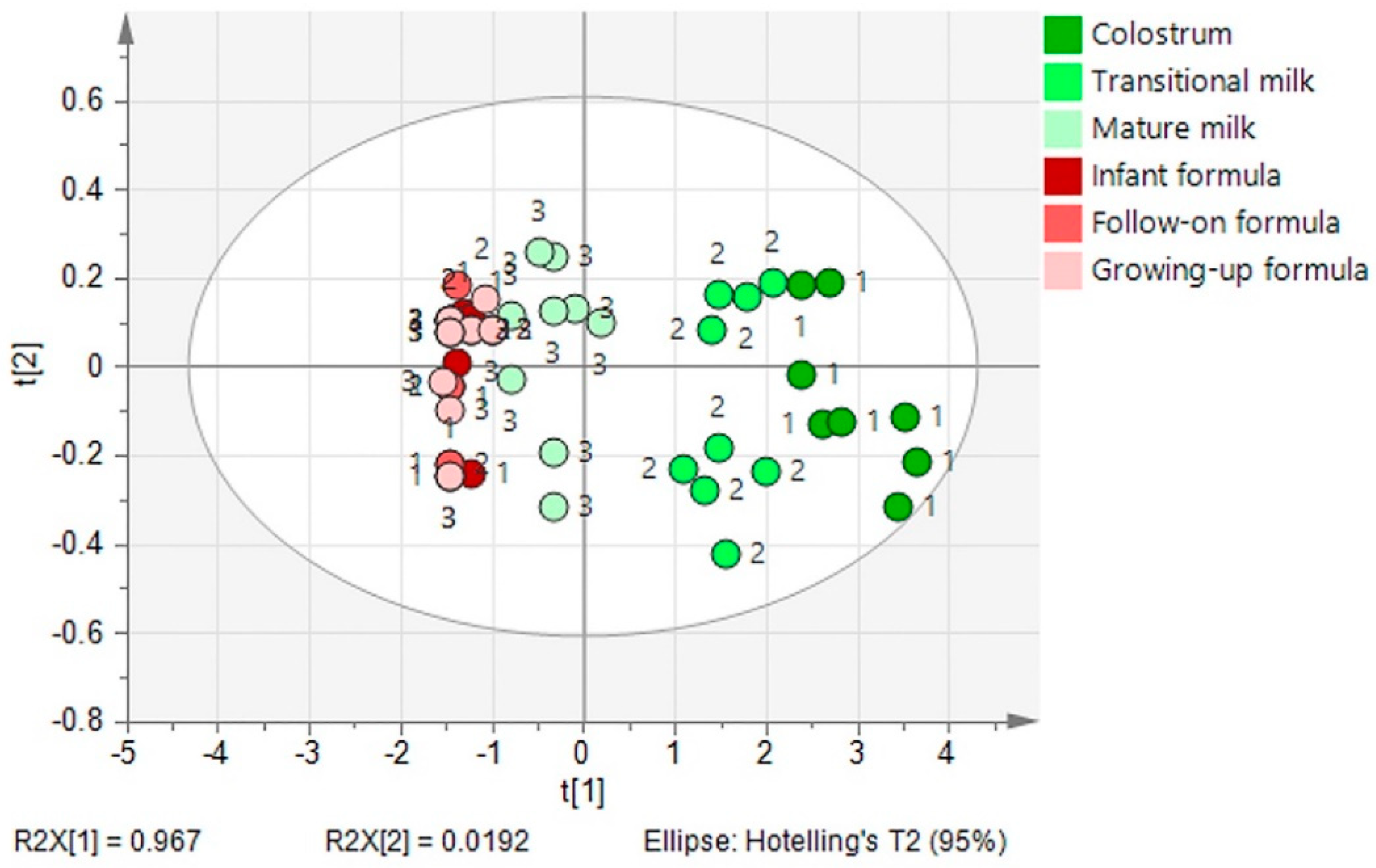
3. Results
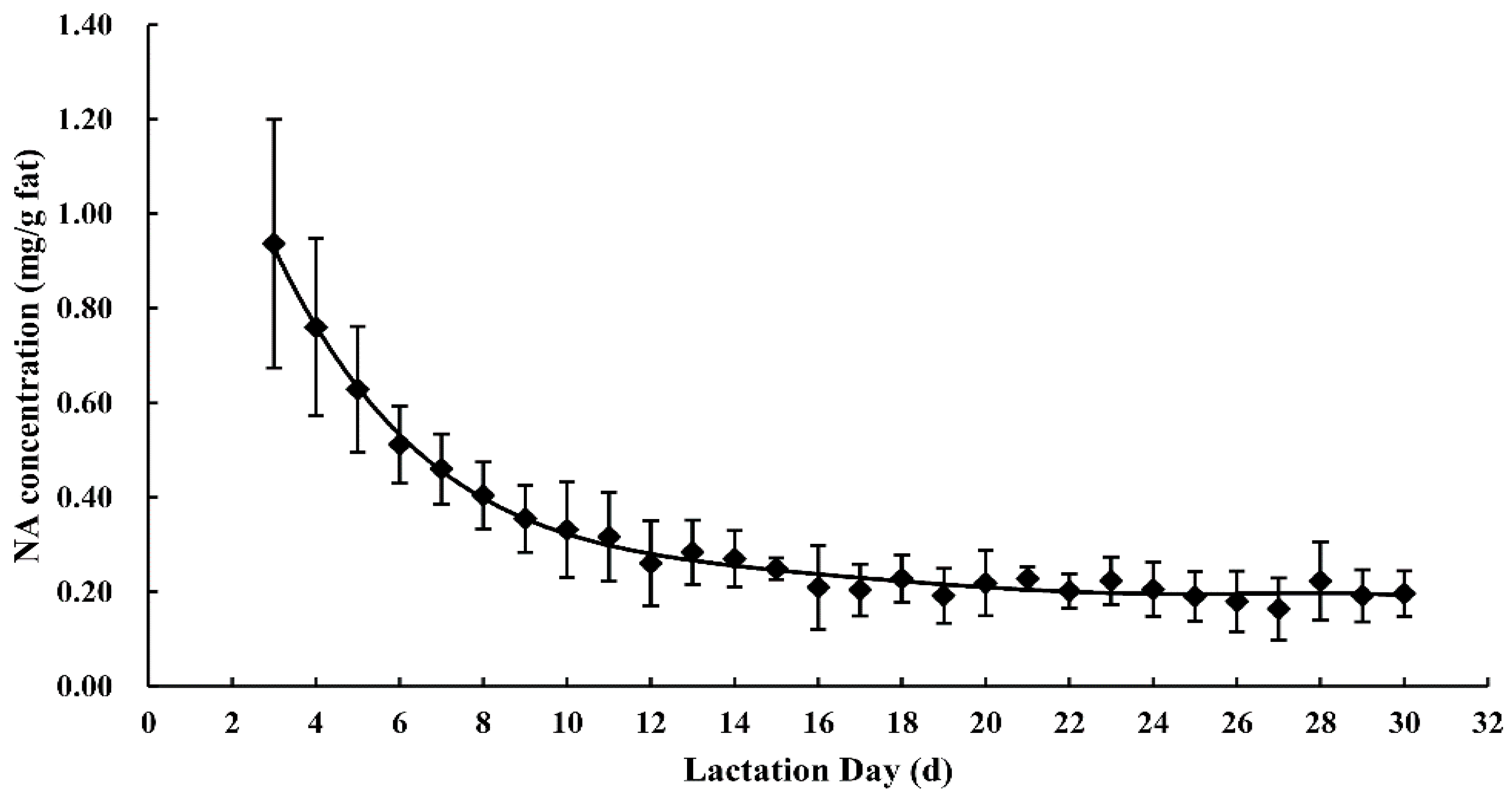
3.1. Nervonic Acid Concentration in Human Milk
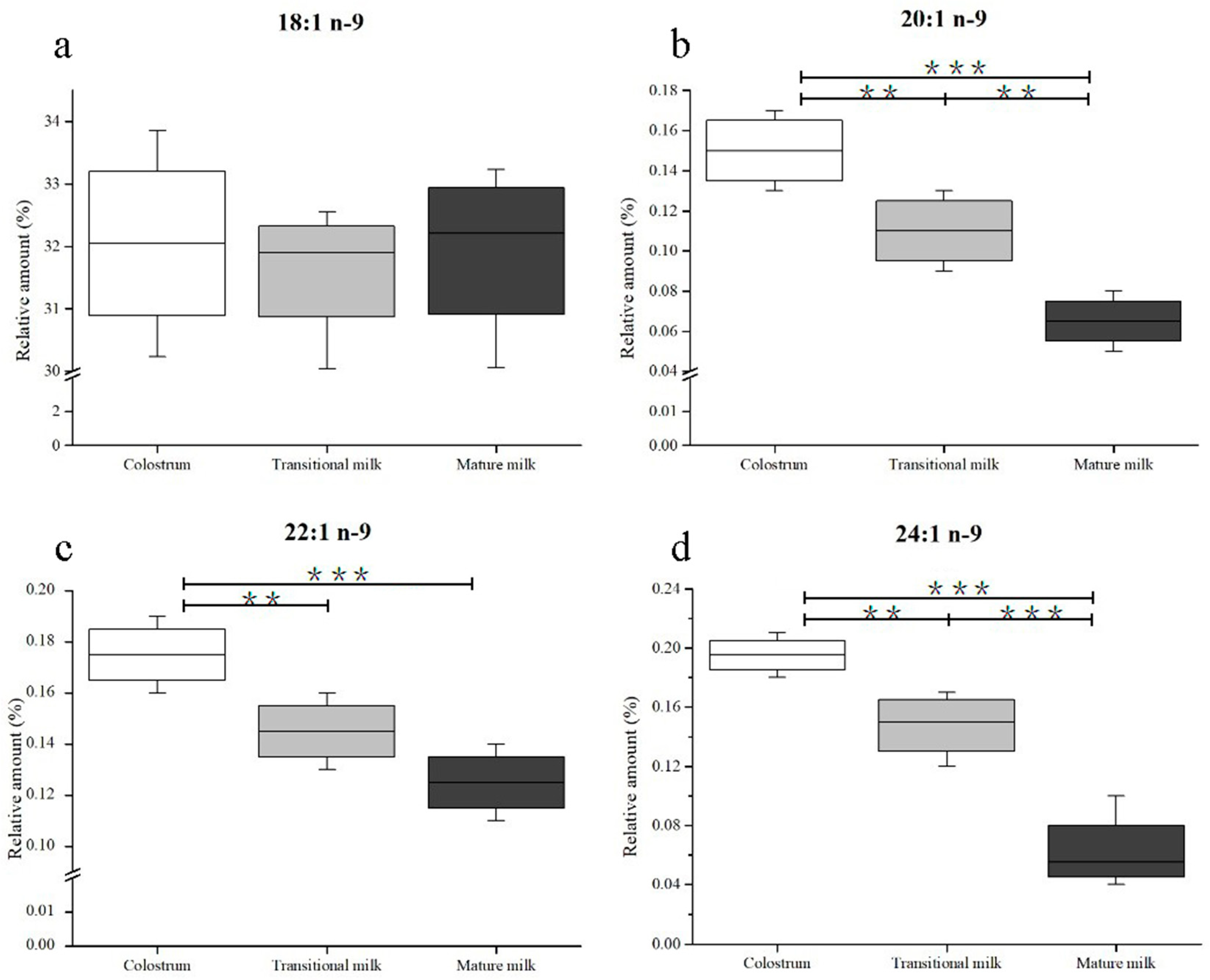
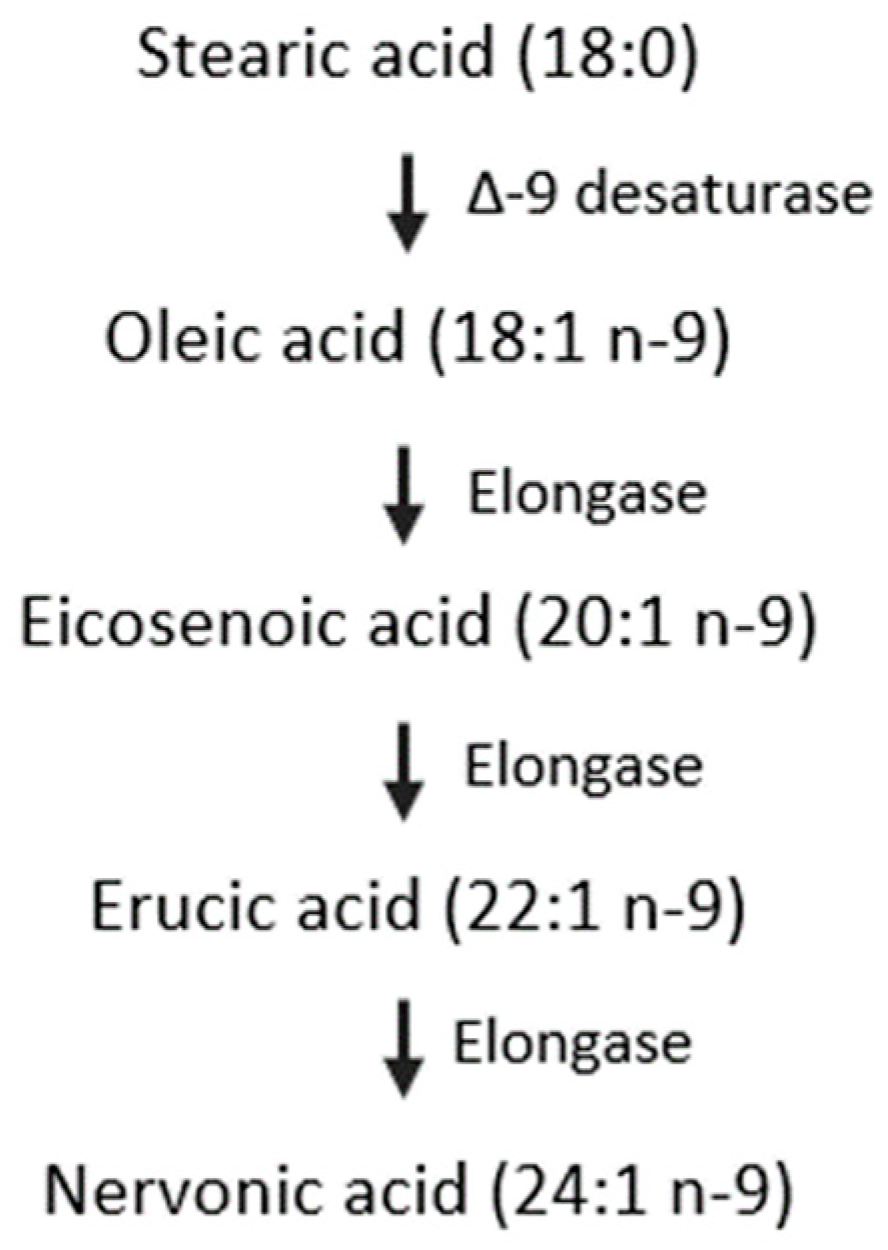
3.2. Composition of n-9 Fatty Acids in Human Milk
3.3. Composition of n-9 Fatty Acids in Infant Formulae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheong, L.Z.; Jiang, C.Y.; He, X.Q.; Song, S.; Lai, O.M. Lipid Profiling, Particle Size Determination, and in Vitro Simulated Gastrointestinal Lipolysis of Mature Human Milk and Infant Formula. J. Agric. Food Chem. 2018, 66, 12042–12050. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jin, Q.; Wang, X. Human milk fat substitutes: Past achievements and current trends. Prog. Lipid Res. 2019, 74, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zou, X.; Yao, Y.; Jin, J.; Xia, Y.; Huang, J.; Jin, Q.; Wang, X. Evaluation of fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Int. Dairy J. 2016, 63, 42–51. [Google Scholar] [CrossRef]
- Amminger, G.P.; Schaefer, M.R.; Klier, C.M.; Slavik, J.M.; Holzer, I.; Holub, M.; Goldstone, S.; Whitford, T.J.; McGorry, P.D.; Berk, M. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol. Psychiatry 2012, 17, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Kinney, H.C.; Brody, B.A.; Kloman, A.S.; Gilles, F.H. Sequence of central nervous system myelination in human infancy. II. Patterns of Myelination in Autopsied Infants. J. Neuropathol. Exp. Neurol. 1988, 47, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.B.; Jones, D.S.; Raymond, G.V.; Moser, H.W. Plasma and red blood cell fatty acids in peroxisomal disorders. Neurochem. Res. 1999, 24, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.B.; Kreiter, N.; Bezman, L.; Lu, S.; Raymond, G.V.; Naidu, S.; Moser, H.W. Plasma very long chain fatty acids in 3000 peroxisome disease patients and 29,000 controls. Ann. Neurol. 1999, 45, 100–110. [Google Scholar] [CrossRef]
- Yeh, Y.Y. Long chain fatty acid deficits in brain myelin sphingolipids of undernourished rat pups. Lipids 1988, 23, 1114–1118. [Google Scholar] [CrossRef]
- Ntoumani, E.; Strandvik, B.; Sabel, K.G. Nervonic acid is much lower in donor milk than in milk from mothers delivering premature infants-Of neglected importance? Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 241–244. [Google Scholar] [CrossRef]
- Wu, K.; Gao, R.; Tian, F.; Mao, Y.; Wang, B.; Zhou, L.; Shen, L.; Guan, Y.; Cai, M. Fatty acid positional distribution (sn-2 fatty acids) and phospholipid composition in Chinese breast milk from colostrum to mature stage. Br. J. Nutr. 2018, 121, 65–73. [Google Scholar] [CrossRef]
- Nyuar, K.B.; Min, Y.; Ghebremeskel, K.; Khalil, A.K.H.; Elbashir, M.I.; Cawford, M.A. Milk of northern Sudanese mothers whose traditional diet is high in carbohydrate contains low docosahexaenoic acid. Acta Pãediatr. 2010, 99, 1824. [Google Scholar] [CrossRef]
- Boersma, E.R.; Offringa, P.J.; Muskiet, F.A.; Chase, W.M.; Simmons, I.J. Vitamin E, lipid fractions, and fatty acid composition of colostrum, transitional milk, and mature milk: An international comparative study. Am. J. Clin. Nutr. 1991, 53, 1197–1204. [Google Scholar] [CrossRef]
- Thakkar, S.K.; De Castro, C.A.; Beauport, L.; Tolsa, J.F.; Fumeaux, C.J.F.; Affolter, M.; Giuffrida, F. Temporal Progression of Fatty Acids in Preterm and Term Human Milk of Mothers from Switzerland. Nutrients 2019, 11, 12. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, S.W.; Liu, L.; Pang, X.Y.; Yang, Y.; Lu, J.; Lv, J.P. Differences in the Triacylglycerol and Fatty Acid Compositions of Human Colostrum and Mature Milk. J. Agric. Food Chem. 2018, 66, 4571–4579. [Google Scholar] [CrossRef]
- Jamieson, E.C.; Farquharson, J.; Logan, R.W.; Howatson, A.G.; Patrick, W.J.A.; Weaver, L.T.; Cockburn, F. Infant cerebellar gray and white matter fatty acids in relation to age and diet. Lipids 1999, 34, 1065–1071. [Google Scholar] [CrossRef]
- Nagae, L.M.; Hoon, A.H.; Stashinko, E.; Lin, D.; Zhang, W.; Levey, E.; Wakana, S.; Jiang, H.; Leite, C.C.; Lucato, L.T. Diffusion tensor imaging in children with periventricular leukomalacia: Variability of injuries to white matter tracts. Ajnr Am. J. Neuroradiol. 2007, 28, 1213. [Google Scholar] [CrossRef]
- De Vries, L.S.; Van Haastert, I.L.C.; Rademaker, K.J.; Corine, K.; Floris, G. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J. Pediatr. 2004, 144, 815–820. [Google Scholar] [CrossRef]
- Liu, Z.; Rochfort, S.; Cocks, B. Milk lipidomics: What we know and what we don’t. Prog. Lipid Res. 2018, 71, 70–85. [Google Scholar] [CrossRef]
- Hua, M.C.; Su, H.M.; Kuo, M.L.; Chen, C.C.; Yao, T.C.; Tsai, M.H.; Liao, S.L.; Lai, S.H.; Chiu, C.Y.; Su, K.W.; et al. Association of maternal allergy with human milk soluble CD14 and fatty acids, and early childhood atopic dermatitis. Pediatr. Allergy Immunol. 2019, 30, 204–213. [Google Scholar] [CrossRef]
- Rueda, R.; Ramírez, M.; Garcíasalmerón, J.L.; Maldonado, J.; Gil, A. Gestational Age and Origin of Human Milk Influence Total Lipid and Fatty Acid Contents. Ann. Nutr. Metab. 1998, 42, 12–22. [Google Scholar] [CrossRef]
- Salavila, A.; Castellote, A.I.; Rodriguezpalmero, M.; Campoy, C.; Lópezsabater, M.C. Lipid composition in human breast milk from Granada (Spain): Changes during lactation. Nutrition 2005, 21, 467–473. [Google Scholar] [CrossRef]
- Zou, L.; Pande, G.; Akoh, C.C. Infant Formula Fat Analogs and Human Milk Fat: New Focus on Infant Developmental Needs. In Annual Review of Food Science and Technology; Doyle, M.P., Klaenhammer, T.R., Eds.; Annual Reviews: Palo Alto, CA, USA, 2016; Volume 7, pp. 139–165. [Google Scholar]
- Billeaud, C.; Boue-Vaysse, C.; Couedelo, L.; Steenhout, P.; Jaeger, J.; Cruz-Hernandez, C.; Ameye, L.; Rigo, J.; Picaud, J.C.; Saliba, E.; et al. Effects on Fatty Acid Metabolism of a New Powdered Human Milk Fortifier Containing Medium-Chain Triacylglycerols and Docosahexaenoic Acid in Preterm Infants. Nutrients 2018, 10, 690. [Google Scholar] [CrossRef]
- Dingess, K.; Valentine, C.; Davidson, B.; Peng, Y.; Guerrero, M.; Ruiz-Palacios, G.; Brenna, J.; McMahon, R.; Morrow, A. Docosahexaenoic acid, nervonic acid and iso-20 (BCFA) concentrations in human milk from the Global Exploration of Human Milk Project. FASEB J. 2014, 28, 623.15. [Google Scholar]
- Sala-Vila, A.; Castellote, A.I.; Campoy, C.M.; Rodriguez-Palmero, M. The source of long-chain PUFA in formula supplements does not affect the fatty acid composition of plasma lipids in full-term infants. J. Nutr. 2004, 134, 868–873. [Google Scholar] [CrossRef]
- Jensen, R.G. The Lipids of Human Milk; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Qi, C.; Sun, J.; Xia, Y.; Yu, R.Q.; Wei, W.; Xiang, J.Y.; Jin, Q.Z.; Xiao, H.; Wang, X.G. Fatty Acid Profile and the sn-2 Position Distribution in Triacylglycerols of Breast Milk during Different Lactation Stages. J. Agric. Food Chem. 2018, 66, 3118–3126. [Google Scholar] [CrossRef]
- The United States Pharmacopeia. Validation of Compendial Procedures. In The United States Pharmacopeia, the National. Formulary, Chapter 1225; Stanford University: Stanford, CA, USA, 2009. [Google Scholar]
- Quintela, M.; Báguena, J.; Gotor, G.; Blanco, M.J.; Broto, F. Estimation of the uncertainty associated with the results based on the validation of chromatographic analysis procedures: Application to the determination of chlorides by high performance liquid chromatography and of fatty acids by high resolution gas chro. J. Chromatogr. A 2012, 1223, 107–117. [Google Scholar] [CrossRef]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trac Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- IOFI Working Group on Methods of Analysis. Guidelines for the quantitative gas chromatography of volatile flavouring substances, from the Working Group on Methods of Analysis of the International Organization of the Flavor Industry (IOFI). Flavour Fragr. J. 2011, 26, 297–299. [Google Scholar]
- Gravador, R.S.; Harrison, S.M.; Monahan, F.J.; Gkarane, V.; Farmer, L.J.; Brunton, N.P. Validation of a Rapid Microwave-Assisted Extraction Method and GC-FID Quantification of Total Branched Chain Fatty Acids in Lamb Subcutaneous Adipose Tissue. J. Food Sci. 2019, 84, 80–85. [Google Scholar] [CrossRef]
- AOAC. AOAC Peer-Verified Methods Program, Manual on Policies and Procedures; The Association of Official Analytical Chemists: Arlington, VA, USA, 1998. [Google Scholar]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN Coordinated International Expert Group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef]
- Xiang, M.; Harbige, L.S.; Zetterstrã, M.R. Long-chain polyunsaturated fatty acids in Chinese and Swedish mothers: Diet, breast milk and infant growth. Acta Pædiatr. 2005, 94, 1543–1549. [Google Scholar] [CrossRef]
- Rydlewski, A.A.; Silva, P.D.; Manin, L.P.; Tavares, C.B.G.; Paula, M.G.; Figueiredo, I.L.; Neia, V.; Santos, O.O.; Visentainer, J.V. Lipid Profile Determination by Direct Infusion ESI-MS and Fatty Acid Composition by GC-FID in Human Milk Pools by Folch and Creamatocrit Methods. J. Braz. Chem. Soc. 2019, 30, 1063–1073. [Google Scholar] [CrossRef]
- Golfetto, I.; Mcgready, R.; Ghebremeskel, K.; Min, Y.; Dubowitz, L.; Nosten, F.; Drury, P.; Simpson, J.A.; Arunjerdja, R.; Crawford, M.A. Fatty acid composition of milk of refugee Karen and urban Korean mothers. Is the level of DHA in breast milk of Western women compromised by high intake of saturated fat and linoleic acid? Nutr. Health 2007, 18, 319. [Google Scholar] [CrossRef]
- Yakes, E.A.; Arsenault, J.E.; Munirul, I.M.; Hossain, M.B.; Ahmed, T.; Bruce, G.J.; Gillies, L.A.; Shafiqur, R.A.; Drake, C.; Jamil, K.M. Intakes and breast-milk concentrations of essential fatty acids are low among Bangladeshi women with 24–48-month-old children. Br. J. Nutr. 2011, 105, 1660–1670. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, T.; Wang, Q.; Liu, P.; Zhang, T.; Zetterström, R.; Strandvik, B. Fatty acid composition of diet, cord blood and breast milk in Chinese mothers with different dietary habits. Prostagland. Leukot. Essent. Fat. Acids 2009, 81, 325–330. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.W.; Zhang, Z.W.; Yu, H.; An, Y.; Kramer, J.K.G.; Deng, Z.Y. Evaluating the trans fatty acid, CLA, PUFA and erucic acid diversity in human milk from five regions in China. Lipids 2009, 44, 257. [Google Scholar] [CrossRef]
- Rocquelin, G.; Tapsoba, S.; Dop, M.C.; Mbemba, F.; Traissac, P.; Martinprével, Y. Lipid content and essential fatty acid (EFA) composition of mature Congolese breast milk are influenced by mothers’ nutritional status: Impact on infants’ EFA supply. Eur. J. Clin. Nutr. 1998, 52, 164–171. [Google Scholar] [CrossRef]
- van Beusekom, C.M.; Nijeboer, H.J.; Van der Veere, C.N.; Luteyn, A.J.; Offringa, P.J.; Muskiet, F.A.; Boersma, E.R. Indicators of long chain polyunsaturated fatty acid status of exclusively breastfed infants at delivery and after 20–22 days. Early Hum. Dev. 1993, 32, 207. [Google Scholar] [CrossRef]
- Van, B.C.; Martini, I.A.; Rutgers, H.M.; Boersma, E.R.; Muskiet, F.A. A carbohydrate-rich diet not only leads to incorporation of medium-chain fatty acids (6:0–14:0) in milk triglycerides but also in each milk-phospholipid subclass. Am. J. Clin. Nutr. 1990, 52, 326. [Google Scholar]
- Nishimura, R.Y.; Barbieiri, P.; Castro, G.S.; Jordão, A.A., Jr.; Perdoná, G.S.; Sartorelli, D.S. Dietary polyunsaturated fatty acid intake during late pregnancy affects fatty acid composition of mature breast milk. Nutrition 2014, 30, 685. [Google Scholar] [CrossRef]
- Bettger, W.J.; DiMichelle-Ranalli, E.; Dillingham, B.; Blackadar, C.B. Nervonic acid is transferred from the maternal diet to milk and tissues of suckling rat pups. J. Nutr. Biochem. 2003, 14, 160–165. [Google Scholar] [CrossRef]
- Bettger, W.J.; Blackadar, C.B. Dietary very long chain fatty acids directly influence the ratio of tetracosenoic (24:1) to tetracosanoic (24:0) acids of sphingomyelin in rat liver. Lipids 1997, 32, 51–55. [Google Scholar] [CrossRef]
- Fulco, A.J.; Mead, J.F. The biosynthesis of lignoceric, cerebronic, and nervonic acids. J. Biol. Chem. 1961, 236, 2416–2420. [Google Scholar]
- Lauritzen, L.; Jorgensen, M.H.; Hansen, H.S.; Michaelsen, K.F. Fluctuations in human milk long-chain PUFA levels in relation to dietary fish intake. Lipids 2002, 37, 237–244. [Google Scholar] [CrossRef]
- Argov-Argaman, N.; Mandel, D.; Lubetzky, R.; Kedem, M.H.; Cohen, B.C.; Berkovitz, Z.; Reifen, R. Human milk fatty acids composition is affected by maternal age. J. Matern. Fetal Neonatal Med. 2017, 30, 34–37. [Google Scholar] [CrossRef]
- Marín, M.C.; Sanjurjo, A.; Rodrigo, M.A.; de Alaniz, M.J. Long-chain polyunsaturated fatty acids in breast milk in La Plata, Argentina: Relationship with maternal nutritional status. Prostagland. Leukot. Essent. Fat. Acids 2005, 73, 355–360. [Google Scholar] [CrossRef]
- Muskiet, F.A.; Hutter, N.H.; Martini, I.A.; Jonxis, J.H.; Offringa, P.J.; Boersma, E.R. Comparison of the fatty acid composition of human milk from mothers in Tanzania, Curacao and Surinam. Hum. Nutr. Clin. Nutr. 1987, 41, 149–159. [Google Scholar]
- Molto-Puigmarti, C.; Plat, J.; Mensink, R.P.; Muller, A.; Jansen, E.; Zeegers, M.P.; Thijs, C. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. Am. J. Clin. Nutr. 2010, 91, 1368–1376. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.A.; Troxler, H.; Klinke, G.; Rogler, D.; Braegger, C.; Hersberger, M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 2013, 12, 89. [Google Scholar] [CrossRef]
- Lópezlópez, A.; Lópezsabater, M.C.; Campoyfolgoso, C.; Riverourgell, M.; Castellotebargalló, A.I. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and in infant formulas. Eur. J. Clin. Nutr. 2002, 56, 1242–1254. [Google Scholar] [CrossRef] [Green Version]
- Salem, N.; Wegher, B.; Mena, P.; Uauy, R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA 1996, 93, 49–54. [Google Scholar] [CrossRef]
- Gibson, R.A.; Makrides, M.; Neumann, M.A.; Simmer, K.; Mantzioris, E.; James, M.J. Ratios of linoleic acid to alpha-linolenic acid in formulas for term infants. J. Pediatr. 1994, 125, 48–55. [Google Scholar] [CrossRef]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129. [Google Scholar] [CrossRef]
- Uzman, L.L.; Rumley, M.K. Changes in the composition of the developing mouse brain during early myelination. J. Neurochem. 2010, 3, 170–184. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, H.-M.; Hu, G.-R.; Li, F. Biosynthesis of nervonic acid and perspectives for its production by microalgae and other microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3027–3035. [Google Scholar] [CrossRef]
- Wang, X.Y.; Fan, J.S.; Wang, S.Q. Development situation and outlook of nervonic acid plants in China. China Oils Fats 2006, 31, 69–71. [Google Scholar]
- Yiming, G.; Elzbieta, M.; Tammy, F.; Vesna, K.; Brost, J.M.; Michael, G.; Barton, D.L.; Taylor, D.C. Increase in nervonic acid content in transformed yeast and transgenic plants by introduction of a Lunaria annua L. 3-ketoacyl-CoA synthase (KCS) gene. Plant Mol. Biol. 2009, 69, 565–575. [Google Scholar]
- Das, S.; Roscoe, T.J.; Delseny, M.; Srivastava, P.S.; Lakshmikumaran, M. Cloning and molecular characterization of the Fatty Acid Elongase 1 (FAE 1) gene from high and low erucic acid lines of Brassica campestris and Brassica oleracea. Plant Sci. 2002, 162, 245–250. [Google Scholar] [CrossRef]
| Fatty Acids | CMF | GMF | POF | p-Value | |||
|---|---|---|---|---|---|---|---|
| (n = 30) | (n = 16) | (n = 51) | F | S | F × S | ||
| 18:1 n-9 | IF | 32.15 ± 5.06 a | 34.81 ± 5.66 a,b | 40.74 ± 8.32 b | *** | NS | NS |
| FF | 30.65 ± 5.95 a | 30.15 ± 5.28 a | 41.61 ± 6.11 b | ||||
| GF | 31.76 ± 5.09 a | 29.66 ± 6.50 a | 40.89 ± 7.31 b | ||||
| 20:1 n-9 | IF | 0.23 ± 0.09 | 0.29 ± 0.09 | 0.31 ± 0.10 | *** | NS | NS |
| FF | 0.17 ± 0.10 a | 0.24 ± 0.09 a,b | 0.33 ± 0.09 b | ||||
| GF | 0.13 ± 0.04 a | 0.21 ± 0.07 a | 0.30 ± 0.09 b | ||||
| 22:1 n-9 | IF | 0.04 ± 0.04 | 0.06 ± 0.04 | 0.04 ± 0.03 | ** | NS | NS |
| FF | 0.03 ± 0.03 | 0.05 ± 0.03 | 0.05 ± 0.03 | ||||
| GF | 0.01 ± 0.01 a | 0.04 ± 0.03 a,b | 0.05 ± 0.04 b | ||||
| 24:1 n-9 | IF | 0.03 ± 0.13 | 0.03 ± 0.02 | 0.03 ± 0.02 | NS | NS | NS |
| FF | 0.02 ± 0.00 a | 0.02 ± 0.01 a | 0.03 ± 0.01 b | ||||
| GF | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | ||||
| Regions | Colostrum | Transitional Milk | Mature Milk | References |
|---|---|---|---|---|
| Wuxi, China | 0.20 (3–6 d) | 0.15 (7–14 d) | 0.06 (15–30 d) | This study |
| Taiwan, China | 0.99 (1–6 d) | - | 0.28 (2 m) | Hua et al. [19] |
| Switzerland | 0.39 (1 week) | 0.13 (2 weeks) | 0.07 (3–8 weeks) | Thakkar et al. [13] |
| Beijing, China | 0.54 (4 d) | - | 0.25 (30 d) | Zhao et al. [14] |
| Thailand | - | - | 0.06 | Golfetto et al. [37] |
| Korean | - | 0.27 | - | |
| Bangladeshi | - | - | 0.20 | Yakes et al. [38] |
| Northern Sudanese | 0.19 | 0.15 | 0.02 | Nyuar et al. [11] |
| Wenzhou, China | 0.45 | - | - | Peng et al. [39] |
| Changzhou, China | 0.25 | - | - | |
| Shanghai, China | - | 0.08 | 0.05 | Jing et al. [40] |
| Guangzhou, China | - | 0.06 | 0.06 | |
| Nanchang, China | - | 0.12 | 0.11 | |
| Harbin, China | - | 0.06 | 0.04 | |
| Hohhot, China | - | 0.21 | 0.19 | |
| Granada, Spain | 0.28 | 0.08 | 0.07 | Sala-Vila A et al. [21] |
| Congolese | - | - | 0.04 | Rocquelin et al. [41] |
| Panama | 0.32 | 0.16 | 0.10 | Rueda et al. [20] |
| Spain | 0.24 | 0.17 | 0.10 | |
| Dominica | - | - | 0.05 | Beusekoma et al. [42] |
| Saint Lucia | 0.41 | 0.11 | 0.04 | Boersma et al. [12] |
| Belize | - | - | 0.06 | Cheristien et al. [43] |
| Dominica | - | - | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Yuan, T.; Zhang, X.; Jin, Q.; Wei, W.; Wang, X. Quantification of Nervonic Acid in Human Milk in the First 30 Days of Lactation: Influence of Lactation Stages and Comparison with Infant Formulae. Nutrients 2019, 11, 1892. https://doi.org/10.3390/nu11081892
Yu J, Yuan T, Zhang X, Jin Q, Wei W, Wang X. Quantification of Nervonic Acid in Human Milk in the First 30 Days of Lactation: Influence of Lactation Stages and Comparison with Infant Formulae. Nutrients. 2019; 11(8):1892. https://doi.org/10.3390/nu11081892
Chicago/Turabian StyleYu, Jiahui, Tinglan Yuan, Xinghe Zhang, Qingzhe Jin, Wei Wei, and Xingguo Wang. 2019. "Quantification of Nervonic Acid in Human Milk in the First 30 Days of Lactation: Influence of Lactation Stages and Comparison with Infant Formulae" Nutrients 11, no. 8: 1892. https://doi.org/10.3390/nu11081892





