Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Data Analysis
3. Results
3.1. Study Selection Process
3.2. Characteristics of Participants
3.3. Characteristics of Interventions
3.4. Risk of Bias
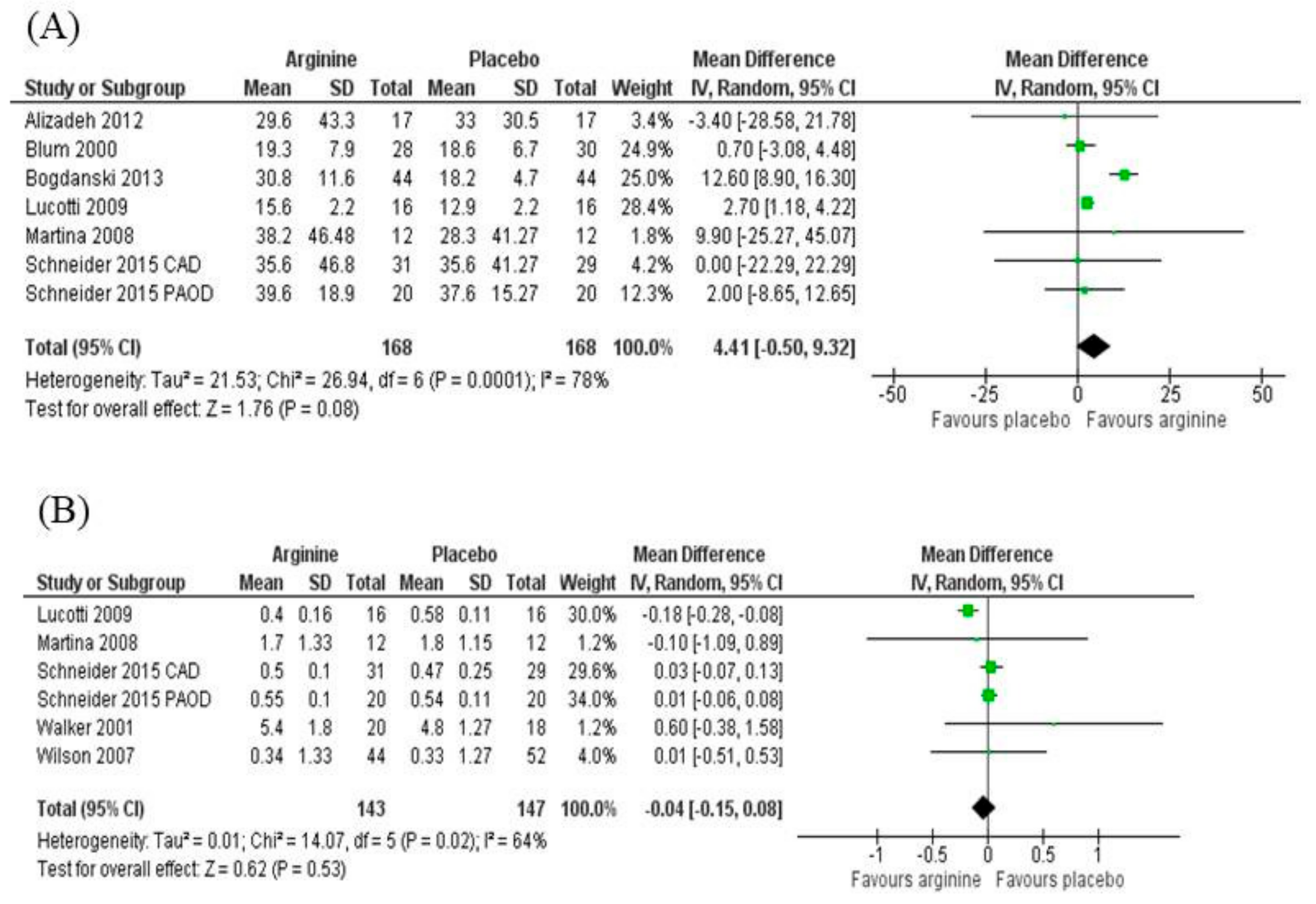
3.5. Effect Measures
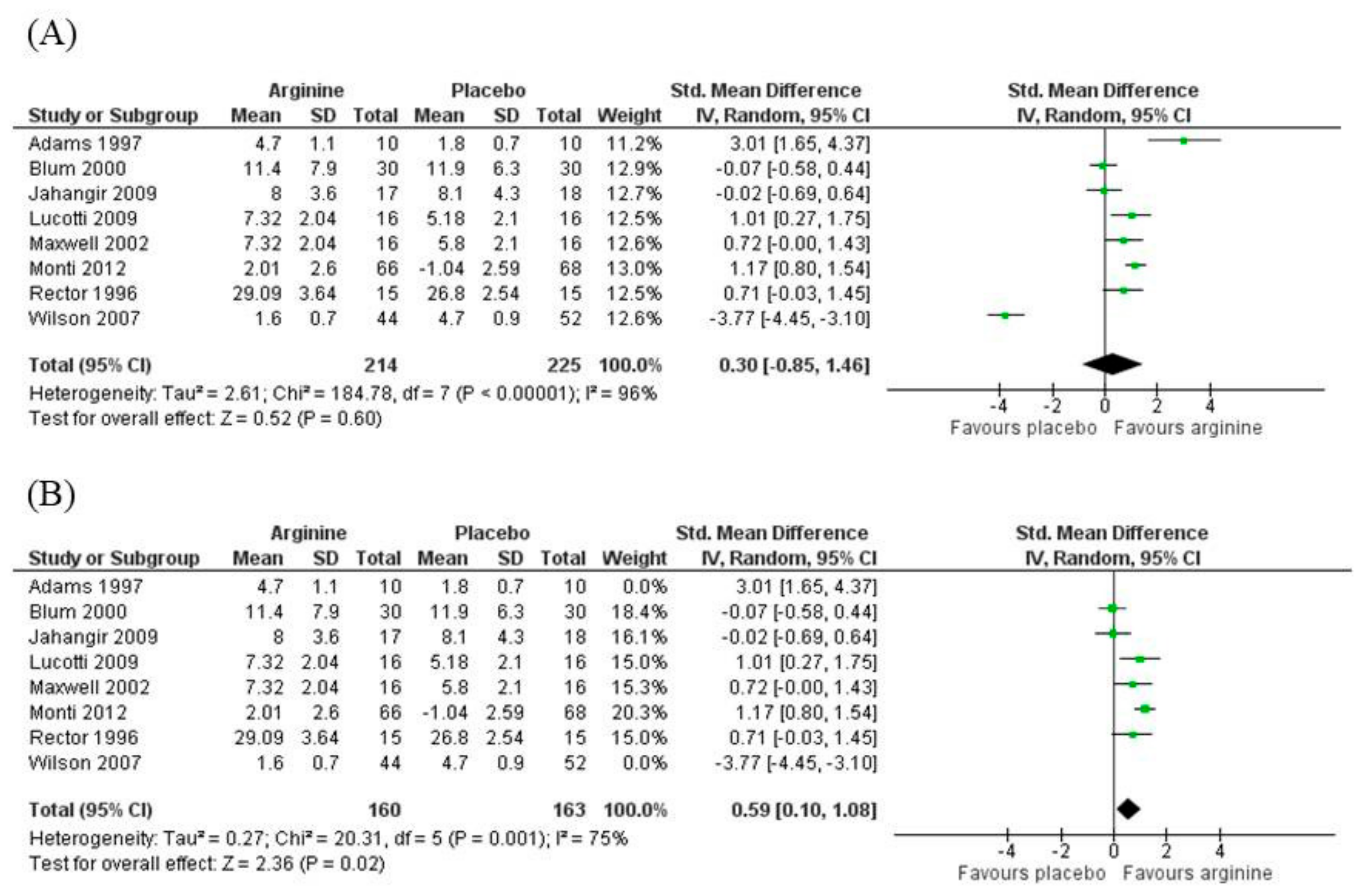
3.5.1. Blood Flow
3.5.2. Nitrites/Nitrates (NOx)
3.5.3. Asymmetric Dimethylarginine (ADMA)
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Newsholme, P.; Homem De Bittencourt, P.I.; C, O.H.; De Vito, G.; Murphy, C.; Krause, M.S. Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: the central role of ROS and nitric oxide. Clin. Sci. (Lond.) 2009, 118, 341–349. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front Endocrinol. (Lausanne) 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Fayh, A.P.; Krause, M.; Rodrigues-Krause, J.; Ribeiro, J.L.; Ribeiro, J.P.; Friedman, R.; Moreira, J.C.; Reischak-Oliveira, A. Effects of L-arginine supplementation on blood flow, oxidative stress status and exercise responses in young adults with uncomplicated type I diabetes. Eur. J. Nutr. 2013, 52, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Rodrigues-Krause, J.; O’Hagan, C.; Medlow, P.; Davison, G.; Susta, D.; Boreham, C.; Newsholme, P.; O’Donnell, M.; Murphy, C.; et al. The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur. J. Appl. Physiol. 2014, 114, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Krause, J.; Farinha, J.B.; Krause, M.; Reischak-Oliveira, A. Effects of dance interventions on cardiovascular risk with ageing: Systematic review and meta-analysis. Complement. Ther. Med. 2016, 29, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitao, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.S.; Lakin, R.O.; Campos, P.; Allemang, M.; Kim, A.; Sarac, T.P.; Hausladen, A.; Stamler, J.S. The LargPAD Trial: Phase IIA evaluation of l-arginine infusion in patients with peripheral arterial disease. J. Vasc. Surg. 2017, 66, 187–194. [Google Scholar] [CrossRef]
- Dimova, R.; Tankova, T.; Chakarova, N. Vitamin D in the Spectrum of Prediabetes and Cardiovascular Autonomic Dysfunction. J. Nutr. 2017, 147, 1607–1615. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Newsholme, P.; Rebelato, E.; Abdulkader, F.; Krause, M.; Carpinelli, A.; Curi, R. Reactive oxygen and nitrogen species generation, antioxidant defenses, and beta-cell function: a critical role for amino acids. J. Endocrinol. 2012, 214, 11–20. [Google Scholar] [CrossRef]
- Krause, M.; Rodrigues-Krause, J.; O’Hagan, C.; De Vito, G.; Boreham, C.; Susta, D.; Newsholme, P.; Murphy, C. Differential nitric oxide levels in the blood and skeletal muscle of type 2 diabetic subjects may be consequence of adiposity: a preliminary study. Metabolism 2012, 61, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.R.; McCredie, R.; Jessup, W.; Robinson, J.; Sullivan, D.; Celermajer, D.S. Oral L-arginine improves endothelium-dependent dilatation and reduces monocyte adhesion to endothelial cells in young men with coronary artery disease. Atherosclerosis 1997, 129, 261–269. [Google Scholar] [CrossRef]
- Blum, A.; Hathaway, L.; Mincemoyer, R.; Schenke, W.H.; Kirby, M.; Csako, G.; Waclawiw, M.A.; Panza, J.A.; Cannon, R.O., 3rd. Oral L-arginine in patients with coronary artery disease on medical management. Circulation 2000, 101, 2160–2164. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, E.; Vita, J.A.; Handy, D.; Holbrook, M.; Palmisano, J.; Beal, R.; Loscalzo, J.; Eberhardt, R.T. The effect of L-arginine and creatine on vascular function and homocysteine metabolism. Vasc. Med. 2009, 14, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Lucotti, P.; Monti, L.; Setola, E.; La Canna, G.; Castiglioni, A.; Rossodivita, A.; Pala, M.G.; Formica, F.; Paolini, G.; Catapano, A.L.; et al. Oral L-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metabolism 2009, 58, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.J.; Zapien, M.P.; Pearce, G.L.; MacCallum, G.; Stone, P.H. Randomized trial of a medical food for the dietary management of chronic, stable angina. J. Am. Coll. Cardiol. 2002, 39, 37–45. [Google Scholar] [CrossRef]
- Monti, L.D.; Setola, E.; Lucotti, P.C.; Marrocco-Trischitta, M.M.; Comola, M.; Galluccio, E.; Poggi, A.; Mammi, S.; Catapano, A.L.; Comi, G.; et al. Effect of a long-term oral l-arginine supplementation on glucose metabolism: a randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2012, 14, 893–900. [Google Scholar] [CrossRef]
- Rector, T.S.; Bank, A.J.; Mullen, K.A.; Tschumperlin, L.K.; Sih, R.; Pillai, K.; Kubo, S.H. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation 1996, 93, 2135–2141. [Google Scholar] [CrossRef]
- Caldwell, R.W.; Rodriguez, P.C.; Toque, H.A.; Narayanan, S.P.; Caldwell, R.B. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol. Rev. 2018, 98, 641–665. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Harada, R.; Nair, N.; Balasubramanian, N.; Cooke, J.P. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation 2007, 116, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Safaeiyan, A.; Ostadrahimi, A.; Estakhri, R.; Daneghian, S.; Ghaffari, A.; Gargari, B.P. Effect of L-arginine and selenium added to a hypocaloric diet enriched with legumes on cardiovascular disease risk factors in women with central obesity: a randomized, double-blind, placebo-controlled trial. Ann. Nutr. Metab. 2012, 60, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Lowas, S.R.; Marks, D.; Malempati, S. Prevalence of transient hyperglycemia during induction chemotherapy for pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2009, 52, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Martina, V.; Masha, A.; Gigliardi, V.R.; Brocato, L.; Manzato, E.; Berchio, A.; Massarenti, P.; Settanni, F.; Della Casa, L.; Bergamini, S.; et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care 2008, 31, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.Y.; Rothmann, S.; Schroder, F.; Langen, J.; Lucke, T.; Mariotti, F.; Huneau, J.F.; Frolich, J.C.; Tsikas, D. Effects of chronic oral L-arginine administration on the L-arginine/NO pathway in patients with peripheral arterial occlusive disease or coronary artery disease: L-Arginine prevents renal loss of nitrite, the major NO reservoir. Amino Acids 2015, 47, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.A.; McGing, E.; Fisher, I.; Boger, R.H.; Bode-Boger, S.M.; Jackson, G.; Ritter, J.M.; Chowienczyk, P.J. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J. Am. Coll. Cardiol. 2001, 38, 499–505. [Google Scholar] [CrossRef]
- Bogdanski, P.; Szulinska, M.; Suliburska, J.; Pupek-Musialik, D.; Jablecka, A.; Witmanowski, H. Supplementation with L-arginine favorably influences plasminogen activator inhibitor type 1 concentration in obese patients. A randomized, double blind trial. J. Endocrinol. Invest. 2013, 36, 221–226. [Google Scholar]
- Sun, T.; Zhou, W.B.; Luo, X.P.; Tang, Y.L.; Shi, H.M. Oral L-arginine supplementation in acute myocardial infarction therapy: a meta-analysis of randomized controlled trials. Clin. Cardiol. 2009, 32, 649–652. [Google Scholar] [CrossRef]
- Dong, J.Y.; Qin, L.Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef]
- Preli, R.B.; Klein, K.P.; Herrington, D.M. Vascular effects of dietary L-arginine supplementation. Atherosclerosis 2002, 162, 1–15. [Google Scholar] [CrossRef]
- Murrant, C.L.; Sarelius, I.H. Local control of blood flow during active hyperaemia: what kinds of integration are important? J. Physiol. 2015, 593, 4699–4711. [Google Scholar] [CrossRef] [PubMed]
- Simundic, A.M.; Bartlett, W.A.; Fraser, C.G. Biological variation: a still evolving facet of laboratory medicine. Ann. Clin. Biochem. 2015, 52, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.D.; Heresztyn, T. An overview of plasma concentrations of asymmetric dimethylarginine (ADMA) in health and disease and in clinical studies: methodological considerations. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 851, 42–50. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Krug, O.; Bode-Boger, S.M. Determination of arginine and asymmetric dimethylarginine (ADMA) in human plasma by liquid chromatography/mass spectrometry with the isotope dilution technique. J. Mass Spectrom. 2004, 39, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Menge, B.A.; Schrader, H.; Ritter, P.R.; Ellrichmann, M.; Uhl, W.; Schmidt, W.E.; Meier, J.J. Selective amino acid deficiency in patients with impaired glucose tolerance and type 2 diabetes. Regul. Pept. 2009, 160, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Pieper, G.M.; Dondlinger, L.A. Plasma and vascular tissue arginine are decreased in diabetes: acute arginine supplementation restores endothelium-dependent relaxation by augmenting cGMP production. J. Pharmacol. Exp. Ther. 1997, 283, 684–691. [Google Scholar]
- Stapleton, P.A.; Goodwill, A.G.; James, M.E.; Brock, R.W.; Frisbee, J.C. Hypercholesterolemia and microvascular dysfunction: interventional strategies. J. Inflamm. (Lond.) 2010, 7, 54. [Google Scholar] [CrossRef]
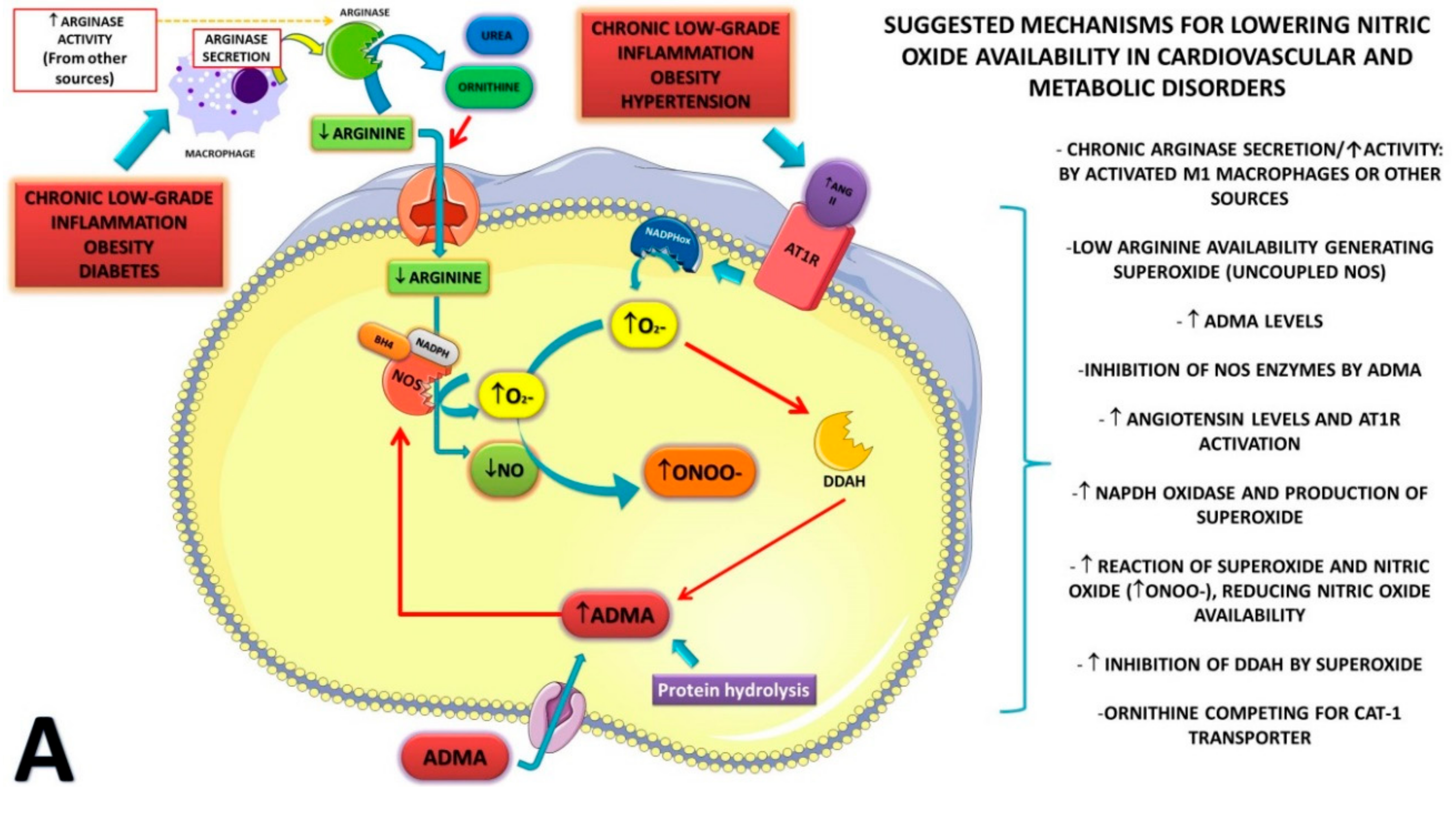
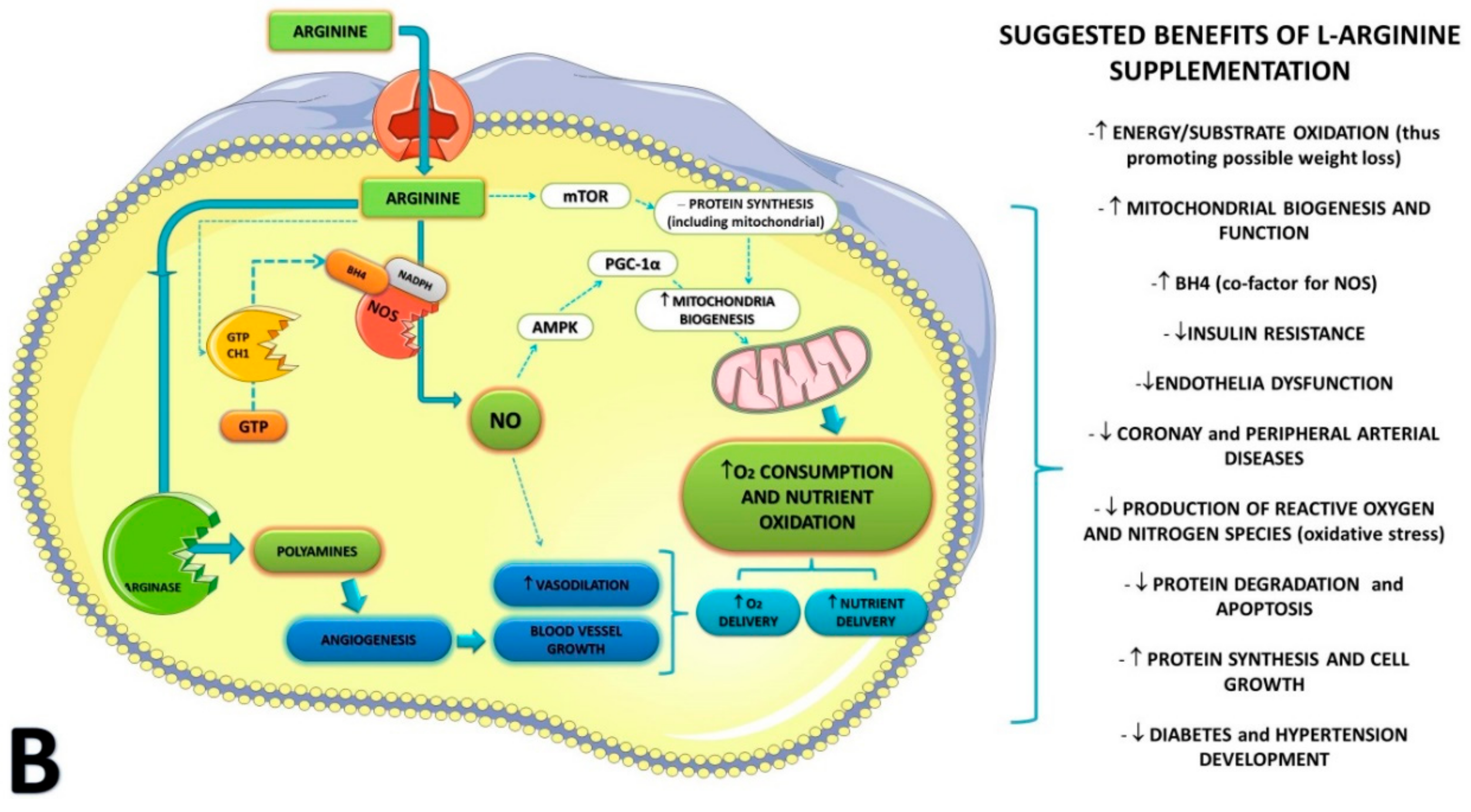
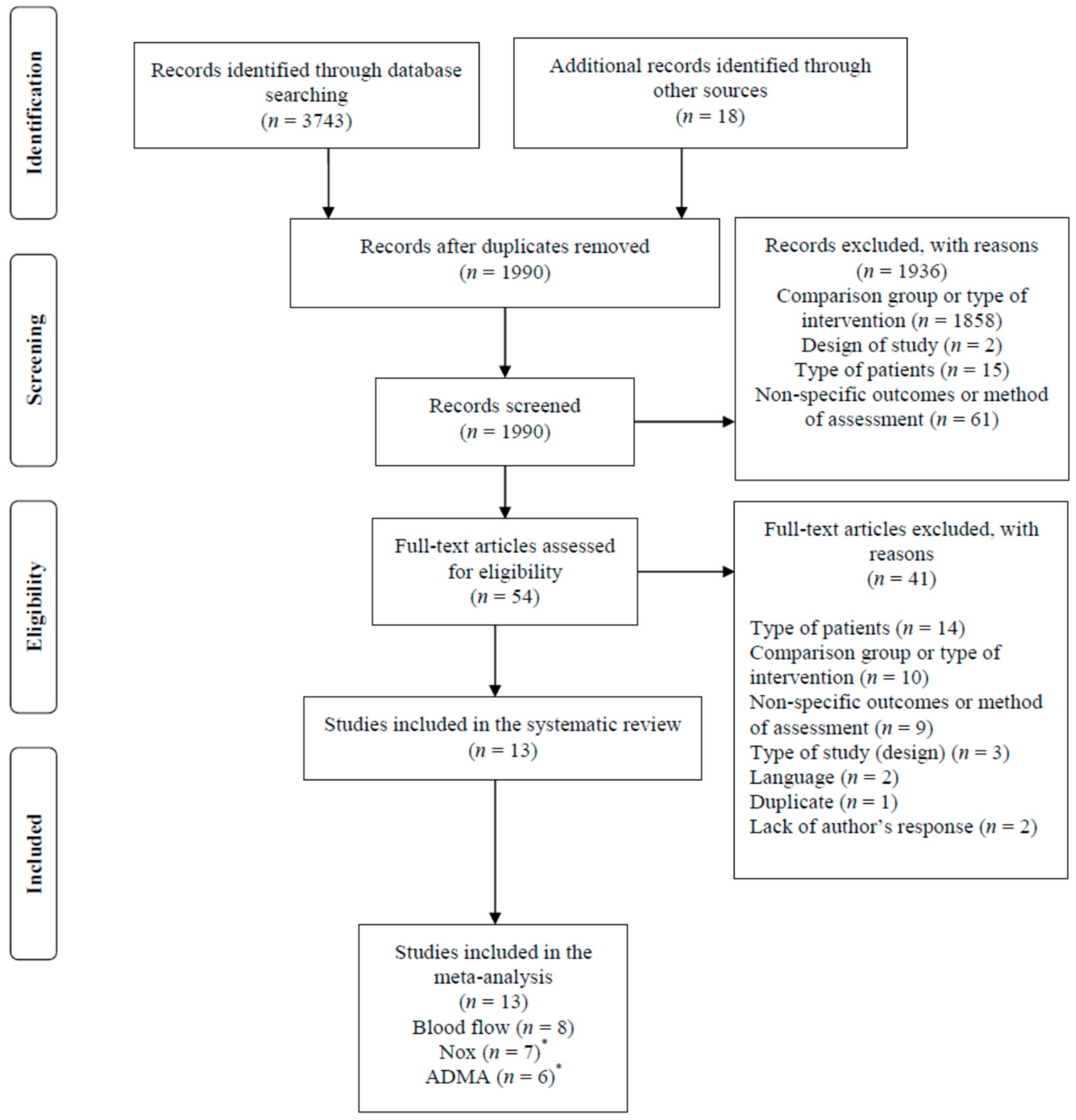
| Reference and Type of Study | Condition and Baseline Characteristics | Supplementation and Dosage | Duration of Supplementation | Outcomes Measured/ Analysis Method | Conclusions | |
|---|---|---|---|---|---|---|
| Arginine Group | Placebo Group | |||||
| Patients with Obesity | ||||||
| Alizadeh et al. [23] Prospective, randomized, double-blind, placebo-controlled trial | Premenopausal women with central obesity (n = 87). | n = 17 33.6 ± 8.6 years Hypocaloric diet enriched in legumes (HDEL) + l-arginine (5 g/day) in form of tablets | n = 17 33.8 ± 9.1 years Hypocaloric diet enriched in legumes (HDEL) + placebo (starch and lactose in form of tablets) | Six weeks | (1) NOx/Griess reaction | HDEL + placebo increased NOx levels, but adding l-arginine eliminated the beneficial effect of HDEL. |
| Bogdanski et al. [28] Prospective, randomized, double-blind, placebo-controlled trial | Obese patients (n = 88) | n = 44 (21 male) 43.1 ± 8.6 years l-arginine (9 g/day) in form of capsules | n = 44 (24 male) 41.5 ± 9.1 years Placebo in form of capsules | Six months | (1) NOx/enzyme immunoassay (ELISA) | Treatment with l-arginine resulted in significant increase in NOx. No significant changes between analyzed variables were noticed in placebo group. |
| Monti et al. [17] Prospective, randomized, double-blind, placebo-controlled trial | Patients with impaired glucose tolerance and metabolic syndrome (n = 144) | n = 72 (42 male) 57.2 ± 11.7 years l-arginine (6.4 g/day) | n = 72 (39 male) 58.2 ± 9.4 years Placebo | 18 months | (1) Blood flow/venous occlusion plethysmography of the forearm artery | Treatment with l-arginine increased post-ischaemic hyperemia, and no changes were observed in placebo group. |
| Patients with Type 2 Diabetes | ||||||
| Martina et al. [25] Prospective, randomized, double-blind, placebo-controlled trial | Male patients with type 2 diabetes and hypertension (n = 24) | n = 12 62.5 (59.3–74.5) years 600 mg N-acetylcysteine (NAC), one tablet twice a day + l-arginine (1,200 mg/day) one vial | n = 12 67.0 (51.0–69.7) years Placebo in form of compounds identical in appearance to NAC and l-arginine | Six months | (1) NOx/ Griess reaction. (2) Blood flow/ultrasound for assessment the endothelial- dependent flow-mediated vasodilation of the brachial artery | In comparison with baseline, l-arginine + NAC reduced intima-media thickness and increase plasma nitrites and nitrates. |
| Patients with Cardiovascular Disease | ||||||
| Adams et al. [12] Prospective, randomized crossover, double-blind, placebo-controlled trial | Men with angiographically documented coronary artery disease in at least two vessels (n = 10) | n = 10 41 ± 2 years l-arginine (7 g/day) in form of powder | The same group (crossover design) Placebo in form of powder with same flavor and appearance | Three days | (1) Blood flow/ultrasound (brachial artery for assessment the endothelial- dependent (reactive hyperemia) and independent (response to glyceryltrinitrate) flow-mediated vasodilation | Treatment with l-arginine improved endothelium-dependent dilatation, but no changes were seen in endothelium-independent dilatation of the brachial artery. |
| Maxwell et al. [16] Prospective, randomized crossover, double-blind, placebo-controlled trial | Patients with angina secondary to atherosclerotic coronary artery disease (n = 36) | n = 36 (28 male) 65.9 ± 10 years. l-arginine (6.6 g/day) in form of two bars with 3.3 g each | The same group (crossover design) Placebo bar with the same weight, appearance and flavor. | Two weeks | (1) Blood flow/ultrasound for assessment the endothelial- dependent flow-mediated vasodilation of the brachial artery | Treatment with l-arginine improved flow-mediated vasodilation. |
| Jahangir et al. [14] Prospective, randomized, double-blind, placebo-controlled trial | Patients with coronary artery disease (n = 109) | n = 26 (23 male) 60 ± 9 years l-arginine (9 g/day) in form of tablets | n = 26 (22 male) 58 ± 12 years Placebo (lactose) in form of tablets | Four days | (1) Blood flow/ultrasound (brachial artery for assessment the endothelial- dependent (reactive hyperemia) and independent (response to glyceryltrinitrate) flow-mediated vasodilation | Treatment with l-arginine had no effects on vascular function. |
| Lucotti et al. [15] Prospective, randomized, double-blind, placebo-controlled trial | Patients with cardiovascular disease, nondiabetic, previously submitted to an aortocoronary bypass (n = 30) | n = 16 (15 male) 65 ± 10 years l-arginine (6.4 g/day) | n = 14 (13 male) 64 ± 11 years Placebo with similar appearance of l-arginine | Six months | (1) NOx/Griess reaction (2) Asymmetric dimethylarginine (ADMA)/high-performance liquid chromatography (3) Blood Flow/ultrasound (brachial artery for assessment the endothelial-dependent (reactive hyperemia) vasodilation | Compared with placebo, l-arginine decreased NOx and ADMA levels, but no changes in basal blood flow were observed. |
| Blum et al. [13] Prospective, randomized crossover, double-blind, placebo-controlled trial | Patients with coronary artery disease (n = 30) | n = 30 (29 men) 67 ± 8 years l-arginine (9 g/d) in form of capsules | The same group (crossover design) Placebo with capsules identical to l-arginine | One month | (1) NOx/chemiluminescent technique (2) Blood flow/ultrasound (brachial artery for assessment the endothelial- dependent (reactive hyperemia) and independent (response to glyceryltrinitrate) flow-mediated vasodilation | No effects were observed on NOx and on brachial artery diameters, flow-mediated dilation, or nitroglycerin-induced dilation. |
| Rector et al. [18] Prospective, randomized crossover, double-blind, placebo-controlled trial | Patients with heart failure (n = 15) | n = 15 (14 male) 56 ± 10 years l-arginine (5.6 g/day (n = 9) or 12.6 g/day (n = 6) | The same group (crossover design) placebo capsules | Six weeks | (1) Blood flow/venous occlusion plethysmography | Treatment with l-arginine did not change forearm blood flow. |
| Schneider et al. [26] Prospective, randomized, double-blind, placebo-controlled trial | Patients suffering from peripheral arterial occlusive disease | n = 20 (16 male) 67.3 ± 8.0 years l-arginine (9.96 g/day) in form of effervescent tablets | n = 20 (15 male) 68.4 ± 8.0 years Placebo in form of tablets | Three months | (1) ADMA, nitrites, and nitrates (plasma and urine)/validated with mass spectrometry-based methods | Treatment with l-arginine increased insignificantly the ADMA concentration in the plasma, but enhanced the excretion rate of ADMA. |
| Schneider et al. [26] Prospective, randomized, double-blind, placebo-controlled trial | Patients suffering from coronary artery disease | n = 31 (24 male) (62 years—no standard deviation was informed) l-arginine (9.96 g/day) in form of effervescent tablets | n = 29 (24 male) (62 years—no standard deviation was informed) Placebo in form of tablets | Six months | (1) ADMA, nitrites, and nitrates (plasma and urine)/validated with mass spectrometry-based methods | Compared to placebo, plasma ADMA, nitrite, and nitrate did not change significantly with oral l-arginine supplementation. Urinary ADMA increased only marginally after three but not after six months of l-arginine supplementation. Urinary excretion of nitrate and nitrite did not significantly change after l-arginine supplementation for 3 and 6 months. |
| Walker et al. [27] Prospective, randomized, double-blind, placebo-controlled trial | Men with stable angina (n = 40) | n = 21 60 ± 2 years l-arginine (15 g/day) | n = 19 63 ± 2 years Placebo (lactose) | Two weeks | (1) ADMA/high-performance liquid chromatography (2) Blood flow/venous occlusion plethysmography of the forearm artery | Treatment with l-arginine supplementations did not alter plasma ADMA, and did not improve endothelium dependent vasodilation. |
| Wilson et al. [22] Prospective, randomized, double-blind, placebo-controlled trial | Patients with intermittent claudication due to peripheral arterial disease (n = 133) | n = 66 (48 male) 73 ± 9 years l-arginine (3 g/day) in the form of capsules | n = 67 (53 male) 72 ± 7 years Placebo in the form of capsules | Six months | (1) NOx/Griess reaction (2) ADMA/immunoassay (3) Blood flow/ultrasound (brachial artery for assessment the endothelial- dependent (reactive hyperemia) and independent (response to glyceryltrinitrate) flow-mediated vasodilation | Treatment with l-arginine did not increase nitric oxide synthesis or improve vascular reactivity. |
| Study | Risk of Bias | ||||||
|---|---|---|---|---|---|---|---|
| Random Sequence Generation | Allocation Concealment | Selective Reporting | Other Bias | Blinding of Participants, Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | |
| Schneider et al. [26] | Low | Low | Low | Low | Low | Low | Low |
| Bogdanski et al. [28] | Low | Low | Low | Low | Unclear | Low | Low |
| Monti et al. [17] | Low | Low | Low | Low | Unclear | Low | Low |
| Alizadeh et al. [23] | Low | Low | Low | Low | Low | Low | Low |
| Jahangir et al. [14] | Unclear | Low | Low | Low | Low | Low | Low |
| Lucotti et al. [15] | Low | Low | Low | Low | Low | Low | Low |
| Martina et al. [25] | Low | Low | Low | Low | Unclear | Low | Low |
| Wilson et al. [22] | Unclear | Unclear | Low | Low | Low | Low | Low |
| Maxwell et al. [16] | Unclear | Low | Low | Low | Unclear | Low | Low |
| Walker et al. [27] | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Blum et al. [13] | Unclear | Low | Low | Low | Unclear | Unclear | Low |
| Rector et al. [18] | Low | Low | Low | Low | Unclear | Unclear | Low |
| Adams et al. [12] | Unclear | Low | Low | Unclear | Low | Unclear | Low |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues-Krause, J.; Krause, M.; Rocha, I.M.G.d.; Umpierre, D.; Fayh, A.P.T. Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 15. https://doi.org/10.3390/nu11010015
Rodrigues-Krause J, Krause M, Rocha IMGd, Umpierre D, Fayh APT. Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis. Nutrients. 2019; 11(1):15. https://doi.org/10.3390/nu11010015
Chicago/Turabian StyleRodrigues-Krause, Josianne, Mauricio Krause, Ilanna Marques Gomes da Rocha, Daniel Umpierre, and Ana Paula Trussardi Fayh. 2019. "Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis" Nutrients 11, no. 1: 15. https://doi.org/10.3390/nu11010015
APA StyleRodrigues-Krause, J., Krause, M., Rocha, I. M. G. d., Umpierre, D., & Fayh, A. P. T. (2019). Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis. Nutrients, 11(1), 15. https://doi.org/10.3390/nu11010015






