Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective
Abstract
1. Introduction
2. Liver Disease: Pathophysiology and Epidemiology
3. Role of Oxidative Stress in Development of Liver Disease
4. Curcumin and Oxidative Stress
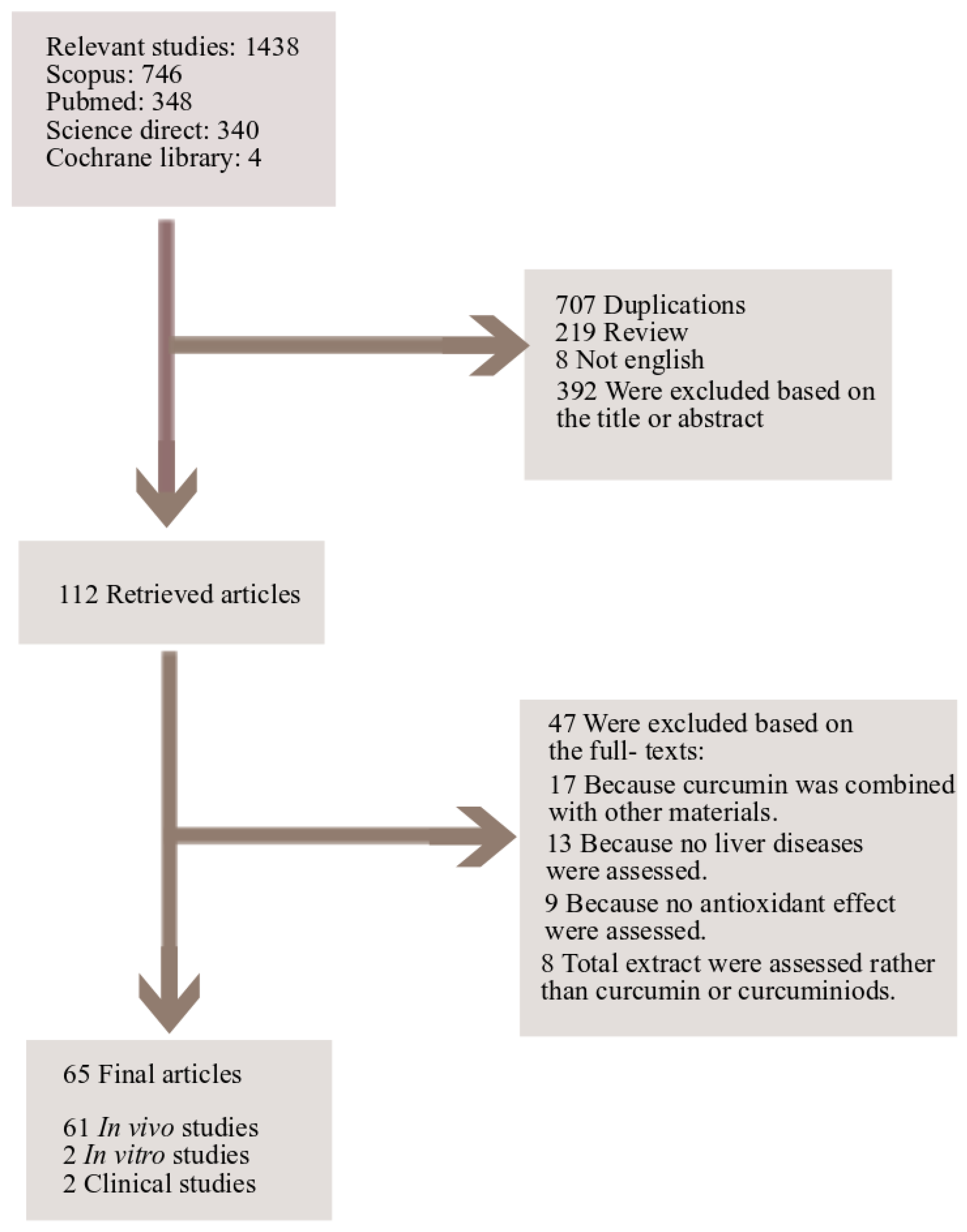
5. Study Design
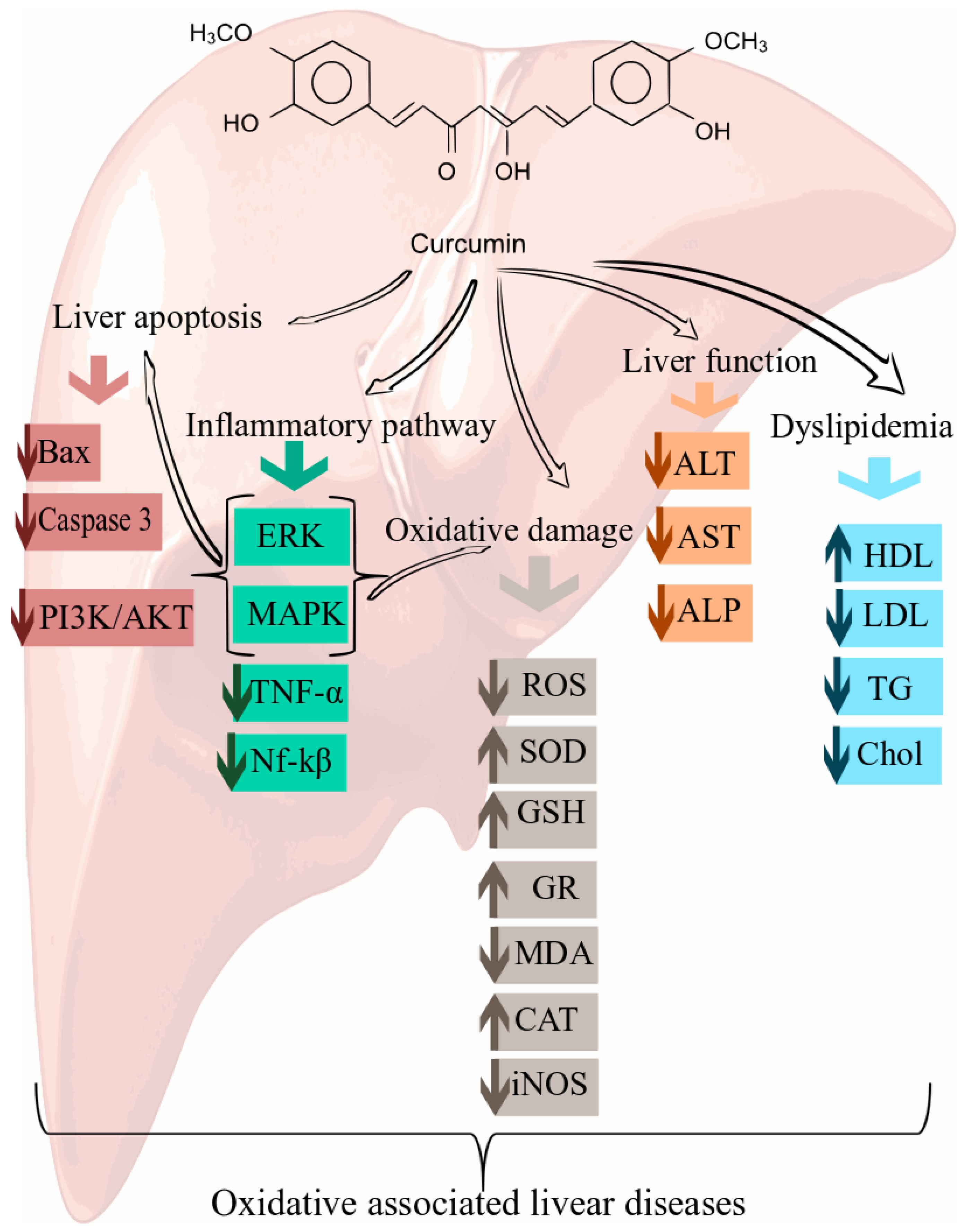
6. Cellular and Molecular Mechanisms of Curcumin in the Prevention of Oxidative Associated Liver Disease
6.1. Curcumin and Non-Alcoholic Steatohepatitis (NASH)
6.2. Curcumin and Alcoholic Liver Disease (ALD)
6.3. Curcumin for the Prevention of the Oxidative Stress in Liver
6.4. Curcumin and Liver Injury
6.5. Curcumin and Hepatotoxicity
6.6. Curcumin and Liver Fibrosis and Cirrhosis
6.7. Role of Epigenetic Pathway in Protective Effect of Curcumin against Oxidative Associated Liver Diseases
7. Nanoformulations of Curcumin in Oxidative Associated Liver Diseases
8. Clinical Studies Supporting the Efficacy of Curcumin in Oxidative Associated Liver Diseases
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muriel, P. The Liver: General Aspects and Epidemiology. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–22. [Google Scholar]
- Cichoż-Lach, H.; Michalak, A. Oxidative Stress as a Crucial Factor in Liver Diseases. World J. Gastroenterol. 2014, 20, 8082. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M. Natural products as a resource for new drugs. Pharm. Res. 1996, 13, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A. Strategies for discovering drugs from previously unexplored natural products. Drug Discov. Today 2000, 5, 294–300. [Google Scholar] [CrossRef]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, X. Recent advances in natural products from plants for treatment of liver diseases. Eur. J. Med. Chem. 2013, 63, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; So, K.F.; Liong, E.C.; Tipoe, G.L. Recent advances in the herbal treatment of non-alcoholic Fatty liver disease. J. Tradit. Complement. Med. 2013, 3, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Sun Kim, M.; Kung, S.; Grewal, T.; D Roufogalis, B. Methodologies for investigating natural medicines for the treatment of nonalcoholic fatty liver disease (NAFLD). Curr. Pharm. Biotechnol. 2012, 13, 278–291. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rahimi, R.; Farzaei, M.H. Pharmacokinetic interactions of curcuminoids with conventional drugs: A review. J. Ethnopharmacol. 2017, 209, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Srimal, R.; Dhawan, B. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Satoskar, R.; Shah, S.; Shenoy, S. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654. [Google Scholar] [PubMed]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985, 29, 197–202. [Google Scholar] [CrossRef]
- Sharma, O. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976, 25, 1811–1812. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Singh, A.K.; Thaloor, D.; Banaudha, K.K.; Patnaik, G.K.; Srimal, R.C.; Maheshwari, R.K. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998, 6, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Blachier, M.; Leleu, H.; Peck-Radosavljevic, M.; Valla, D.-C.; Roudot-Thoraval, F. The Burden of Liver Disease in Europe: A Review of Available Epidemiological Data. J. Hepatol. 2013, 58, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, V.; Nakamura, T.; Ueno, T.; Torimura, T.; Aguirre-García, J. Structure and Ultrastructure of the Normal and Diseased Liver. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–44. [Google Scholar]
- Ueno, T.; Sugawara, H.; Sujaku, K.; Hashimoto, O.; Tsuji, R.; Tamaki, S.; Torimura, T.; Inuzuka, S.; Sata, M.; Tanikawa, K. Therapeutic Effects of Restricted Diet and Exercise in Obese Patients with Fatty Liver. J. Hepatol. 1997, 27, 103–107. [Google Scholar] [CrossRef]
- Aita, K.; Jin, Y.; Irie, H.; Takahashi, I.; Kobori, K.; Nakasato, Y.; Kodama, H.; Yanagawa, Y.; Yoshikawa, T.; Shiga, J. Are There Histopathologic Characteristics Particular to Fulminant Hepatic Failure Caused by Human Herpesvirus-6 Infection? A Case Report and Discussion. Hum. Pathol. 2001, 32, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Ueno, T.; Morita, Y.; Nagata, E.; Sata, M. Usefulness of Serum Hepatic Fibrosis Markers in the Diagnosis of Nonalcoholic Steatohepatitis (NASH). Hepatogastroenterology 2006, 53, 678–681. [Google Scholar] [PubMed]
- Morita, Y.; Ueno, T.; Sasaki, N.; Kuhara, K.; Yoshioka, S.; Tateishi, Y.; Nagata, E.; Kage, M.; Sata, M. Comparison of Liver Histology Between Patients with Non-Alcoholic Steatohepatitis and Patients with Alcoholic Steatohepatitis in Japan. Alcohol. Clin. Exp. Res. 2005, 29. [Google Scholar] [CrossRef]
- Wanless, I.R.; Lentz, J.S. Fatty liver hepatitis (steatohepatitis) and obesity: An autopsy study with analysis of risk factors. Hepatology 1990, 12, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Saccoccio, G.; Masutti, F.; Crocè, L.S.; Brandi, G.; Sasso, F.; Cristanini, G.; Tiribelli, C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann. Intern. Med. 2000, 132, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Loria, P.; Leonardi, F.; Borsatti, A.; Neri, P.; Pulvirenti, M.; Verrone, A.M.; Bagni, A.; Bertolotti, M.; Ganazzi, D.; et al. Fasting insulin and uric acid levels but not indices of iron metabolism are independent predictors of non-alcoholic fatty liver disease. A case-control study. Dig. Liver Dis. 2002, 34, 204–211. [Google Scholar] [CrossRef]
- Omagari, K.; Kadokawa, Y.; Masuda, J.; Egawa, I.; Sawa, T.; Hazama, H.; Ohba, K.; Isomoto, H.; Mizuta, Y.; Hayashida, K.; et al. Fatty liver in non-alcoholic non-overweight Japanese adults: Incidence and clinical characteristics. J. Gastroenterol. Hepatol. 2002, 17, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Promrat, K.; Lutchman, G.; Uwaifo, G.I.; Freedman, R.J.; Soza, A.; Heller, T.; Doo, E.; Ghany, M.; Premkumar, A.; Park, Y. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004, 39, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Mofrad, P.S.; Contos, M.J.; Sargeant, C.; Luketic, V.A.; Sterling, R.K.; Stravitz, R.T.; Shiffman, M.L.; Clore, J.; Mills, A.S. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2004, 2, 1107–1115. [Google Scholar] [CrossRef]
- Nesto, R.W.; Bell, D.; Bonow, R.O.; Fonseca, V.; Grundy, S.M.; Horton, E.S.; le Winter, M.; Porte, D.; Semenkovich, C.F.; Smith, S. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association. Circulation 2003, 108, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. Treatment of non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2002, 17, S385–S388. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Schwank, J.; Staib, F.; Wang, X.W.; Harris, C.C. TP53 Mutations and Hepatocellular Carcinoma: Insights into the Etiology and Pathogenesis of Liver Cancer. Oncogene 2007, 26, 2166. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M.; Cunningham, C.C. Contribution of Mitochondria to Oxidative Stress Associated with Alcoholic Liver disease1. Free Radic. Biol. Med. 2002, 32, 11–16. [Google Scholar] [CrossRef]
- Palma, H.E.; Wolkmer, P.; Gallio, M.; Corrêa, M.M.B.; Schmatz, R.; Thomé, G.R.; Pereira, L.B.; Castro, V.S.P.; Pereira, A.B.; Bueno, A. Oxidative Stress Parameters in Blood, Liver, and Kidney of Diabetic Rats Treated with Curcumin And/or Insulin. Mol. Cell. Biochem. 2014, 386, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M.; Cunningham, C.C. Acute and Chronic Ethanol Increases Reactive Oxygen Species Generation and Decreases Viability in Fresh, Isolated Rat Hepatocytes. Hepatology 1998, 28, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, X.; Su, Y.; Paueksakon, P.; Hu, W.; Zhang, M.-Z.; Harris, R.C.; Blackwell, T.S.; Zent, R.; Pozzi, A. p47phox Contributes to Albuminuria and Kidney Fibrosis in Mice. Kidney Int. 2015, 87, 948–962. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A. Oxidative Stress Signaling Underlying Liver Disease and Hepatoprotective Mechanisms. World J. Hepatol. 2009, 1, 72. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, A.B.; Gül, M.; Karabulut, E.; Kiran, T.R.; Ocak, S.G.; Otlu, O. Oxidant and Antioxidant Activity in Rabbit Livers Treated with Zoledronic Acid. Transpl. Proc. 2010, 42, 3820–3822. [Google Scholar]
- Ozgur, E.; Güler, G.; Seyhan, N. Mobile Phone Radiation-Induced Free Radical Damage in the Liver Is Inhibited by the Antioxidants N-Acetyl Cysteine and Epigallocatechin-Gallate. Int. J. Radiat. Biol. 2010, 86, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Bando, I.; Reus, M.I.S.; Andrés, D.; Cascales, M. Endogenous Antioxidant Defence System in Rat Liver Following Mercury Chloride Oral Intoxication. J. Biochem. Mol. Toxicol. 2005, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.S.; Kumar, J.K.; Luqman, S.; Shanker, K.; Gupta, M.M.; Khanuja, S.P.S. Recent Advances in Plant Hepatoprotectives: A Chemical and Biological Profile of Some Important Leads. Med. Res. Rev. 2008, 28, 746–772. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.A.C.; Leon, L.L. Biological Activities of Curcuma Longa L. Mem. Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Zhongfa, L.; Chiu, M.; Wang, J.; Chen, W.; Yen, W.; Fan-Havard, P.; Yee, L.D.; Chan, K.K. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother. Pharmacol. 2012, 69, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and Radical Scavenging Properties of Curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Martín-Aragón, S.; Benedí, J.M.; Villar, A.M. Modifications on Antioxidant Capacity and Lipid Peroxidation in Mice under Fraxetin Treatment. J. Pharm. Pharmacol. 1997, 49, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.N.A. Nitric Oxide Scavenging by Curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. In The molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin, Germany, 2007; pp. 105–125. [Google Scholar]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin Activates the Haem Oxygenase-1 Gene via Regulation of Nrf2 and the Antioxidant-Responsive Element. Biochem. J. 2003, 371 Pt 3, 887. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.S.; Mishra, S.; Jha, S.K.; Surolia, A. Antioxidant Activity and Electrochemical Elucidation of the Enigmatic Redox Behavior of Curcumin and Its Structurally Modified Analogues. Electrochim. Acta 2015, 151, 574–583. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and Anti-Inflammatory Potential of Curcumin Accelerated the Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S. Predicting the Antioxidant Activity of Curcumin and Curcuminoids. J. Mol. Struct. THEOCHEM 2002, 591, 207–217. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the Antioxidant Mechanism of Curcumin: Classical Methods Are Needed to Determine Antioxidant Mechanism and Activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Andrea, M.; Cinzia, C.; Sergio, L.; van Beek Teris, A.; Luca, G.; Francesco, R.S.; Jules, B. Production of Novel Antioxidative Phenolic Amides through Heterologous Expression of the Plant’s Chlorogenic Acid Biosynthesis Genes in Yeast. Metab. Eng. 2010, 12, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of Phenolic OH and Methylene Hydrogen on the Free Radical Reactions and Antioxidant Activity of Curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, H.-Y.; Ji, H.-F. Successful Application of TD-DFT in Transient Absorption Spectra Assignment. Org. Lett. 2005, 7, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The Story so Far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Hismiogullari, S.E.; Hismiogullari, A.A.; Sunay, F.B.; Paksoy, S.; Can, M.; Aksit, H.; Karaca, O.; Yavuz, O. The protective effect of curcumin on carbon tetrachloride induced liver damage. Revue Méd. Vét. 2014, 165, 194–200. [Google Scholar]
- Vizzutti, F.; Provenzano, A.; Galastri, S.; Milani, S.; Delogu, W.; Novo, E.; Caligiuri, A.; Zamara, E.; Arena, U.; Laffi, G.; et al. Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab. Investig. 2010, 90, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, L.; Lu, Q.; Da, W. Liver injury attenuation by curcumin in a rat NASH model: An Nrf2 activation-mediated effect? Ir. J. Med. Sci. 2016, 185, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Afrin, R.; Arumugam, S.; Rahman, A.; Wahed, M.I.; Karuppagounder, V.; Harima, M.; Suzuki, H.; Miyashita, S.; Suzuki, K.; Yoneyama, H.; et al. Curcumin ameliorates liver damage and progression of NASH in NASH-HCC mouse model possibly by modulating HMGB1-NF-kappaB translocation. Int. Immunopharmacol. 2017, 44, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Dadabhai, A.S.; Jang, Y.Y.; Gurakar, A.; Mezey, E. Current Management of Alcoholic Hepatitis and Future Therapies. J. Clin. Transl. Hepatol. 2016, 4, 113–122. [Google Scholar] [PubMed]
- Samuhasaneeto, S.; Thong-Ngam, D.; Kulaputana, O.; Suyasunanont, D.; Klaikeaw, N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J. Biomed. Biotechnol. 2009, 2009, 981963. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; McGregor, R.A.; Choi, M.S.; Seo, K.I.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Low doses of curcumin protect alcohol-induced liver damage by modulation of the alcohol metabolic pathway, CYP2E1 and AMPK. Life Sci. 2013, 93, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Li, K.; Rong, S.; Yao, P.; Hao, L.; Ying, C.; Zhang, X.; Nussler, A.; Liu, L. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J. Ethnopharmacol. 2010, 128, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Zhao, Y.; Bao, W.; Xiao, X.; Wang, D.; Nussler, A.K.; Yan, H.; Yao, P.; Liu, L. Curcumin prevents chronic alcohol-induced liver disease involving decreasing ROS generation and enhancing antioxidative capacity. Phytomedicine 2012, 19, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.E.; Dong, W.G.; Wang, B.Y.; Tong, Q.Y.; Li, Z.Y. Curcumin attenuates chronic ethanol-induced liver injury by inhibition of oxidative stress via mitogen-activated protein kinase/nuclear factor E2-related factor 2 pathway in mice. Pharmacogn. Mag. 2015, 11, 707–715. [Google Scholar] [PubMed]
- Varatharajalu, R.; Garige, M.; Leckey, L.C.; Reyes-Gordillo, K.; Shah, R.; Lakshman, M.R. Protective role of dietary curcumin in the prevention of the oxidative stress induced by chronic alcohol with respect to hepatic injury and antiatherogenic markers. Oxid. Med. Cell Longev. 2016, 2016, 5017460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Song, C.H.; Chai, O.H. Negative effects of curcumin on liver injury induced by alcohol. Phytother. Res. 2012, 26, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaei, Z.M.; Mohammad, T.U.; Ali, L.K. Effects of local curcumin on oxidative stress and total antioxidant capacity in vivo study. Pak. J. Biol. Sci. 2014, 17, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Faten, R.A.; Ibrahim, A.E.; Khaled, A.E. Protective and modulatory effects of Curcumin and L-Carnitine against Methotrexate-induced Oxidative stress in albino rats. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 744–754. [Google Scholar]
- Hemeida, R.A.; Mohafez, O.M. Curcumin attenuates methotraxate-induced hepatic oxidative damage in rats. J. Egypt. Natl. Cancer Inst. 2008, 20, 141–148. [Google Scholar]
- AL-Harbi, S.M.; Hamza, Z.R.; Dwary, A.A. Ameliorative effect of selenium and curcumin on sodium fluoride induced hepatotoxicity and oxidative stress in male mice. J. Chem. Pharm. Res. 2014, 6, 984–998. [Google Scholar]
- Alp, H.; Aytekin, I.; Hatipoglu, N.K.; Alp, A.; Ogun, M. Effects of sulforophane and curcumin on oxidative stress created by acute malathion toxicity in rats. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 144–148. [Google Scholar] [PubMed]
- Hussein, A.S.; El-Said Azab, M.; El-Shall, K.S. Protective effect of curcumin on antioxidant defense system and oxidative stress in liver tissue of iron overloading rats. Asian J. Clin. Nutr. 2014, 6, 1–17. [Google Scholar] [CrossRef]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative study of natural antioxidants–curcumin, resveratrol and melatonin-in cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.; Ozdemir, I.; Tanyildizi, S.; Yildiz, S.; Oguzturk, H. Antioxidative effects of curcumin, β-myrcene and 1, 8-cineole against 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol. Ind. Health 2011, 27, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Coneac, A.; Meda, S.O.; Leucuta, D.C.; Decea, N.; Filip, M.; Mihu, C.M.; Muresan, A.; Remus, I.O.; Moldovan, M. Effect of Curcumin on Oxidative Stress in a Model of Turpentine Induced Acute Experimental Inflammation. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45. [Google Scholar] [CrossRef]
- Dai, C.; Tang, S.; Li, D.; Zhao, K.; Xiao, X. Curcumin attenuates quinocetone-induced oxidative stress and genotoxicity in human hepatocyte L02 cells. Toxicol. Mech. Methods 2015, 25, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dou, W.; Zheng, Y.; Wen, Q.; Qin, M.; Wang, X.; Tang, H.; Zhang, R.; Lv, D.; Wang, J.; et al. Curcumin upregulates Nrf2 nuclear translocation and protects rat hepatic stellate cells against oxidative stress. Mol. Med. Rep. 2016, 13, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzová, D.; Bludovská, M. The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol. Lett. 2004, 151, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zheng, S.; Lin, J.; Ryerse, J.; Chen, A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol. Pharmacol. 2008, 73, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gordillo, K.; Segovia, J.; Shibayama, M.; Vergara, P.; Moreno, M.G.; Muriel, P. Curcumin protects against acute liver damage in the rat by inhibiting NF-κB, proinflammatory cytokines production and oxidative stress. Biochim. Biophys. Acta 2007, 1770, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sharma, P. Hepatoprotective effect of curcumin on lindane-induced oxidative stress in male wistar rats. Toxicol. Int. 2011, 18, 124. [Google Scholar] [PubMed]
- Sankar, P.; Telang, A.G.; Manimaran, A. Protective effect of curcumin on cypermethrin-induced oxidative stress in Wistar rats. Exp. Toxicol. Pathol. 2012, 64, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Fukui, T. Suppressive effect of curcumin on trichloroethylene-induced oxidative stress. J. Nutr. Sci. Vitaminol. 2000, 46, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Cerný, D.; Lekić, N.; Váňová, K.; Muchová, L.; Hořínek, A.; Kmoníčková, E.; Zídek, Z.; Kameníková, L.; Farghali, H. Hepatoprotective effect of curcumin in lipopolysaccharide/-galactosamine model of liver injury in rats: Relationship to HO-1/CO antioxidant system. Fitoterapia 2011, 82, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xue, C.G.; Chen, T.H.; Wang, J.L.; Sun, C.S. Chemopreventive effect of curcuma and curcumin on liver injury induced by microcystins in mice. Chin. Pharmacol. Bull. 2005, 21, 1517–1519. [Google Scholar]
- Tokaç, M.; Taner, G.; Aydın, S.; Ozkardeş, A.B.; Dündar, H.Z.; Taşlıpınar, M.Y.; Arıkök, A.T.; Kılıç, M.; Başaran, A.A.; Basaran, N. Protective effects of curcumin against oxidative stress parameters and DNA damage in the livers and kidneys of rats with biliary obstruction. Food Chem. Toxicol. 2013, 61, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ghoreshi, Z.A.; Kabirifar, R.; Safari, F.; Karimollah, A.; Moradi, A.; Eskandari-Nasab, E. Hepatoprotective effects of curcumin in rats after bile duct ligation via downregulation of Rac1 and NOX1. Nutrition 2017, 36, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Lee, J.F.; Chiang, L.L.; Chen, C.F.; Wang, D.; Su, C.L. The protective effect of curcumin on ischemia-reperfusion-induced liver injury. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2012; Volume 44, pp. 974–977. [Google Scholar]
- Jayakumara, T.; Sakthivel, M.; Thomasb, P.A.; Geraldinea, P. Pleurotus ostreatus, an oyster mushroom, decreases the oxidative stress induced by carbon tetrachloride in rat kidneys, heart and brain. Chem.-Biol. Interact. 2008, 176, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Fadda, L.M.; Al-Rasheed, N.M.; Hasan, I.H.; Ali, H.M.; Al-Rasheed, N.M.; Al-Fayez, M.; Ahmed, A.M.; Almutlaq, N.; Qasem, N.; Reem Khalaf, R. Bax and CD68 expression in response to liver injury induced by acetaminophen: The hepatoprotective role of thymoquinone and curcumin. Pak. J. Zool. 2017, 49, 85–93. [Google Scholar] [CrossRef]
- Galaly, S.R.; Ahmed, O.M.; Mahmoud, A.M. Thymoquinone and curcumin prevent gentamicin-induced liver injury by attenuating oxidative stress, inflammation and apoptosis. J. Physiol. Pharmacol. 2014, 65, 823–832. [Google Scholar] [PubMed]
- El-Agamy, D.S. Comparative effects of curcumin and resveratrol on aflatoxin B 1-induced liver injury in rats. Arch. Toxicol. 2010, 84, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Madkour, N.K. Protective effect of curcumin on oxidative stress and DNA fragmentation against lambda cyhalothrin-induced liver damage in rats. J. Appl. Pharm. Sci. 2012, 2, 76–81. [Google Scholar]
- Lee, H.Y.; Kim, S.W.; Lee, G.H.; Choi, M.K.; Jung, H.W.; Kim, Y.J.; Kwon, H.J.; Chae, H.J. Turmeric extract and its active compound, curcumin, protect against chronic CCl4-induced liver damage by enhancing antioxidation. BMC Complement. Altern. Med. 2016, 16, 316. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Jeon, C.H.; Ko, G.; Kim, J.; Sohn, D.H. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J. Pharm. Pharmacol. 2000, 52, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, Z.; Li, H.; Guo, M.; Yang, T.; Feng, S.; Xu, B.; Deng, Y. Protective effects of curcumin against mercury-induced hepatic injuries in rats, involvement of oxidative stress antagonism, and Nrf2-ARE pathway activation. Hum. Exp. Toxicol. 2017, 36, 949–966. [Google Scholar] [CrossRef] [PubMed]
- García-Niño, W.R.; Pedraza-Chaverrí, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ding, W.; Du, J.; Jia, R.; Liu, Y.; Zhao, C.; Shen, Y.; Yin, G. Effects of curcumin on antioxidative activities and cytokine production in Jian carp (Cyprinus carpio var. Jian) with CCl4-induced liver damage. Fish Shellfish Immunol. 2015, 43, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Khanlarkhani, N. Melatonin application in targeting oxidative-induced liver injuries: A review. J. Cell. Physiol. 2017, 233, 4015–4032. [Google Scholar] [CrossRef] [PubMed]
- Vera-Ramirez, L.; Pérez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and liver disease. Biofactors 2013, 39, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.M.; El-Kordy, E.A. The protective effect of curcumin on paracetamol-induced liver damage in adult male rabbits: Biochemical and histological studies. Egypt. J. Histol. 2014, 37, 629–639. [Google Scholar] [CrossRef]
- Dattani, J.J.; Rajput, D.K.; Moid, N.; Highland, H.N.; George, L.B.; Desai, K.R. Ameliorative effect of curcumin on hepatotoxicity induced by chloroquine phosphate. Environ. Toxicol. Pharmacol. 2010, 30, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Otuechere, C.A.; Abarikwu, S.O.; Olateju, V.I.; Animashaun, A.L.; Kale, O.E. Protective effect of curcumin against the liver toxicity caused by propanil in rats. Int. Sch. Res. Notices 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Fazal, Y.; Fatima, S.N.; Shahid, S.M.; Mahboob, T. Effects of curcumin on angiotensin-converting enzyme gene expression, oxidative stress and anti-oxidant status in thioacetamide-induced hepatotoxicity. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Ashkenazi, M.; Weizman, N.; Shahmurov, M.; Aeed, H.; Bruck, R. Curcumin ameliorates acute thioacetamide-induced hepatotoxicity. J. Gastroenterol. Hepatol. 2006, 21, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Tirkey, N.; Bharrhan, S.; Chanana, V.; Rishi, P.; Chopra, K. Inhibition of oxidative stress and cytokine activity by curcumin in amelioration of endotoxin-induced experimental hepatoxicity in rodents. Am. J. Clin. Exp. Immunol. 2006, 145, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, L.; Mansouri, E.; Orazizadeh, M.; Jozi, Z. Curcumin Attenuates Hepatotoxicity Induced by Zinc Oxide Nanoparticles in Rats. Balk. Med. J. 2016, 33, 252. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.H.; Nabavi, S.F.; Nabavi, S.M.; Loizzo, M.R.; Roohbakhsh, A.; Setzer, W.N. Ameliorative effects of curcumin against sodium fluoride-induced hepatotoxicity. Prog. Nutr. 2015, 17, 324–330. [Google Scholar]
- El-Desoky, G.E.; Abdel-Ghaffar, A.; Al-Othman, Z.A.; Habila, M.A.; Al-Sheikh, Y.A.; Ghneim, H.K.; Giesy, J.P.; Aboul-Soud, M.A. Curcumin protects against tartrazine-mediated oxidative stress and hepatotoxicity in male rats. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 635–645. [Google Scholar] [PubMed]
- García-Niño, W.R.; Tapia, E.; Zazueta, C.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Vega-García, C.C.; Pedraza-Chaverrí, J. Curcumin pretreatment prevents potassium dichromate-induced hepatotoxicity, oxidative stress, decreased respiratory complex I activity, and membrane permeability transition pore opening. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- García-Niño, W.R.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Vega-García, C.C.; Tapia, E.; Pedraza-Chaverri, J. Oxidative stress markers and histological analysis in diverse organs from rats treated with a hepatotoxic dose of Cr (VI): Effect of curcumin. Biol. Trace Elem. Res. 2015, 167, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Bruck, R.; Ashkenazi, M.; Weiss, S.; Goldiner, I.; Shapiro, H.; Aeed, H.; Genina, O.; Helpern, Z.; Pines, M. Prevention of liver cirrhosis in rats by curcumin. Liver Int. 2007, 27, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gordillo, K.; Segovia, J.; Shibayama, M.; Tsutsumi, V.; Vergara, P.; Moreno, M.G.; Muriel, P. Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: Role of TGF-β modulation and oxidative stress. Fundam. Clin. Pharmacol. 2008, 22, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Chenari, S.; Safari, F.; Moradi, A. Curcumin enhances liver SIRT3 expression in the rat model of cirrhosis. Iran. J. Basic Med. Sci. 2017, 20, 1306. [Google Scholar] [PubMed]
- Akila, G.; Rajakrishnan, V.; Viswanathan, P.; Rajashekaran, K.N.; Menon, V.P. Effects of curcumin on lipid profile and lipid peroxidation status in experimental hepatic fibrosis. Hepatol. Res. 1998, 11, 147–157. [Google Scholar] [CrossRef]
- Erenoğlu, C.; Kanter, M.; Aksu, B.; Sağıroğlu, T.; Ayvaz, S.; Aktaş, C.; Erboğa, M. Protective effect of curcumin on liver damage induced by biliary obstruction in rats. Balk. Med. J. 2011, 28, 352–357. [Google Scholar] [CrossRef]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Friso, S.; Choi, S.W. Epigenetic mechanisms underlying the link between non-alcoholic fatty liver diseases and nutrition. Nutrients 2014, 6, 3303–3325. [Google Scholar] [CrossRef] [PubMed]
- Podrini, C.; Borghesan, M.; Greco, A.; Pazienza, V.; Mazzoccoli, G.; Vinciguerra, M. Redox homeostasis and epigenetics in non-alcoholic fatty liver disease (NAFLD). Curr. Pharm. Des. 2013, 19, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397. [Google Scholar]
- de Mello, V.D.; Matte, A.; Perfilyev, A.; Männistö, V.; Rönn, T.; Nilsson, E.; Käkelä, P.; Ling, C.; Pihlajamäki, J. Human liver epigenetic alterations in non-alcoholic steatohepatitis are related to insulin action. Epigenetics 2017, 12, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.S.; Heidor, R.; Pogribny, I.P. Nutritional epigenetics and the prevention of hepatocellular carcinoma with bioactive food constituents. Nutr. Cancer 2016, 68, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Momtazi, A.A.; Derosa, G.; Maffioli, P.; Banach, M.; Sahebkar, A. Role of microRNAs in the therapeutic effects of curcumin in non-cancer diseases. Mol. Diagn. Ther. 2016, 20, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, C.; Lin, Z.; Guo, Y.; Shi, L.; Dong, P.; Lu, Z.; Gao, S.; Liao, Y.; Chen, B.; Yu, F. Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation—A novel mechanism suppressing liver fibrosis. FEBS J. 2014, 281, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Hu, Y.; Wang, J.; Yuan, C. Curcumin inhibits growth of human breast cancer cells through demethylation of DLC1 promoter. Mol. Cell. Biochem. 2017, 425, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Tang, D.; Du, Y.L.; Cao, C.Y.; Nie, Y.Q.; Cao, J.; Zhou, Y.J. Fatty liver mediated by PPAR-α DNA methylation can be reversed by a methylation inhibitor and curcumin. J. Dig. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1, 7-bis-(4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, V.; Chandrasekhara, N. Metabolism of curcumn-studies with [3H] curcumin. Toxicology 1981, 22, 337–344. [Google Scholar] [CrossRef]
- Pan, M.-H.; Huang, -T.; Lin, -J. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Algandaby, M.M.; Al-Sawahli, M.M.; Ahmed, O.A.; Fahmy, U.A.; Abdallah, H.M.; Hattori, M.; Ashour, O.M.; Abdel-Naim, A.B. Curcumin-Zein Nanospheres Improve Liver Targeting and Antifibrotic Activity of Curcumin in Carbon Tetrachloride-Induced Mice Liver Fibrosis. J. Biomed. Nanotechnol. 2016, 12, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Q.; Wang, H.; Zhang, L.; Wang, -J. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials 2005, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Guo, H. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Taweel, G.M.A.; Hidayathulla, S. Nano-composites chitosan-curcumin synergistically inhibits the oxidative stress induced by toxic metal cadmium. Int. J. Biol. Macromol. 2018, 108, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Khullar, N.; Kakkar, V.; Kaur, I.P. Attenuation of carbon tetrachloride-induced hepatic injury with curcumin-loaded solid lipid nanoparticles. BioDrugs 2014, 28, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Hwang, K.H.; Ahn, J.; Ha, T.Y. Curcumin Attenuates Diet-Induced Hepatic Steatosis by Activating AMP-Activated Protein Kinase. Basic Clin. Pharmacol. Toxicol. 2013, 113, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Zhuge, L.; Su, D.; Yang, M.; Tao, S.; Li, J. Comparison between the efficacies of curcumin and puerarin in C57BL/6 mice with steatohepatitis induced by a methionine-and choline-deficient diet. Exp. Ther. Med. 2014, 7, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.-K.; Luo, J.-L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2011, 61, 1058–1064. [Google Scholar]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, J.; Mayorga, M.; Crespo, J. Nonalcoholic Fatty Liver Disease: A Pathological View. In Liver Biopsy—Indications, Procedures, Results; InTech: Rijeka, Croatia, 2012; Chapter 8. [Google Scholar]
- Huang, Y.; Cao, S.; Zhang, Q.; Zhang, H.; Fan, Y.; Qiu, F.; Kang, N. Biological and pharmacological effects of hexahydrocurcumin, a metabolite of curcumin. Arch. Biochem. Biophys. 2018, 646, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, X.; Zhang, L.; Wang, L.; Peng, Z.; Chen, Y. The pharmacokinetics and tissue distribution of curcumin and its metabolites in mice. Biomed. Chromatogr. 2018, e4267. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Vitek, L.; Schwertner, H.A. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv. Clin. Chem. 2007, 43, 1–57. [Google Scholar] [PubMed]
- Zheng, S.; Anping, C.H. Activation of PPARγ is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem. J. 2004, 384, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, A. Curcumin suppresses the expression of extracellular matrix genes in activated hepatic stellate cells by inhibiting gene expression of connective tissue growth factor. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G883–G893. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yumei, F.; Chen, A. De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic. Biol. Med. 2007, 43, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Gukovsky, I.; Reyes, C.N.; Vaquero, E.C.; Gukovskaya, A.S.; Pandol, S.J. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G85–G95. [Google Scholar] [CrossRef] [PubMed]
| Liver Disease Type | Experimental Model Used (Animal, Strain, Genetic or Dietary Liver Injury) | Curcumin Source | Dose and Formulation (Injection) | Duration of Treatment | Reference |
|---|---|---|---|---|---|
| Non-Alcoholic Steatohepatitis (NASH) | |||||
| Ccl4 0.5 mL/kg/every other day/SC/3 weeks | Male Albino Wistar rat | Sigma, St. Louis, MO, USA | 200 mg/kg/day in olive oil (oral) | 3 weeks | [58] |
| Methionine and choline deficient diet (MCD diet) | Male C57BL/6 mice | Sigma, St. Louis, MO, USA | 25 µg/every other day/in DMSO (IP) | 4, 8 or 10 days | [59] |
| High fat diet (HFD) | Adult Sprague-Dawley rats | Sigma, St. Louis, MO, USA | 50 mg/kg/day/suspended in 0.5% CMC | 6 weeks | [60] |
| 200 μg STZ/single dose/SC/2 days after birth (HFD 32) | C57BL/6 J mice | Sigma-Aldrich, Tokyo, Japan | 100 mg/kg/day in 1% gum Arabic (oral) | 14 weeks | [61] |
| Alcoholic Liver Disease | |||||
| 50% ethanol (7.5 g/kg/day/4 weeks/oral) | Female Sprague-Dawley rats | Cayman Chemical Company, USA | 400 or 600 mg/kg/twice a day/in 50% ethanol | 4 weeks | [63] |
| 25% ethanol (5 g/kg/day/6 weeks/oral) | Male ICR mice | Sigma, St. Louis, MO, USA | 19.7 or 47.5 mg/kg/day (oral) | 6 weeks | [64] |
| 100 mm ethanol for 8 h | Primary rat hepatocytes from male Sprague–Dawley rats | diferuloylmethane; CAS No. 458-37-7, Sigma, St. Louis, MO, USA | 5–50 M/dissolved in 0.5 N NaOH then diluted in PBS | 0–5 h before ethanol treatment | [65] |
| 2.4 g/kg/day ethanol for the initial 4 weeks and 4 g/kg/day for another 2 weeks | Male Balb/c mice | Diferuloylmethane; CAS No. 458-37-7), Sigma, St. Louis, MO, USA | 75 mg/kg/day/in DMSO (oral) | 6 weeks along with ethanol intake | [66] |
| 2.4 g/kg/day ethanol/daily/6 weeks | Adult Male Balb/C mice | Purity >98.0%, from the National Institute for Food and Drug Control (Beijing, China) | 75 or 150 mg/kg/in olive oil/(oral) | 1 h before ethanol administration for 6 | [67] |
| Low ω-3 poly unsaturated fatty acids (PUFA) diet + ethanol High 𝜔 − 3 PUFA + ethanol | Female Wistar-Furth rats | - | 150 mg/kg/day | 8 weeks | [68] |
| 5% ethanol/IV, 5 times a week/100 µL/mouse/for 2 weeks | Male C57BL/6 mice | Sigma-Aldrich, USA | 1 mM or 1 mM/100 µL/mouse (IV) dissolved in 0.5 N NaOH then diluted in PBS | 5 times a week for 2 weeks | [69] |
| Oxidative Stress Inducers | |||||
| H2O2 0.5% (v/v)/day/for 60 days | Male Wistar rats | Crude curcumin was purchased from local wet market in Baghdad, Iraq | 200 mg/kg/day | 1st model = 30 days after induction of oxidative stress 2nd model = 15 days then followed by receiving H2O2 for 60 days | [70] |
| Methotrexate 20 mg/kg/I.P./single dose | Rat | 100 mg/kg/day (I.P.) | 5 days | [72] | |
| Melathion (MAL) 200 mg/kg/oral | Female Sprague Dawley rats | Curcuma longa Turmeric, Sigma, St. Louis, MO, USA (C1386) | 1 g/kg (oral) | 1 days | [76] |
| Iron overload (Haemojet®) containing ferric hydroxide polymaltose 100 mg/Kg/I.P./3 doses per week/for 2 weeks | Male albino rats | Purity~95%, Indian production, purchased from El-Goumhoria Co., Cairo-Egypt | 100 mg/kg/day/dissolved in DMSO | 3, 4 or 5 weeks | [75] |
| 2,3,7,8-tetrachlorodibenzo-p-dioxin 2 mg/kg/week/oral diluted in corn oil | Female Sprague Dawley rats | Sigma Chemical Co., St. Louis, Missouri, USA | 100 mg/kg/day/dissolved in corn oil | 30 and 60 days | [77] |
| Turpentine oil 0.6 mL/kg/I.M. | Wistar Bratislava albino rats | Purity >98%, Abcam (Cambridge, United Kingdom) | 150 mg/kg/dissolved in 0.5% CMC (oral) | 1st model = 60 min prior Turpentine injection 2nd model = 120 min after Turpentine injection | [78] |
| Cdcl2 0.025 mmol/kg to rats and 0.03 mmol/kg to mice/S.C. | Adult male Wistar rats and male CD mice | Sigma–Aldrich, St. Louis, MO, USA | 50 mg/kg/day/dispersed in 0.25% methylcellulose | 3 days | [81] |
| Immobilization-induced stress, rats kept in the restrainers for 1 h every day, for 21 consecutive days | Wistar albino rats | Sigma-Aldrich Chemical (St. Louis, USA) | 10 or 20 or 30 mg/kg/day/IP | 21 days | [82] |
| Ccl4 1 mL/kg (1:1) in olive oil/every other day for 8 weeks./I.P. | Male Sprague-Dawley rats | - | 200 or 400 mg/kg/suspended in PBS | 48 h | [83,84] |
| Lindane 1st model = 60 mg/kg/for 24 h/oral 2nd model = 30 mg/kg/for 14 d | Male Wistar rats | Sigma Aldrich (St. Louis, MO, USA) | 100 or 200 mg/kg/day/dissolved in DMSO (oral) | 1st model = pretreatment for 14 days 2nd model = posttreatment for 14 days | [85] |
| Cypermethrin 25 mg/kg/day/for 28 days | Adult male Wistar rats | Sigma Chemicals, USA and SRL Chemicals, India. | 100 mg/kg (oral) | 28 days | [86] |
| Trichloroethylene (TCE) 1.2 mmol/kg/diluted in corn oil/24 h | Male ddY mice | - | 10, 50 or 100 µM/dissolved in DMSO (I.P.) | 24 h | [87] |
| In Vitro Study | |||||
| Quinocetone (QCT)- | Human hepatocyte L02 cells | Purity 98%, Aladdin Reagent Co., Ltd. (Shanghai, China) | 2.5 or 5 mM/0.1% DMSO | 2 h pretreatment then incubated for 4 or 24 h with QCT | [79] |
| Glucose oxidase (GO) 100 mu/mL/2 h | Rat HSCs-6 | Sigma-Aldrich (St. Louis, MO, USA) | 0.15 µM | 3 h pretreatment then incubated with GO for 2 h | [80] |
| Liver Injury | |||||
| LPS (10 μg/kg/I.P.)/D-galactosamine (400 mg/kg/I.P.)/24 h | Male Wistar rats | Sigma-Aldrich (Prague, Czech Republic) | 100 mg/kg (I.P.) | Pretreatment for 1 h | [88] |
| Microcystins 38.11 μg/kg/3 h/I.P. | Male Swiss mice | 300 mg/kg (oral) | 7 days pretreatment | [89] | |
| Biliary duct ligation (BDL) | Male Wistar albino rats (Rattus novegiccus) | curcumin (97%, purity) from Sigma Chemicals | 50 mg/kg/day in corn oil (oral) | 14 days | [90] |
| Male Wistar rats | Curcumin (purity >80%) from Sigma Chemicals | 100 mg/kg/day in Carboxymethyl cellulose (CMC) (oral) | 28 days | [91] | |
| Ischemia/reperfusion (I/R) | Female Wistar Albino rat | Sigma Chemical Co., USA | 100 mg/kg (I.P.) | 30 min pretreatment before I/R | [92] |
| Acetaminophen (APAP) (750 mg/kg/single dose/oral | Male Albino Wistar rats | Armal company | 200 mg/kg in corn oil (oral) | 1st = 24 h before APA 2nd = 2 h after APAP 3rd = 12 h after APAP | [94] |
| Gentamicin (100 mg/kg/I.P.) | Male albino rats | Sigma Chemical Co. | 20 mg/kg/every other day in 1% CMC (oral) | 21 days | [95] |
| Xenobiotics | |||||
| Aflatoxin B1 (25 μg/kg) | Male Fischer rats | Sigma Chemical Company | 200 mg/kg | 90 days | [96] |
| Lambda cyhalothrin | Male albino rats (Rattus norvegicus) | Sigma-Aldrich Chemical Co | 200 mg/kg/day suspended in PBS (oral) | 4 weeks | [97] |
| Hg (0.6, 1.2, or 2.4 mg/kg in saline/IP/daily/3 days) | Male and female Adult Wistar rat | Curcumin (98%) was provided by Sigma, Saint Louis, Missouri, USA | 100 mg/kg/in DMSO (SC) | 2 h pretreatment before Hg | [100] |
| CCl4 | |||||
| (1:1 in olive oil) 1 mL/kg/every other day/IP/4 weeks | Male Sprague-Dawley rats | Curcuma longa L. (CLL, turmeric) | 200 mg/kg/day in PBS (oral) | 4 weeks | [98] |
| 1st model = 0.2 mL/kg/24 h 2nd model = 1 mL/kg (1:1 in corn oil)/2 times per week, oral/4 weeks | Male Sprague-Dawley rats | Curcuma longa L. (Zingiberaceae) | 50, 100 or 200 mg kg/day in corn oil (oral) orally for 4 consecutive days | 1st = 4 days before CCl4 treatment 2nd = during CCl4 treatment for 4 weeks | [99] |
| 30% CCl4 in olive oil (0.05 mL/10 g/IP | Fish Cyprinus carpio var. Jian (Jian carp) | Sigma-Aldrich Chemical Co | 0.1%, 0.5%, or 1.0% | 60 days before CCl4 treatment | [102] |
| Hepatotoxicity | |||||
| Propanil 20 mg/kg/3 times a week/in olive oil (oral) | Albino rat | Sigma Chemical, USA | 50 mg/kg/3 times a week/in olive oil | 28 days | [95] |
| Paracetamol 500 mg/kg/day for 15 days (oral) | Adult male rabbits | Sigma Chemical | 50 and 100 mg/kg/in corn oil (oral) | 15 days | [105] |
| Chloroquine phosphate (CQ) 100, 200 or 300/daily/45 d | Male Swiss Albino mice | - | 80 mg/kg/day (oral) | 45 days during CQ treatment | [107] |
| TAA 200 mg/kg/I.P. for 12 weeks | Male Wistar albino rats | - | 75 mg/kg (oral) | 12 weeks after discontinuation of TAA | [108] |
| TAA 300 mg/kg/2 days/I.P./dissolved in a solution of glycerol formal, chremaphore and H2O (5:2:2) | Male Wistar rats | Sigma Chemical | 200 or 400 mg/kg/day dissolved in glycerol formal, chremaphore and H2O (5:2:2) (oral) | 48 h before TAA administration then continued during the two days of TAA injection | [109] |
| LPS 1 mg/kg/I.P. | Male Wistar rats | Sigma Aldrich Chemicals Private Ltd., New Delhi, India | 5, 30 or 60 mg/kg/suspended in 0·5% CMC (oral) | 6 days before LPS injection and sacrificed after 6 h post LPS injection | [110] |
| Nzno 50 mg/kg/on 7th day of saline administration (oral) | Male Wistar rats | 200 mg/kg/day/in corn oil (oral) | 7 days prior NZnO and continued for 21 days | [111] | |
| Naf 600 ppm via drinking water/7 days | Male Wistar rats | Sigma-Aldrich Chemical, USA | 10 or 20 mg/kg/dissolved in 5% DMSO (I.P.) | 7 days then exposed for 7 days NaF | [112] |
| (Tz, azo dye) 7.5 mg/kg/diet/90 days | Male Wistar Albino rats | Local markets, Saudi Arabia | 1, 2 or 4 g/kg | 90 days | [113] |
| K2Cr2O7 15 mg/kg/I.P./single dose | Male Wistar rats | Sigma-Aldrich (St. Louis, MO, USA). | 400 mg/kg/suspended in 0.5% CMC (oral) | 10 days prior single dose of K2Cr2O7 for 24 h or 48 h | [114,115] |
| Fibrosis and Cirrhosis | |||||
| TAA 200 mg/kg/I.P./twice a week for 12 weeks | Male Wistar rats | - | 300 mg/kg/day/in solvent/2 mL per rat (intragingival) | 12 weeks along with TAA or 4 or 6 weeks after TAA discontinuation | [116] |
| Biliary duct ligation | Male Wistar Albino rats | Sigma-Aldrich, USA | 100 mg/kg/day (oral) | 1st dose = 3 days before BDL and terminated after 14 days | [120] |
| Male Wistar Albino rats | Sigma Chemicals Co Purity (HPLC) >80%, USA | 100 mg/kg/day/suspended in 5% CMC (oral) | 28 days after BDL surgery | [118] | |
| Male Wistar rats | Sigma Chemicals Co, USA | 100 mg/kg/day/suspended in 0.7% CMC (oral) | 28 days | [117] | |
| CCl4 0.4 g/kg/3 times per week/dissolved in mineral oil/for 3 months | Male Wistar rats | Sigma Chemicals Co, USA | 100 mg/kg/day (oral) | 2 months | [117] |
| LPS 5 mg/kg/I.P. | Male C57BL/6 mice | - | 20, 40 or 80 mg/kg/day (oral | 4 weeks | [121] |
| Dose | Study Design | No. of Patients | Duration of Treatment | Result | Reference |
|---|---|---|---|---|---|
| 1000 mg/day | Patients with NAFLD were randomly assigned to the curcumin (n = 44) or placebo group (n = 43) | 87 | 8 weeks | ↓ the body mass index, AST, ALT, SGOT, SGPT | [144] |
| 500 mg/day | Patients with NAFLD were randomly assigned to the curcumin (n = 40) or placebo group (n = 40) | 80 | 8 weeks | ↓ Total cholesterol, LDL-C, ALT, AST ↑ HDL-C | [143] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. https://doi.org/10.3390/nu10070855
Farzaei MH, Zobeiri M, Parvizi F, El-Senduny FF, Marmouzi I, Coy-Barrera E, Naseri R, Nabavi SM, Rahimi R, Abdollahi M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients. 2018; 10(7):855. https://doi.org/10.3390/nu10070855
Chicago/Turabian StyleFarzaei, Mohammad Hosein, Mahdi Zobeiri, Fatemeh Parvizi, Fardous F. El-Senduny, Ilias Marmouzi, Ericsson Coy-Barrera, Rozita Naseri, Seyed Mohammad Nabavi, Roja Rahimi, and Mohammad Abdollahi. 2018. "Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective" Nutrients 10, no. 7: 855. https://doi.org/10.3390/nu10070855
APA StyleFarzaei, M. H., Zobeiri, M., Parvizi, F., El-Senduny, F. F., Marmouzi, I., Coy-Barrera, E., Naseri, R., Nabavi, S. M., Rahimi, R., & Abdollahi, M. (2018). Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients, 10(7), 855. https://doi.org/10.3390/nu10070855








