Chitosan Nanoparticles as a Promising Nanomaterial for Encapsulation of Pomegranate (Punica granatum L.) Peel Extract as a Natural Source of Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Pomegranate Peel Extract (PPE)
2.3. Preparation of Chitosan Nanoparticles (CSNPs) Loaded with PPE
2.4. Characterization of the CNPs
2.4.1. Total Phenolic Content (TPC)
2.4.2. Mean Particle Size, Zeta Potential, and Polydispersity Index
2.4.3. Scanning Electron Microscopy Observations
2.4.4. Encapsulation Efficiency and Loading Capacity
2.4.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4.6. Antioxidant Activity
2.4.7. Antibacterial Activity
2.4.8. Gas Chromatography (GC)–Mass Spectrometer Analysis
2.5. Statistical Analysis
3. Results and Discussion
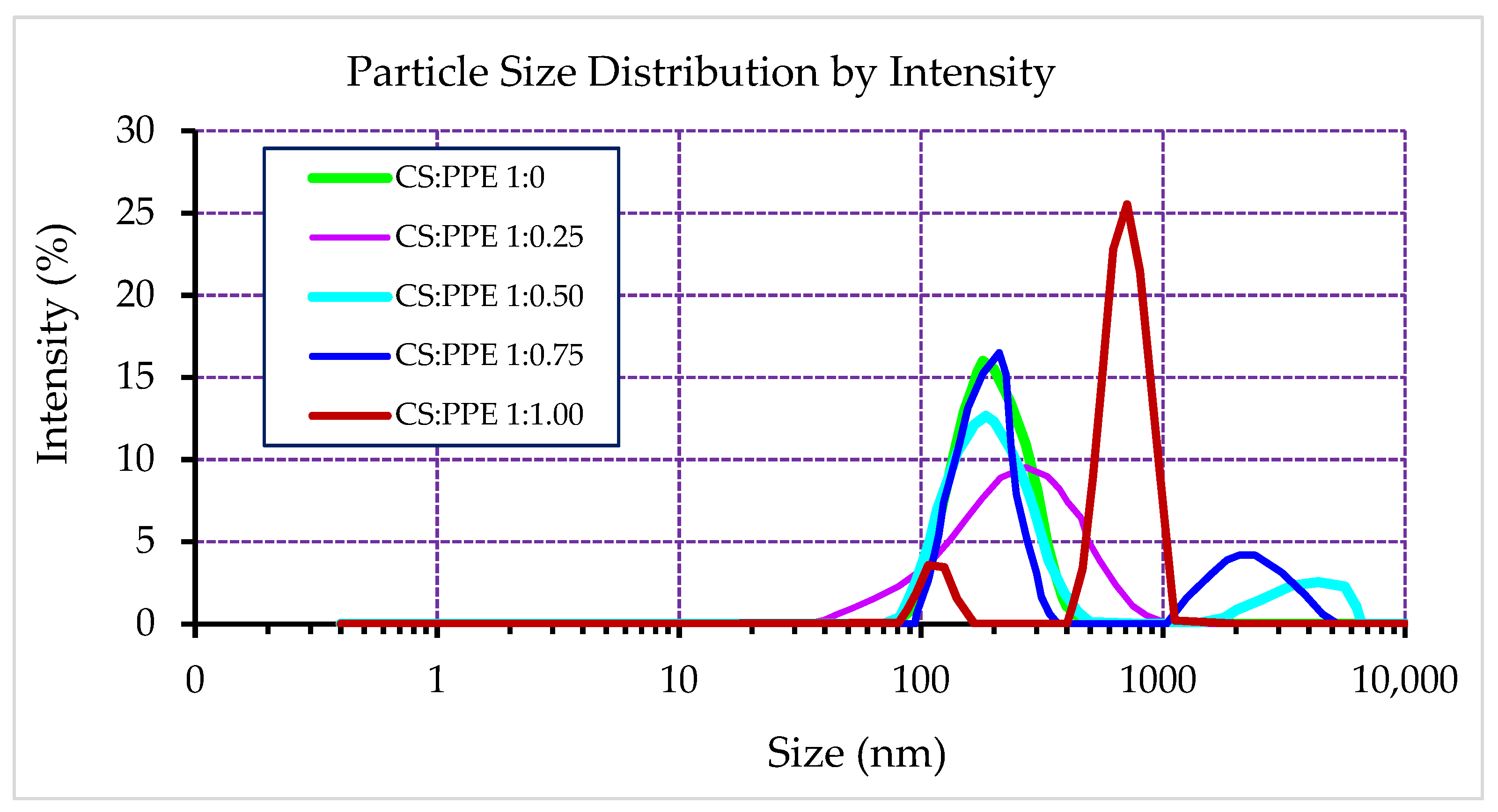
3.1. Particle Size Distribution, Average Diameter, Zeta Potential, and Poly Disparity Index
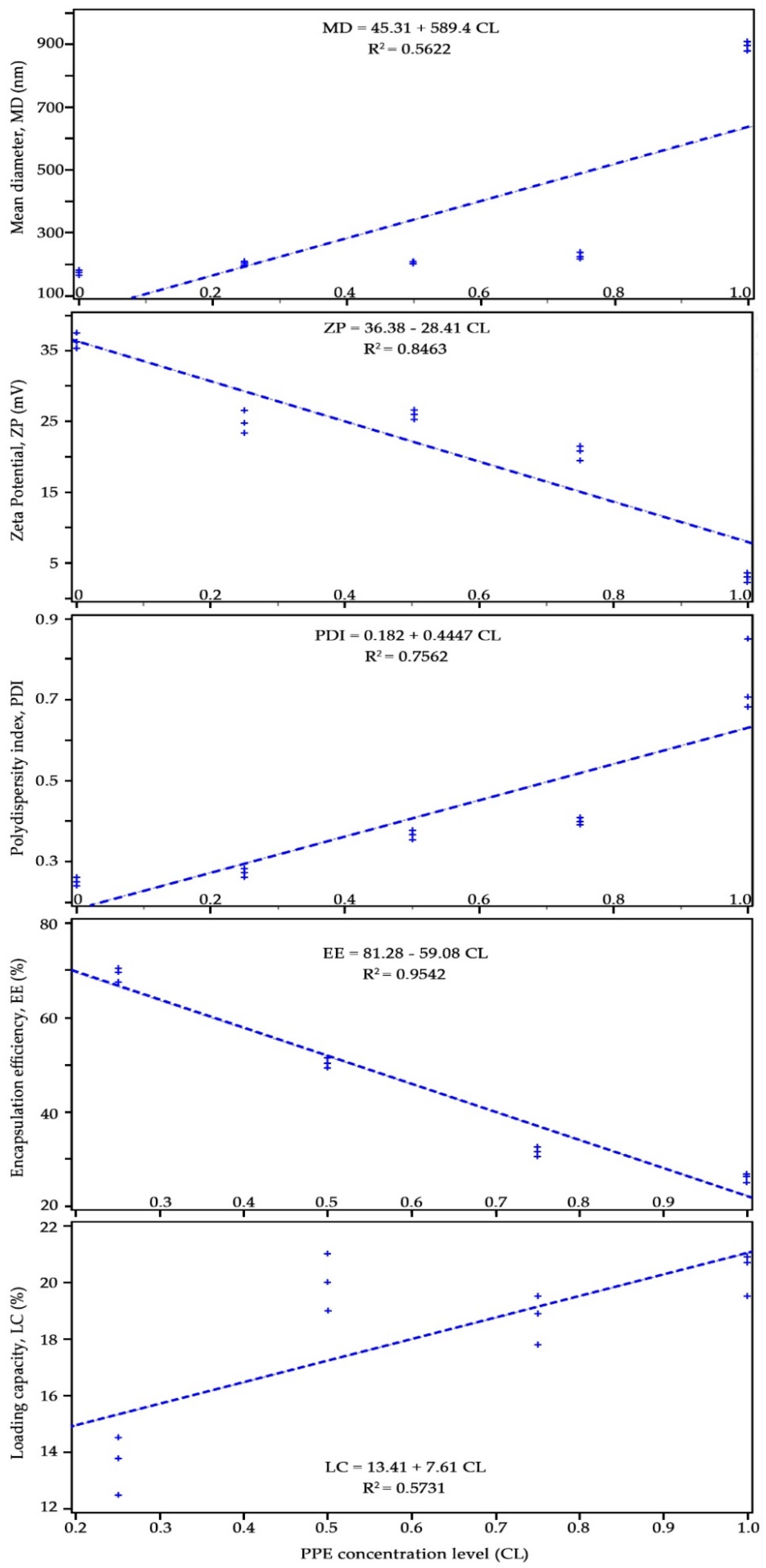
3.2. Pearson’s Correlation Between the Independent Variable (PPE Concentration Levels, CL) and Dependent Variables
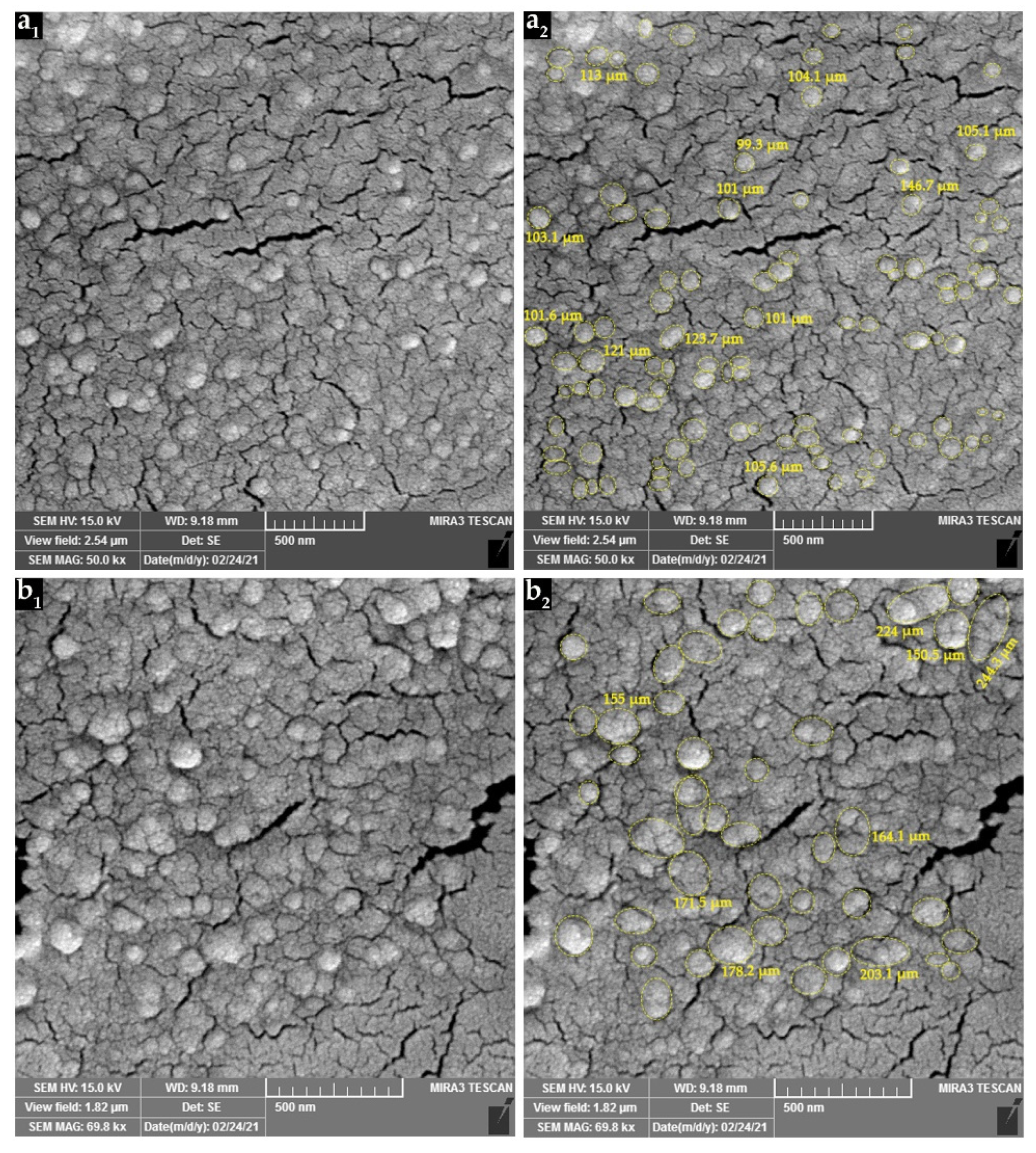
3.3. Scanning Electron Microscopy Observations
3.4. Encapsulation Efficiency (EE) and Loading Capacity (LC)
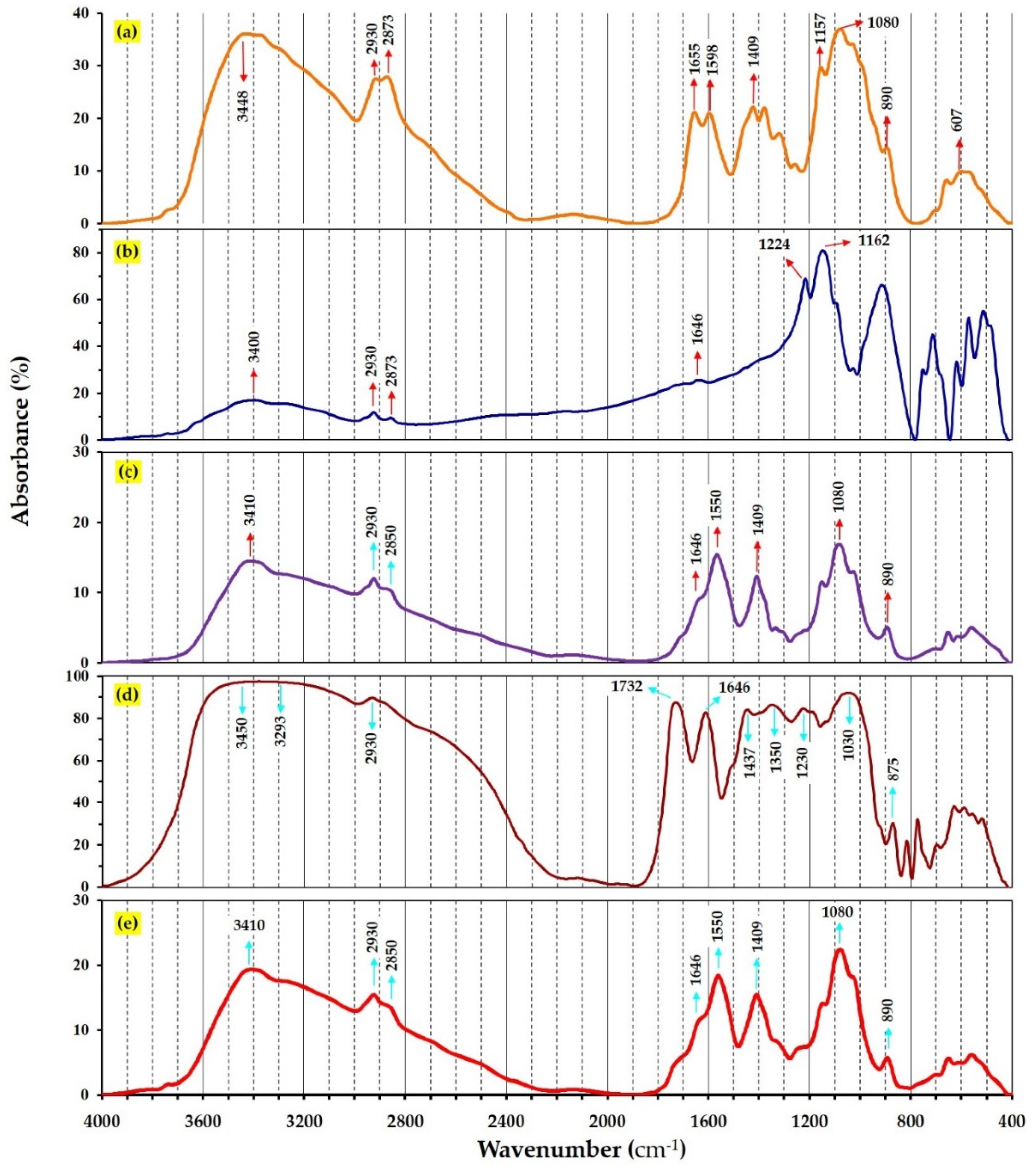
3.5. FTIR Spectroscopy
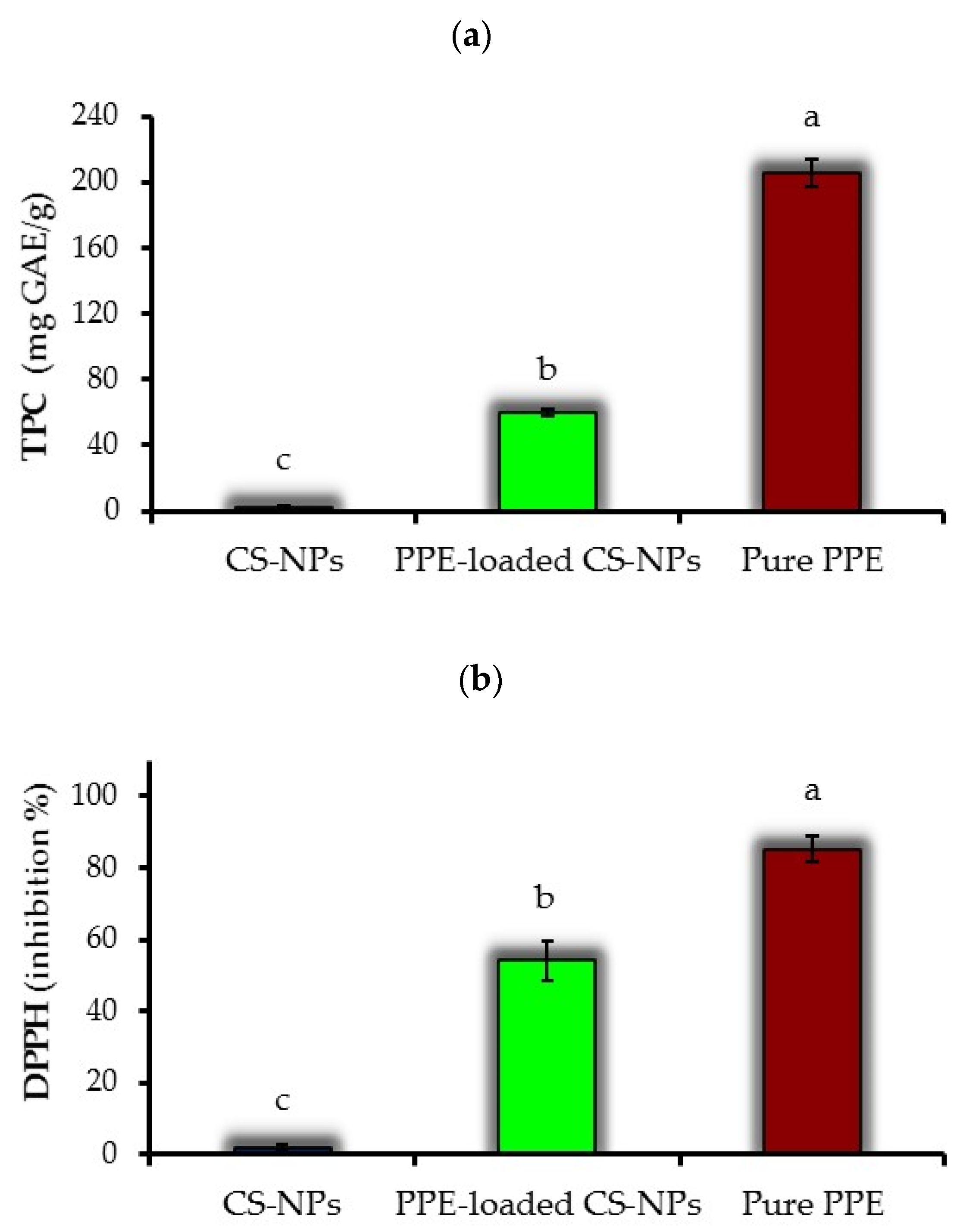
3.6. Total Phenolic Content
3.7. DPPH Radical Scavenging Activity
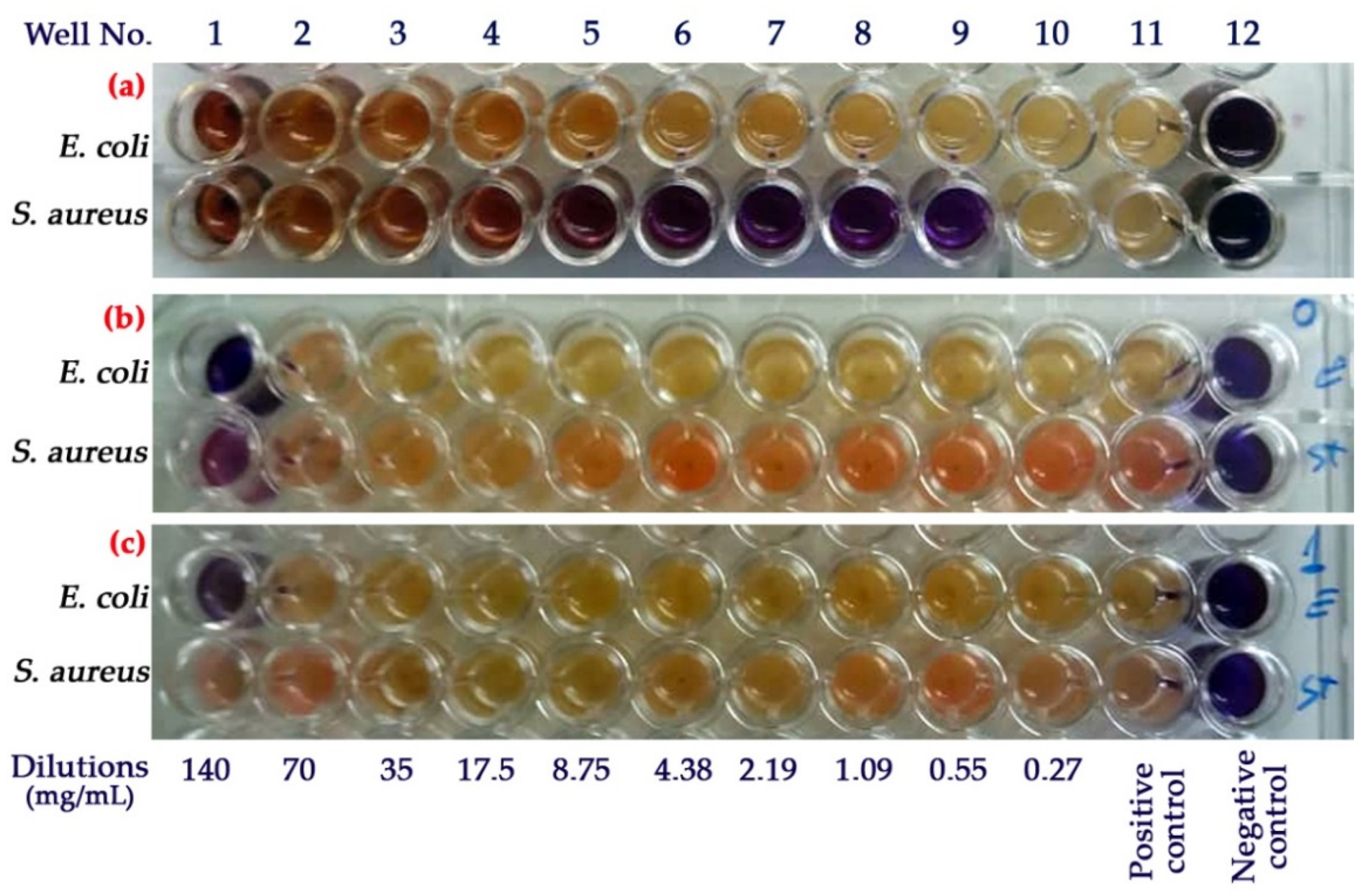
3.8. Antimicrobial Activity
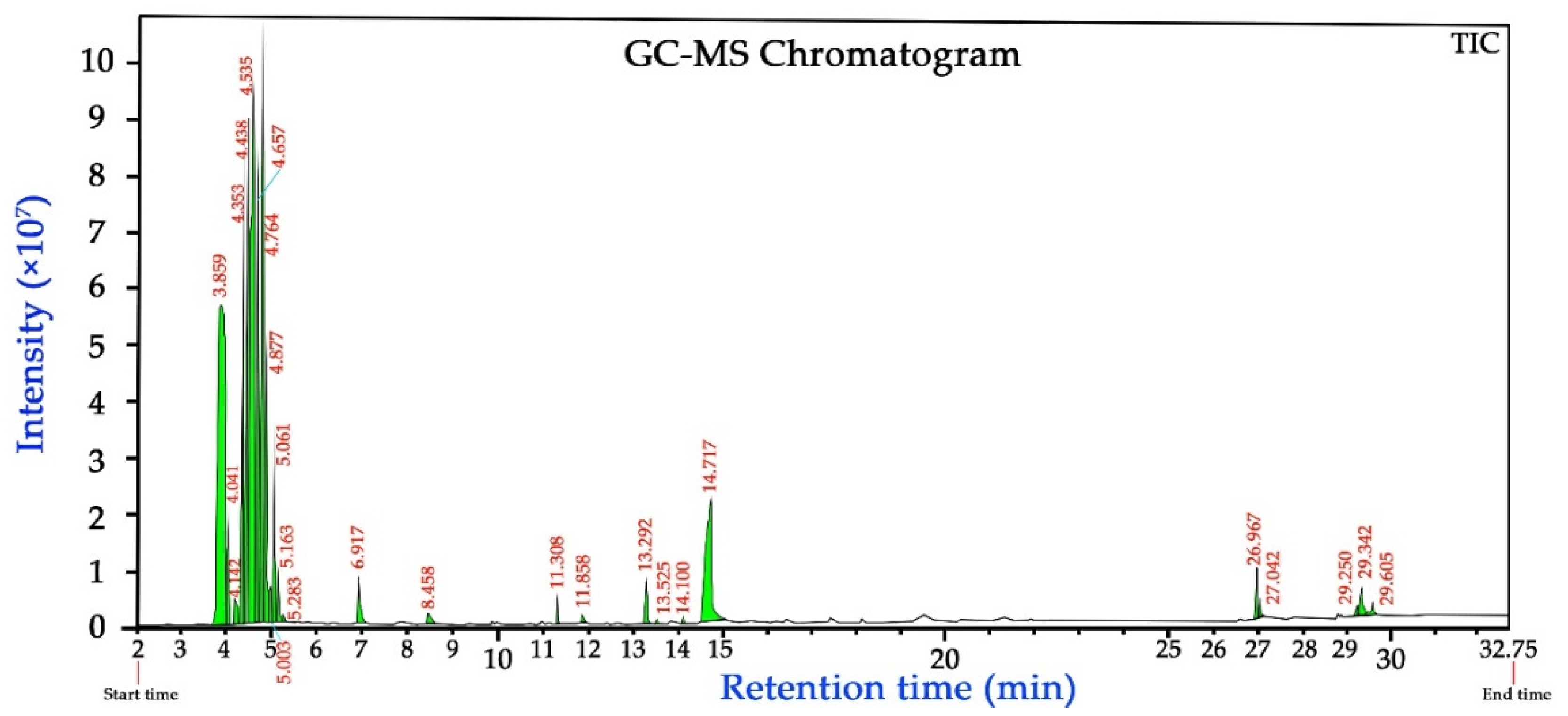
3.9. GS-MS Analysis of the PPE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Upadhyay, N.; Singh, P.; Sharma, S.; Dubey, N.K. Encapsulation in chitosan-based nanomatrix as an efficient green technology to boost the antimicrobial, antioxidant and in situ efficacy of Coriandrum sativum essential oil. Int. J. Biol. Macromol. 2019, 133, 294–305. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Licciardello, F.; Siracusa, L.; Muratore, G.; Hamdi, M.; Restuccia, C. Antimicrobial and antioxidant features of ‘Gabsi’ pomegranate peel extracts. Ind. Crops Prod. 2018, 111, 345–352. [Google Scholar] [CrossRef]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan Nanoparticles with Encapsulated Natural and UF-Purified Annatto and Saffron for the Preparation of UV Protective Cosmetic Emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef] [Green Version]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Jalili, S.; Tabatabee Naini, A.; Ashrafi, M.; Aminlari, M. Antioxidant Activity of Pericarp Extract from Different Varieties of Pomegranate Fruit. J. Agric. Sci. Technol. 2020, 22, 95–107. [Google Scholar]
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules 2020, 25, 2859. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Chowdhury, N.R.; Dandapat, P.; Gagaoua, M.; Chauhan, P.; Pateiro, M.; Lorenzo, J.M. Application of Pomegranate by-Products in Muscle Foods: Oxidative Indices, Colour Stability, Shelf Life and Health Benefits. Molecules 2021, 26, 467. [Google Scholar] [CrossRef]
- Licciardello, F.; Kharchoufi, S.; Muratore, G.; Restuccia, C. Effect of edible coating combined with pomegranate peel extract on the quality maintenance of white shrimps (Parapenaeus longirostris) during refrigerated storage. Food Packag. Shelf Life 2018, 17, 114–119. [Google Scholar] [CrossRef]
- Chen, J.; Liao, C.; Ouyang, X.; Kahramanoğlu, I.; Gan, Y.; Li, M. Antimicrobial Activity of Pomegranate Peel and Its Applications on Food Preservation. J. Food Qual. 2020, 2020, 8850339. [Google Scholar] [CrossRef]
- Howell, A.B.; D’Souza, D.H. The Pomegranate: Effects on Bacteria and Viruses That Influence Human Health. Evid. Based Complement. Altern. Med. 2013, 2013, 606212. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Guerrero-Solano, J.A.; Jaramillo-Morales, O.A.; Velázquez-González, C.; De la O-Arciniega, M.; Castañeda-Ovando, A.; Betanzos-Cabrera, G.; Bautista, M. Pomegranate as a Potential Alternative of Pain Management: A Review. Plants 2020, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Ravash, N.; Peighambardoust, S.H.; Soltanzadeh, M.; Pateiro, M.; Lorenzo, J.M. Impact of high-pressure treatment on casein micelles, whey proteins, fat globules and enzymes activity in dairy products: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Peighambardoust, S.H.; Hesari, J.; Akbari-Adergani, B.; Andreu, D. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Biosci. 2019, 32, 100450. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Gullon, P.; Hesari, J.; Gullón, B.; Alirezalu, K.; Lorenzo, J. Quality aspects and safety of pulsed electric field (PEF) processing on dairy products: A comprehensive review. Food Rev. Int. 2020, 1–22. [Google Scholar] [CrossRef]
- Gómez, B.; Barba, F.J.; Domínguez, R.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Toldrá, F.; Lorenzo, J.M. Microencapsulation of antioxidant compounds through innovative technologies and its specific application in meat processing. Trends Food Sci. Technol. 2018, 82, 135–147. [Google Scholar] [CrossRef]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Kulabhusan, P.; Danquah, M. Biofunctional Nanoparticles for Protein Separation, Purification and Detection; Springer: Berlin/Heidelberg, Germany, 2019; pp. 113–156. ISBN 978-3-030-29068-9. [Google Scholar]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surfaces B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010. [Google Scholar] [CrossRef]
- Cahyono, B.; A’yun, Q.; Suzery, M.; Hadiyanto, H. Characteristics of eugenol loaded chitosan-tripolyphosphate particles as affected by initial content of eugenol and their in-vitro release characteristic. IOP Conf. Ser. Mater. Sci. Eng. 2018, 349, 12010. [Google Scholar] [CrossRef] [Green Version]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 699. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef] [Green Version]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surfaces B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, S.; Peighambardoust, S.H.; Peighambardoust, S.J.; Hosseini, S.V.; Regenstein, J.M. Improved mechanical and antibacterial properties of active LDPE films prepared with combination of Ag, ZnO and CuO nanoparticles. Food Packag. Shelf Life 2019, 22. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J. Nanocomposite films containing organoclay nanoparticles as an antimicrobial (active) packaging for potential food application. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Mohammadzadeh Pakdel, P. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Zahed-Karkaj, S.; Peighambardoust, S.H.; Ebrahimi, Y.; Peressini, D. Characterization of carboxymethyl cellulose-based active films incorporating non-modified and Ag or Cu-modified Cloisite 30B and montmorillonite nanoclays. Iran. Polym. J. 2020. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Tajali Ardakani, M.; Hajipour Verdom, B.; Bagheri, A.; Mohammad-Beigi, H.; Aliakbari, F.; Salehpour, Z.; Alipour, M.; Afrouz, S.; Bardania, H. Chitosan nanoparticles containing Physalis alkekengi-L extract: Preparation, optimization and their antioxidant activity. Bull. Mater. Sci. 2019, 42, 131. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.K.; Kesharwani, S.; Sharma, N.K.; Gupta, M.K. Formulation and Evaluation of Herbal Extract of Allivum sativum (Garlic) Loaded Chitosan Nanoparticles. J. Drug Deliv. Ther. 2019, 9. [Google Scholar] [CrossRef]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-Based Nanoparticles Containing Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress. Nutrients 2018, 10, 1598. [Google Scholar] [CrossRef] [Green Version]
- Safer, A.-M.; Hanafy, N.; Bharali, D.; Cui, H.; Mousa, S. Effect of Green Tea Extract Encapsulated Into Chitosan Nanoparticles on Hepatic Fibrosis Collagen Fibers Assessed by Atomic Force Microscopy in Rat Hepatic Fibrosis Model. J. Nanosci. Nanotechnol. 2015, 15, 6452–6459. [Google Scholar] [CrossRef]
- Othman, A.I.; El-Sherbiny, I.M.; ElMissiry, M.A.; Ali, D.A.; AbdElhakim, E. Polyphenon-E encapsulated into chitosan nanoparticles inhibited proliferation and growth of Ehrlich solid tumor in mice. Egypt. J. Basic Appl. Sci. 2018, 5, 110–120. [Google Scholar] [CrossRef]
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Cranberry proanthocyanidin-chitosan hybrid nanoparticles as a potential inhibitor of extra-intestinal pathogenic Escherichia coli invasion of gut epithelial cells. Int. J. Biol. Macromol. 2018, 111, 415–420. [Google Scholar] [CrossRef]
- Servat-Medina, L.; González-Gómez, A.; Reyes-Ortega, F.; Oliveira Sousa, I.; Queiroz, N.; Zago, P.; Jorge, M.; Monteiro, K.; Carvalho, J.; San Roman, J.; et al. Chitosan–tripolyphosphate nanoparticles as Arrabidaea chica standardized extract carrier: Synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int. J. Nanomed. 2015, 10, 3897–3909. [Google Scholar] [CrossRef] [Green Version]
- Omwenga, E.O.; Hensel, A.; Shitandi, A.; Goycoolea, F.M. Chitosan nanoencapsulation of flavonoids enhances their quorum sensing and biofilm formation inhibitory activities against an E.coli Top 10 biosensor. Colloids Surfaces B Biointerfaces 2018, 164, 125–133. [Google Scholar] [CrossRef]
- Mekawey, A.A.I.; El-Metwally, M.M. Impact of nanoencapsulated natural bioactive phenolic metabolites on chitosan nanoparticles as aflatoxins inhibitor. J. Basic Microbiol. 2019, 59, 599–608. [Google Scholar] [CrossRef]
- Jayaram, U.; Sindhu, R.; Sakthivel, S.; Gurusamy, A. Herbal extract encapsulated in chitosan nanoparticle: A novel strategy for the treatment of urolithiasi. IAJPBB 2018, 5, 1955–1961. [Google Scholar]
- Varasteh, F.; Arzani, K.; Zamani, Z.; Mohseni, A. Evaluation of the Most Important Fruit Characteristics of Some Commercial Pomegranate (Punica granatum L.) Cultivars Grown in Iran. Acta Hortic. 2009, 818, 103–108. [Google Scholar] [CrossRef]
- El-Hadary, A.E.; Ramadan, M.F. Phenolic profiles, antihyperglycemic, antihyperlipidemic, and antioxidant properties of pomegranate (Punica granatum) peel extract. J. Food Biochem. 2019, 43, e12803. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Soltanzadeh, M.; Peressini, D. Properties of dried apricots pretreated by ultrasound-assisted osmotic dehydration and application of active coatings. Food Technol. Biotechnol. 2020, 58. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.H.; Beigmohammadi, F.; Peighambardoust, S.J. Application of organoclay nanoparticle in low-density polyethylene films for packaging of UF cheese. Packag. Technol. Sci. 2016, 29, 355–363. [Google Scholar] [CrossRef]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016, 10, 54107. [Google Scholar] [CrossRef] [Green Version]
- Hajizadeh, H.; Peighambardoust, S.J.; Peighambardoust, S.H.; Peressini, D. Physical, mechanical, and antibacterial characteristics of bio-nanocomposite films loaded with Ag-modified SiO2 and TiO2 nanoparticles. J. Food Sci. 2020, 85, 1193–1202. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration analysis, antioxidant, and mechanical characterization of polypropylene-based active food packaging films loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef]
- Tamara, F.R.; Lin, C.; Mi, F.-L.; Ho, Y.-C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, Y.; Peighambardoust, S.J.; Peighambardoust, S.H.; Karkaj, S.Z. Development of Antibacterial Carboxymethyl Cellulose-Based Nanobiocomposite Films Containing Various Metallic Nanoparticles for Food Packaging Applications. J. Food Sci. 2019, 84, 2537–2548. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [Green Version]
- Hasheminya, S.-M.; Dehghannya, J. Composition, phenolic content, antioxidant and antimicrobial activity of Pistacia atlantica subsp. kurdica hulls’ essential oil. Food Biosci. 2020, 34, 100510. [Google Scholar] [CrossRef]
- Tadros, T. Chapter 2—Colloid and Interface Aspects of Pharmaceutical Science. In Colloid and Interface Science in Pharmaceutical Research and Development; Ohshima, H., Makino, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 29–54. ISBN 978-0-444-62614-1. [Google Scholar]
- Mazzoli, A.; Favoni, O. Particle size, size distribution and morphological evaluation of airborne dust particles of diverse woods by Scanning Electron Microscopy and image processing program. Powder Technol. 2012, 225, 65–71. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; van Brenk, S.; van der Goot, A.J.; Hamer, R.J.; Boom, R.M. Dough processing in a Couette-type device with varying eccentricity: Effect on glutenin macro-polymer properties and dough micro-structure. J. Cereal Sci. 2007, 45, 34–48. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; van der Goot, A.J.; Boom, R.M.; Hamer, R.J. Mixing behaviour of a zero-developed dough compared to a flour–water mixture. J. Cereal Sci. 2006, 44, 12–20. [Google Scholar] [CrossRef]
- Van der Goot, A.J.J.; Peighambardoust, S.H.H.; Akkermans, C.; Van Oosten-Manski, J.M.M. Creating novel structures in food materials: The role of well-defined shear flow. Food Biophys. 2008, 3, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int. J. Pharm. 2005, 295, 235–245. [Google Scholar] [CrossRef]
- Xu, Y.; Du, Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 2003, 250, 215–226. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Yan, X.-Q.; Zhang, H.-M.; Yao, S.-J.; Jiang, L. Novel double-walled microspheres based on chitosan, sodium cellulose sulfate and sodium tripolyphosphate: Preparation, characterization and in vitro release study. Korean J. Chem. Eng. 2015, 32, 369–372. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Akermi, A.; Mabrouk, M.; Ouederni, A. Optimization of extraction process and chemical characterization of pomegranate peel extract. Chem. Pap. 2018, 72, 2087–2100. [Google Scholar] [CrossRef]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–290. [Google Scholar] [CrossRef]
- Barzegar, M.; Ghaderi Ghahfarokhi, M.; Sahari, M.A.; Azizi, M.H. Enhancement of thermal stability and antioxidant activity of thyme essential oil by encapsulation in chitosan nanoparticles. J. Agric. Sci. Technol. 2016, 18, 1781–1792. [Google Scholar]
- Chen, F.; Shi, Z.; Neoh, K.G.; Kang, E.T. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol. Bioeng. 2009, 104, 30–39. [Google Scholar] [CrossRef]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef] [PubMed]
| Treatments | LMW−CS (mL)(solids, mg) | PPE (mL)(solids, mg) | TPP (mL)(solids, mg) | Total (mL)(solids, mg) |
|---|---|---|---|---|
| CS:PPE of 1:0 | 10.0 (50) | 0 | 10 (20) | 20 (70.00) |
| CS:PPE of 1:0.25 | 9.0 (45) | 1.0 (11.25) | 10 (20) | 20 (76.25) |
| CS:PPE of 1:0.50 | 9.0 (45) | 1.0 (22.50) | 10 (20) | 20 (87.50) |
| CS:PPE of 1:0.75 | 9.0 (45) | 1.0 (33.75) | 10 (20) | 20 (98.75) |
| CS:PPE of 1:1.00 | 9.0 (45) | 1.0 (45.00) | 10 (20) | 20 (110.0) |
| Wells (column no.) → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Stages | Concentrations (%) | 100 | 50 | 25 | 12.5 | 6.25 | 3.13 | 1.56 | 0.78 | 0.39 | 0.19 | Positive control (Broth + Bacteria) | Negative control (Broth) |
| 1 | Addition of MH broth (µL) | - | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 2 | Addition of extract or NPs (µL) | 100 | 100 | Serial two-fold dilutions for wells 3–10 | 0 | 0 | |||||||
| 3 | Addition of Bacteria (µL) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 |
| 4 | Addition of resazurin (µL) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Chitosan:PPE (w/w) | Z-average Diameter (nm) | Zeta Potential (mV) | Poly-Dispersity Index (PDI) | Encapsulation Efficiency (EE%) | Loading Capacity (LC%) |
|---|---|---|---|---|---|
| 1:0 | 173.9 ± 3.6 *e | 36.3 ± 0.85 a | 0.250 ± 0.011 d | - | - |
| 1:0.25 | 198.0 ± 4.1 d | 24.8 ± 0.89 b | 0.260 ± 0.015 d | 69.7 ± 1.05 a | 13.8 ± 0.15 c |
| 1:0.50 | 208.2 ± 4.8 c | 26.5 ± 1.80 b | 0.368 ± 0.012 c | 50.5 ± 1.25 b | 20.0 ± 0.38 a |
| 1:0.75 | 224.1 ± 5.8 b | 20.8 ± 0.85 c | 0.399 ± 0.013 b | 31.7 ± 0.95 c | 18.9 ± 0.21 b |
| 1:1.00 | 897.7 ± 35.4 a | 2.95 ± 0.22 d | 0.682 ± 0.035 a | 26.3 ± 1.55 d | 20.7 ± 0.57 a |
| Chitosan:PPE (w/w) | n | Particle Diameter or Length (nm) | |||
|---|---|---|---|---|---|
| Average | St. Dev | Min | Max | ||
| Empty CSNPs | 100 | 90.6 | 21.5 | 51.5 | 148.0 |
| PPE-loaded CSNPs | 46 | 127.3 | 38.7 | 66.9 | 244.3 |
| Microbial strain→ | E. coli | S. aureus | ||
| Samples | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) |
| PPE | - | - | 0.27 | 0.55 |
| PPE-loaded CS-NPs | - | - | 1.09 | 2.19 |
| Peak No. | RT (min) | Percentage | Identified Compounds | Molecular Weight (Da) | Molecular Formula |
|---|---|---|---|---|---|
| 1 | 3.701 | 0.05 | Unknown | - | - |
| 2 | 3.859 | 18.09 | Methanol | 32 | CH4O |
| 3 | 4.041 | 1.57 | Ethanol | 46 | C2H6O |
| 4 | 4.142 | 0.43 | Glycidol | 74 | C3H6O2 |
| 5 | 4.189 | 0.84 | Glycolamide; 2-hydroxy-acetamide | 75 | C2H5NO2 |
| 6 | 4.353 | 6.64 | Heptane | 100 | C7H16 |
| 7 | 4.438 | 10.13 | 2,3-Dimethyl pentane | 100 | C7H16 |
| 8 | 4.535 | 20.57 | n-Hexane | 86 | C6H14 |
| 9 | 4.657 | 8.96 | Hexane | 86 | C6H14 |
| 10 | 4.764 | 12.67 | 1-Hexene | 84 | C6H12 |
| 11 | 4.877 | 4.71 | Methyl-cyclopentane | 84 | C6H12 |
| 12 | 5.003 | 0.62 | Methyl-cyclopentane | 84 | C6H12 |
| 13 | 5.061 | 2.84 | Cyclohexane | 84 | C6H12 |
| 14 | 5.163 | 0.92 | Cyclohexane | 84 | C6H12 |
| 15 | 5.283 | 0.13 | Cyclohexane | 84 | C6H12 |
| 16 | 6.917 | 0.92 | Furfural (furan derivatives) | 96 | C5H4O2 |
| 17 | 8.458 | 0.29 | 2,5-Furandione; 3-methyl-citraconic anhydride | 112 | C5H4O3 |
| 18 | 11.305 | 0.32 | 1,8-Cineole; terpene; eucalyptol; p-cineole | 154 | C10H18O |
| 19 | 11.860 | 0.14 | Furancarboxylic acid; furoic acid; methyl furate | 126 | C6H6O3 |
| 20 | 13.296 | 0.78 | 2,3-Dihydro-,3,5-dihydroxy-4H-pyran-4-one, | 144 | C6H8O4 |
| 21 | 13.522 | 0.07 | Camphor; 1,7,7-trimethyl-bicyclo[2.2.1] heptan-2-one | 152 | C10H16O |
| 22 | 14.103 | 0.07 | Borneol; 1,7,7-trimethyl-(1s endo)-bicyclo[2.2.1]heptan-2-one | 154 | C10H18O |
| 23 | 14.720 | 6.05 | 5-Hydroxymethyl-2-furancarboxaldehyde; hydroxymethyl furfurole (HMF) | 126 | C6H6O3 |
| 24 | 26.968 | 0.73 | 2-Methoxy-phenol; guaiacol | 124 | C7H8O2 |
| 25 | 27.039 | 0.29 | n-Hexadecanoic acid; palmitic acid | 256 | C16H32O2 |
| 26 | 29.249 | 0.23 | (z,z)-9,12-Octadecadiennoic acid; linoleic acid | 280 | C18H32O2 |
| 27 | 29.399 | 0.64 | (z)-9-Octadecenoic acid; oleic acid | 282 | C18H34O2 |
| 28 | 29.605 | 0.3 | n-Octadecanoic acid; stearic acid | 284 | C18H36O2 |
| Total | - | 100% | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan Nanoparticles as a Promising Nanomaterial for Encapsulation of Pomegranate (Punica granatum L.) Peel Extract as a Natural Source of Antioxidants. Nanomaterials 2021, 11, 1439. https://doi.org/10.3390/nano11061439
Soltanzadeh M, Peighambardoust SH, Ghanbarzadeh B, Mohammadi M, Lorenzo JM. Chitosan Nanoparticles as a Promising Nanomaterial for Encapsulation of Pomegranate (Punica granatum L.) Peel Extract as a Natural Source of Antioxidants. Nanomaterials. 2021; 11(6):1439. https://doi.org/10.3390/nano11061439
Chicago/Turabian StyleSoltanzadeh, Maral, Seyed Hadi Peighambardoust, Babak Ghanbarzadeh, Maryam Mohammadi, and José M. Lorenzo. 2021. "Chitosan Nanoparticles as a Promising Nanomaterial for Encapsulation of Pomegranate (Punica granatum L.) Peel Extract as a Natural Source of Antioxidants" Nanomaterials 11, no. 6: 1439. https://doi.org/10.3390/nano11061439
APA StyleSoltanzadeh, M., Peighambardoust, S. H., Ghanbarzadeh, B., Mohammadi, M., & Lorenzo, J. M. (2021). Chitosan Nanoparticles as a Promising Nanomaterial for Encapsulation of Pomegranate (Punica granatum L.) Peel Extract as a Natural Source of Antioxidants. Nanomaterials, 11(6), 1439. https://doi.org/10.3390/nano11061439








