Djulis (Chenopodium formosanum) Extract as a Promising Natural Agent Against Skin Aging
Abstract
1. Introduction
2. Mechanisms of Photoaging
2.1. Ultraviolet Radiation
2.2. Oxidative Stress and Reactive Oxygen Species Generation
2.3. Inflammatory Responses
2.4. Matrix Metalloproteinases Activation and Extracellular Matrix Degradation
2.5. DNA Damage
2.6. Cellular Senescence
2.7. Advanced Glycation End Products and Skin Aging
3. Djulis and Phytochemical Composition of Djulis Extract
3.1. Phenolic Compounds
3.2. Betalains
3.3. Phytoecdysteroids
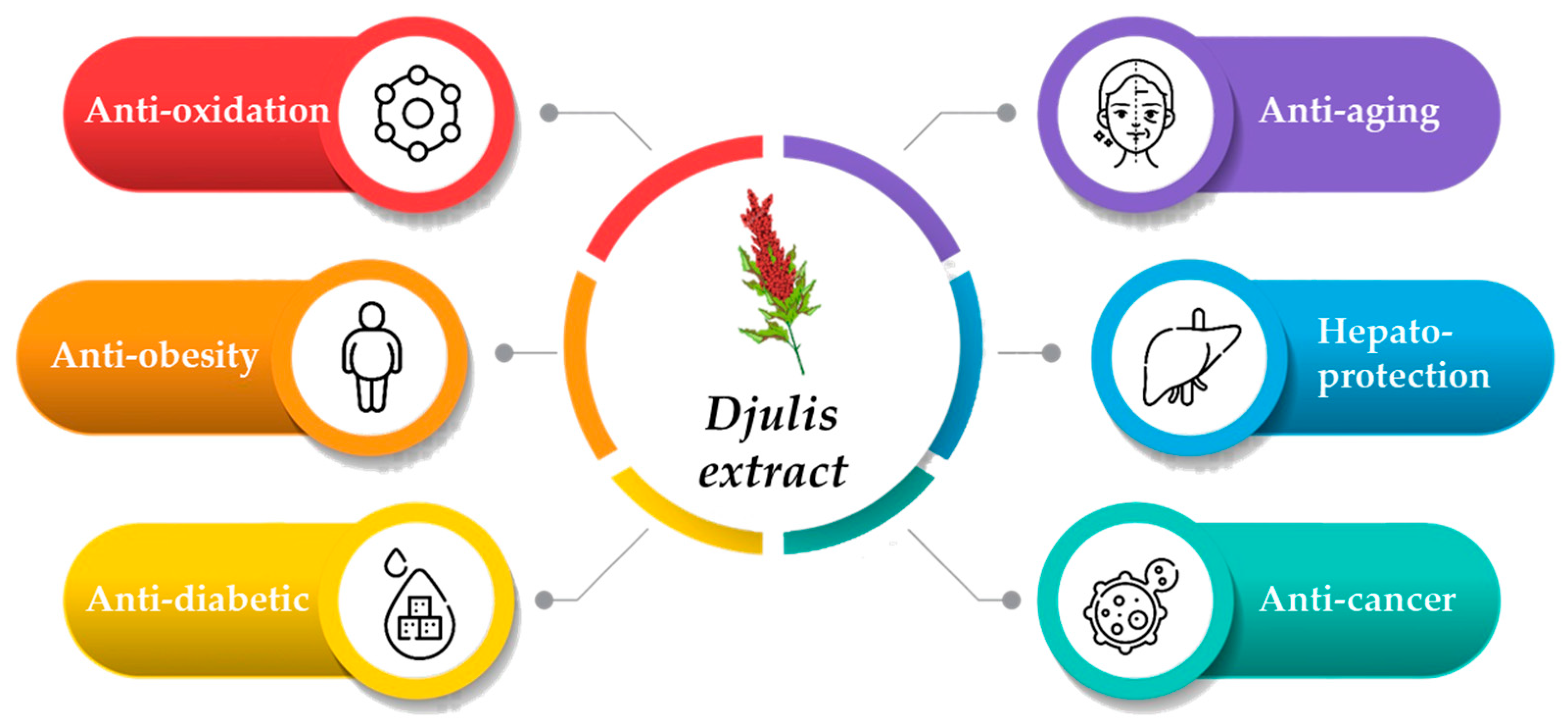
4. Bioactivity of Djulis Extract
4.1. Antioxidant Activity
4.2. Activation of the Antioxidant Defense System Nrf2/HO-1 Signaling Pathway
4.3. Regulation of MAPK/Matrix Metalloproteinases/Collagen Pathway
4.4. Anti-Inflammatory Activity
4.5. Cellular Protection
4.6. Inhibition of Advanced Glycation End Products Formation
4.7. Clinical Trial Design and Validation Studies
5. Safety of Djulis
5.1. In Vitro Studies
5.2. Animal Studies
5.3. Traditional Use and Clinical Trial
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-4PPs | 6-4 photoproducts |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| AGEs | advanced glycation end products |
| AP-1 | activator protein-1 |
| CML | Nε-carboxymethyl-lysine |
| COX-2 | cyclooxygenase-2 |
| CPDs | cyclobutane pyrimidine dimers |
| ECM | extracellular matrix |
| IKK | IκB kinase |
| IL | interleukin |
| MAPKs | mitogen-activated protein kinases |
| MMPs | matrix metalloproteinases |
| NF-κB | nuclear factor-κB |
| RAGE | receptor of advanced glycation end products |
| ROS | reactive oxygen species |
| SASP | senescence-associated secretory phenotype |
| TNF-α | tumor necrosis factor-α |
| UV | ultraviolet |
References
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Attard, N.R.; Karran, P. UVA photosensitization of thiopurines and skin cancer in organ transplant recipients. Photochem. Photobiol. Sci. 2012, 11, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Zouboulis, C.C. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007, 214, 352–360. [Google Scholar] [CrossRef]
- Anwar, S.; Saleem, H.; Azmat, T.; Khurshid, U.; Khan, K.M.; Chohan, T.A.; Khursheed, A.; Alamri, A.; Awadh Ali, N.A. Crotalaria burhia Buch. -Ham.: A comprehensive review of its botany, traditional uses, phytochemistry, and pharmacology. Nat. Prod. Res. 2025, 39, 2277–2292. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Soudek, P.; Pavicic, A.; Langhansova, L. The traditional utilization, biological activity and chemical composition of edible fern species. J. Ethnopharmacol. 2024, 324, 117818. [Google Scholar] [CrossRef]
- Garg, P.; Pundir, S.; Ali, A.; Panja, S.; Chellappan, D.K.; Dua, K.; Kulshrestha, S.; Negi, P. Exploring the potential of Moringa oleifera Lam in skin disorders and cosmetics: Nutritional analysis, phytochemistry, geographical distribution, ethnomedicinal uses, dermatological studies and cosmetic formulations. Naunyn. Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3635–3662. [Google Scholar] [CrossRef]
- Tsai, P.-J.; Chen, Y.-S.; Sheu, C.-H.; Chen, C.-Y. Effect of nanogrinding on the pigment and bioactivity of djulis (Chenopodium formosanum Koidz.). J. Agric. Food Chem. 2011, 59, 1814–1820. [Google Scholar] [CrossRef]
- Brem, R.; Guven, M.; Karran, P. Oxidatively-generated damage to DNA and proteins mediated by photosensitized UVA. Free Radic. Biol. Med. 2017, 107, 101–109. [Google Scholar] [CrossRef]
- Kullavanijaya, P.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 937–958. [Google Scholar] [CrossRef]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem. Photobiol. Sci. 2012, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Stoykova, I.D.; Koycheva, I.K.; Binev, B.K.; Mihaylova, L.V.; Georgiev, M.I. Molecular approaches to prevent UV-induced premature skin aging: Focus on phytochemicals as photo-protectants. Phytochem. Rev. 2025, 24, 119–150. [Google Scholar] [CrossRef]
- Young, A.R.; Harrison, G.I.; Chadwick, C.A.; Nikaido, O.; Ramsden, J.; Potten, C.S. The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema. J. Investig. Dermatol. 1998, 111, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.G.; Gabrielli, A.; Keszenman, D.J. Impact of ecological UV radiation on the photochemistry of nuclear DNA. Biophys. Rev. 2025, 17, 537–545. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef]
- Fraikin, G.Y.; Belenikina, N.; Rubin, A. Photochemical processes of cell DNA damage by UV radiation of various wavelengths: Biological consequences. Mol. Biol. 2024, 58, 1–16. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.; Jiang, G. Research progress on skin photoaging and oxidative stress. Adv. Dermatol. Allergol. Postępy Dermatol. I Alergol. 2021, 38, 931–936. [Google Scholar] [CrossRef]
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Chiang, H.-L.; Wu, P.-Y.; Chu, Y.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Protection against Ultraviolet A-induced skin apoptosis and carcinogenesis through the oxidative stress reduction effects of N-(4-bromophenethyl) caffeamide, a propolis derivative. Antioxidants 2020, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wu, P.-Y.; Chen, C.-W.; Lyu, J.-L.; Liu, Y.-J.; Wen, K.-C.; Lin, C.-Y.; Kuo, Y.-H.; Chiang, H.-M. Protective effects and mechanisms of N-Phenethyl Caffeamide from UVA-induced skin damage in human epidermal keratinocytes through Nrf2/HO-1 regulation. Int. J. Mol. Sci. 2019, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-Y.; Lin, T.-Y.; Hou, C.-W.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. 1, 2-bis [(3-methoxyphenyl) methyl] ethane-1, 2-dicarboxylic acid reduces UVB-induced photodamage in vitro and in vivo. Antioxidants 2019, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Wu, P.-Y.; Hou, C.-W.; Chien, T.-Y.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Protective effects of sesamin against UVB-induced skin inflammation and photodamage in vitro and in vivo. Biomolecules 2019, 9, 479. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in understanding oxidative stress, aging, and aging-related diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid peroxidation-derived aldehydes, 4-hydroxynonenal and malondialdehyde in aging-related disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef]
- Li Pomi, F.; Gammeri, L.; Borgia, F.; Di Gioacchino, M.; Gangemi, S. Oxidative Stress and Skin Diseases: The Role of Lipid Peroxidation. Antioxidants 2025, 14, 555. [Google Scholar] [CrossRef]
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.-P.; Ravanat, J.-L.; Sauvaigo, S. Hydroxyl radicals and DNA base damage. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 424, 9–21. [Google Scholar] [CrossRef]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 571, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Lan, C.C.E. Photocarcinogenesis of the skin: Current status and future trends. Kaohsiung J. Med. Sci. 2025, 41, e12946. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Song, S.; Li, F.; Zhao, B.; Zhou, M.; Wang, X. Ultraviolet light causes skin cell senescence: From mechanism to prevention principle. Adv. Biol. 2025, 9, 2400090. [Google Scholar] [CrossRef] [PubMed]
- Berneburg, M.; Plettenberg, H.; Krutmann, J. Photoaging of human skin. Photodermatol. Photoimmunol. Photomed. Rev. Artic. 2000, 16, 239–244. [Google Scholar] [CrossRef]
- Liu, H.; Dong, J.; Du, R.; Gao, Y.; Zhao, P. Collagen study advances for photoaging skin. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12931. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Chen, X.; Yin, X.; Jiang, Y.; Zhao, C. Matrix metalloproteinases on skin photoaging. J. Cosmet. Dermatol. 2024, 23, 3847–3862. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Sárdy, M. Role of matrix metalloproteinases in skin ageing. Connect. Tissue Res. 2009, 50, 132–138. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative Stress and Human Skin Connective Tissue Aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, K.S.; Ahn, H.J.; Kang, I.H.; Shin, M.K. Reduced matrix metalloproteinase and collagen transcription mediated by the TGF-β/Smad pathway in passaged normal human dermal fibroblasts. J. Cosmet. Dermatol. 2020, 19, 1211–1218. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, X.-J. TGFβ signaling in photoaging and UV-induced skin cancer. J. Investig. Dermatol. 2021, 141, 1104–1110. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Li, S.; Ruan, D.; Xiong, L.; Tang, J.; Hu, M.; Wang, Y.; Huang, W.; Li, L.; et al. Skin-derived precursor conditioned medium alleviated photoaging via early activation of TGF-β/Smad signaling pathway by thrombospondin1: In vitro and in vivo studies. J. Photochem. Photobiol. B Biol. 2024, 253, 112873. [Google Scholar] [CrossRef]
- Goukassian, D.; Gad, F.; Yaar, M.; Eller, M.S.; Nehal, U.S.; Gilchrest, B.A. Mechanisms and implications of the age-associated decrease in DNA repair capacity. FASEB J. 2000, 14, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Lane, D.P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 275–283. [Google Scholar] [CrossRef]
- Nanz, L.; Keim, U.; Katalinic, A.; Meyer, T.; Garbe, C.; Leiter, U. Epidemiology of keratinocyte skin cancer with a focus on cutaneous squamous cell carcinoma. Cancers 2024, 16, 606. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef]
- Ibragimova, M.; Kussainova, A.; Aripova, A.; Bersimbaev, R.; Bulgakova, O. The molecular mechanisms in senescent cells induced by natural aging and ionizing radiation. Cells 2024, 13, 550. [Google Scholar] [CrossRef]
- Herr, L.M.; Schaffer, E.D.; Fuchs, K.F.; Datta, A.; Brosh Jr, R.M. Replication stress as a driver of cellular senescence and aging. Commun. Biol. 2024, 7, 616. [Google Scholar] [CrossRef]
- Ahmad, A.; Braden, A.; Khan, S.; Xiao, J.; Khan, M.M. Crosstalk between the DNA damage response and cellular senescence drives aging and age-related diseases. Semin. Immunopathol. 2024, 46, 10. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation–a review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Wyles, S.P.; Vyas, K.; Wasserburg, J.R.; Ansaf, R.; Kirkland, J.L. Age-related disease: Skin. Aging 2024, 147–164. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Papadopoulou, A.; Pratsinis, H.; Kletsas, D. Senescence-associated alterations in the extracellular matrix: Deciphering their role in the regulation of cellular function. Am. J. Physiol.-Cell Physiol. 2023, 325, C633–C647. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Derm.-Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Dorosz, A.; Skoczeń, A.; Kulesza, M.; Wawrzynów, W.; Jakubowska, M.M.; Kruk, A.; Rutecka, N.; Miłoś, M.; Kuśnierz-Gibała, A.; Kaczmarek, B. The Impact of Environmental Factors on Skin and Tissue Ageing: Mechanisms, Effects, and Preventive Strategies. J. Educ. Health Sport 2025, 79, 58282. [Google Scholar] [CrossRef]
- Hussein, R.S.; Bin Dayel, S.; Abahussein, O.; El-Sherbiny, A.A. Influences on skin and intrinsic aging: Biological, environmental, and therapeutic insights. J. Cosmet. Dermatol. 2025, 24, e16688. [Google Scholar] [CrossRef]
- Lyu, J.-L.; Liu, Y.-J.; Wen, K.-C.; Chiu, C.-Y.; Lin, Y.-H.; Chiang, H.-M. Protective Effect of Djulis (Chenopodium formosanum) Extract against UV-and AGEs-Induced Skin Aging via Alleviating Oxidative Stress and Collagen Degradation. Molecules 2022, 27, 2332. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Zhao, C. The effects of advanced glycation end-products on skin and potential anti-glycation strategies. Exp. Dermatol. 2024, 33, e15065. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Mori, T.; Yamamoto, Y.; Kaji, Y.; Yonei, Y. Significance of advanced glycation end products in aging-related disease. Anti-Aging Med. 2010, 7, 112–119. [Google Scholar] [CrossRef]
- Malik, P.; Rani, R.; Mukherjee, T.K. The Biology of Advanced Glycation End Products. In Glycosylation and Glycation in Health and Diseases; Bentham Science Publishers: Sharjah, United Arab Emirates, 2025; pp. 120–189. [Google Scholar]
- Li, W.; Chen, Q.; Peng, C.; Yang, D.; Liu, S.; Lv, Y.; Jiang, L.; Xu, S.; Huang, L. Roles of the Receptor for Advanced Glycation End Products and Its Ligands in the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 403. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.A.; Zainab, S.; Muthyalaiah, Y.S.; John, C.M.; Arockiasamy, S. Mechanism and implications of advanced glycation end products (AGE) and its receptor RAGE axis as crucial mediators linking inflammation and obesity. Mol. Biol. Rep. 2025, 52, 556. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Tu, C.; Chen, X.; He, R. Advanced Glycation End Products in Disease Development and Potential Interventions. Antioxidants 2025, 14, 492. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Gueniche, A.; Yatskayer, M.; Nelson, D.B. A Single-center, Double-blinded, Randomized, Placebo-controlled Trial Evaluating the Safety and Efficacy of a Dietary Supplement Containing Rosemary Extract on Visible Facial Skin Quality. J. Clin. Aesthetic Dermatol. 2025, 18, 28. [Google Scholar]
- Huang, Y.-C.; Tung, C.-L.; Ho, S.-T.; Li, W.-S.; Li, S.; Tung, Y.-T.; Wu, J.-H. Nutraceutical Potential of Djulis (Chenopodium formosanum) Hull: Phytochemicals, Antioxidant Activity, and Liver Protection. Antioxidants 2024, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-J.; Sheu, C.-H.; Wu, P.-H.; Sun, Y.-F. Thermal and pH stability of betacyanin pigment of djulis (Chenopodium formosanum) in Taiwan and their relation to antioxidant activity. J. Agric. Food Chem. 2009, 58, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-S.; Chan, Y.-J.; Wu, Y.-Z.; Lu, W.-C.; Chiang, P.-Y.; Li, P.-H. Bioactive Compounds and Antioxidant Efficacy of Djulis (Chenopodium formosanum) Leaves: Implications for Sustainable Cosmeceutical Development. Antioxidants 2025, 14, 202. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Huang, Y.-L.; Liu, Y.-C.; Tsai, P.-J. Djulis (Chenopodium formosanum Koidz.) water extract and its bioactive components ameliorate dermal damage in UVB-irradiated skin models. BioMed Res. Int. 2016, 2016, 7368797. [Google Scholar] [CrossRef]
- Noor, S.N.M.; Musa, M.; Azlina, A.; Gan, S.H.; Thirumulu, K.P. Polyphenols in bee products and prevention of cell senescence. Biomedicine 2024, 14, 1. [Google Scholar] [CrossRef]
- McClain, G.E.; Watson, R.R. The role of polyphenols in skin health. In Bioactive Dietary Factors and Plant Extracts in Dermatology; Watson, R.R., Zibadi, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 169–175. [Google Scholar]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; K Katiyar, S. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar]
- Chyau, C.-C.; Chu, C.-C.; Chen, S.-Y.; Duh, P.-D. The inhibitory effects of djulis (Chenopodium formosanum) and its bioactive compounds on adipogenesis in 3T3-L1 adipocytes. Molecules 2018, 23, 1780. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chu, C.-C.; Chyau, C.-C.; Yang, J.-W.; Duh, P.-D. Djulis (Chenopodium formosanum) and its bioactive compounds affect vasodilation, angiotensin converting enzyme activity, and hypertension. Food Biosci. 2019, 32, 100469. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, S.-N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Sci. 2016, 38, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Laurean, D.; Schramm, D.D.; Jacobson, E.L.; Halaweish, I.; Bruckner, G.G.; Boissonneault, G.A. Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites. J. Nutr. Biochem. 2006, 17, 531–540. [Google Scholar] [CrossRef]
- Allegra, M.; Furtmüller, P.G.; Jantschko, W.; Zederbauer, M.; Tesoriere, L.; Livrea, M.A.; Obinger, C. Mechanism of interaction of betanin and indicaxanthin with human myeloperoxidase and hypochlorous acid. Biochem. Biophys. Res. Commun. 2005, 332, 837–844. [Google Scholar] [CrossRef]
- Hankey, G.J.; Eikelboom, J.W. Homocysteine and vascular disease. Lancet 1999, 354, 407–413. [Google Scholar] [CrossRef]
- Wu, L.-c.; Hsu, H.-W.; Chen, Y.-C.; Chiu, C.-C.; Lin, Y.-I.; Ho, J.-A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Betalains a new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Chu, C.-C.; Chen, S.-Y.; Chyau, C.-C.; Wu, Y.-C.; Chu, H.-L.; Duh, P.-D. Anticancer activity and mediation of apoptosis in hepatoma carcinoma cells induced by djulis and its bioactive compounds. J. Funct. Foods 2020, 75, 104225. [Google Scholar] [CrossRef]
- Tu, D.-G.; Chyau, C.-C.; Chen, S.-Y.; Chu, H.-L.; Wang, S.-C.; Duh, P.-D. Antiproliferative Effect and mediation of apoptosis in human hepatoma HepG2 cells induced by djulis husk and its bioactive compounds. Foods 2020, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.L.; Cheng, D.M.; Esposito, D.; Shertel, T.; Poulev, A.; Plundrich, N.; Itenberg, D.; Dayan, N.; Lila, M.; Raskin, I. Compounds leached from quinoa seeds inhibit matrix metalloproteinase activity and intracellular reactive oxygen species. Int. J. Cosmet. Sci. 2015, 37, 212–221. [Google Scholar] [CrossRef]
- Nsimba, R.Y.; Kikuzaki, H.; Konishi, Y. Ecdysteroids act as inhibitors of calf skin collagenase and oxidative stress. J. Biochem. Mol. Toxicol. 2008, 22, 240–250. [Google Scholar] [CrossRef]
- Chyau, C.-C.; Chu, C.-C.; Chen, S.-Y.; Duh, P.-D. Djulis (Chenopodiun formosaneum) and its bioactive compounds protect against oxidative stress in human HepG2 cells. J. Funct. Foods 2015, 18, 159–170. [Google Scholar] [CrossRef]
- Lin, T.-A.; Ke, B.-J.; Cheng, C.-S.; Wang, J.-J.; Wei, B.-L.; Lee, C.-L. Red quinoa bran extracts protects against carbon tetrachloride-induced liver injury and fibrosis in mice via activation of antioxidative enzyme systems and blocking TGF-β1 pathway. Nutrients 2019, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-K.; Chung, Y.-M.; Lin, Y.-H.; Lin, Y.-H.; Hu, W.-C.; Chiang, C.-F. Health functional properties of unhulled red djulis (Chenopodium formosanum) in anti-aging. Int. J. Food Prop. 2021, 24, 833–844. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chu, C.-C.; Chyau, C.-C.; Fu, Z.-H.; Duh, P.-D. Effect of water extract of Djulis (Chenopodium formosaneum) and its bioactive compounds on alcohol-induced liver damage in rats. Int. J. Food Nutr. Sci. 2018, 5, 55–63. [Google Scholar]
- Chen, S.; Chu, C.-C.; Lin, Y.-C.; Duh, P.-D. Djulis (Chenopodium formosanum) and its bioactive compounds for management of hyperlipidemia and hyperglycemia in high-fat diet-fed mice. J. Food Nutr. Res. 2019, 7, 452–457. [Google Scholar] [CrossRef]
- Chu, C.-C.; Chen, S.-Y.; Chyau, C.-C.; Fu, Z.-H.; Liu, C.-C.; Duh, P.-D. Protective effect of Djulis (Chenopodium formosanum) and its bioactive compounds against carbon tetrachloride-induced liver injury, in vivo. J. Funct. Foods 2016, 26, 585–597. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Wu, P.-Y.; Lyu, J.-L.; Liu, Y.-J.; Chien, T.-Y.; Hsu, H.-C.; Wen, K.-C.; Chiang, H.-M. Fisetin regulates Nrf2 expression and the inflammation-related signaling pathway to prevent UVB-induced skin damage in hairless mice. Int. J. Mol. Sci. 2017, 18, 2118. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Kumar, V.; Singh, C.; Singh, A. Crosstalk between phytochemicals and inflammatory signaling pathways. Inflammopharmacology 2023, 31, 1117–1147. [Google Scholar] [CrossRef] [PubMed]
- Vervaeke, A.; Lamkanfi, M. MAP Kinase Signaling at the Crossroads of Inflammasome Activation. Immunol. Rev. 2025, 329, e13436. [Google Scholar] [CrossRef] [PubMed]
- Piperigkou, Z.; Kyriakopoulou, K.; Koutsakis, C.; Mastronikolis, S.; Karamanos, N.K. Key matrix remodeling enzymes: Functions and targeting in cancer. Cancers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis. 2018, 9, 880. [Google Scholar] [CrossRef]
- Bhat, A.M.; Abdullah, S.T. Abstract A013: Shikonin, a strong inhibitor of Phosphoinositide 3-Kinase (PI3K) and Mitogen-Activated Protein (MAP) Kinase pathways, induces cell death in UV-B irradiated B16F10 melanoma cells. Clin. Cancer Res. 2025, 31, A013. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ha, N.G.; Lee, W.J.; Boo, Y.C. Synthetic and Natural Agents Targeting Advanced Glycation End-Products for Skin Anti-Aging: A Comprehensive Review of Experimental and Clinical Studies. Antioxidants 2025, 14, 498. [Google Scholar] [CrossRef]
- Lin, P.; Alexander, R.A.; Liang, C.H.; Liu, C.; Lin, Y.H.; Lin, Y.H.; Chan, L.P.; Kuan, C.M. Collagen formula with Djulis for improvement of skin hydration, brightness, texture, crow’s feet, and collagen content: A double-blind, randomized, placebo-controlled trial. J. Cosmet. Dermatol. 2021, 20, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Lin, Y.-K.; Lin, Y.-H.; Lin, Y.-H.; Hu, W.-C.; Chiang, C.-F. Hydrolyzed Collagen Combined with Djulis and Green Caviar Improve Skin Condition: A Randomized, Placebo-Controlled Trial. Curr. Res. Nutr. Food Sci. J. 2021, 9, 533–541. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Lin, R.J.; Liu, C.; Tseng, Y.P.; Chan, L.P.; Liang, C.H. Djulis supplementation against oxidative stress and ultraviolet radiation-induced cell damage: The influence of antioxidant status and aging of skin in healthy subjects. J. Cosmet. Dermatol. 2021, 21, 2945–2953. [Google Scholar] [CrossRef] [PubMed]

| Plant | Extract Solvent | Cell Model | Animal Model | Anti-Aging Biological Activity | Reference |
|---|---|---|---|---|---|
| Djulis seeds | Water | Inducer: UVB Cell line: HaCaT cells (immortalized human keratinocyte cell line) | Inducer: UVB Animal: BALB/c mice |
| [70] |
| Unhulled djulis | Water | Cell line: CCD-966SK cells (human skin fibroblast cell line) | - |
| [89] |
| Unhulled djulis | Water | Inducer: UVB and CML Cell line: Hs68 cells (human skin fibroblast cell line) | - |
| [58] |
| Plant | Extract Solvent | Cell Model | Animal Model | Hepatoprotective Biological Activity | Reference |
| Djulis | Water | Inducer: t-BHP Cell line: HepG2 cells (human hepatoma cell line) | - |
| [87] |
| Djulis | Water | - | Inducer: CCl4 Animal: Wistar rats |
| [90] |
| Djulis | Water | - | Inducer: EtOH Animal: Wistar rats |
| [91] |
| Djulis bran | Water or ethanol | - | Inducer: CCl4 Animal: BALB/c mice |
| [88] |
| Plant | Extract Solvent | Cell Model | Animal Model | Anti-Obesity and Anti-Diabetic Biological Activity | Reference |
| Djulis | Ethanol | - | Inducer: High-fat diet Animal: C57BL/6 mice |
| [92] |
| Djulis | Water | Cell line: SVEC endothelial cells | Animal: Spontaneously hypertensive rats and Wistar Kyoto rats |
| [76] |
| Djulis | Ethanol | Cell line: 3T3-L1 cells (dipocyte cell line) | - |
| [75] |
| Plant | Extract Solvent | Cell Model | Animal Model | Anticarcinogenic Biological Activity | Reference |
| Djulis husk | Ethanol | Cell line: HepG2 cells (human hepatoma cell line) |
| [84] |
| Participants and Groups | Treatment | Duration | Skin Parameters | Reference |
|---|---|---|---|---|
| 50 subjects (average age 53 years) (25 subjects in collagen drink group) (25 subjects in placebo drink group) | Collagen drink (main ingredient: 12% fish collagen, 2% djulis extract, 1% green caviar) Placebo drink | 28 days | Upregulation by collagen drink:
| [104] |
| 50 subjects (35–50 years old) (25 subjects in collagen drink group) (25 subjects in placebo drink group) | Collagen drink (main ingredient: 11% fish collagen, 2% djulis extract) Placebo drink | 8 weeks | Upregulation by collagen drink:
| [103] |
| 30 subjects (35–55 years old) (15 subjects in djulis functional drink group) (15 subjects in placebo drink group) | Djulis functional drink Placebo drink | 8 weeks | Upregulation by djulis functional drink:
| [105] |
| 30 subjects | Gel formula with djulis leaf extract (0.0625% to 0.25%) | 20 min |
| [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, J.-L.; Wu, P.-Y.; Liao, H.-F.; Lee, C.-L.; Wen, K.-C.; Chang, C.-C.; Chiang, H.-M. Djulis (Chenopodium formosanum) Extract as a Promising Natural Agent Against Skin Aging. Molecules 2025, 30, 3209. https://doi.org/10.3390/molecules30153209
Lyu J-L, Wu P-Y, Liao H-F, Lee C-L, Wen K-C, Chang C-C, Chiang H-M. Djulis (Chenopodium formosanum) Extract as a Promising Natural Agent Against Skin Aging. Molecules. 2025; 30(15):3209. https://doi.org/10.3390/molecules30153209
Chicago/Turabian StyleLyu, Jia-Ling, Po-Yuan Wu, Hsiao-Fang Liao, Chia-Lin Lee, Kuo-Ching Wen, Chang-Cheng Chang, and Hsiu-Mei Chiang. 2025. "Djulis (Chenopodium formosanum) Extract as a Promising Natural Agent Against Skin Aging" Molecules 30, no. 15: 3209. https://doi.org/10.3390/molecules30153209
APA StyleLyu, J.-L., Wu, P.-Y., Liao, H.-F., Lee, C.-L., Wen, K.-C., Chang, C.-C., & Chiang, H.-M. (2025). Djulis (Chenopodium formosanum) Extract as a Promising Natural Agent Against Skin Aging. Molecules, 30(15), 3209. https://doi.org/10.3390/molecules30153209









