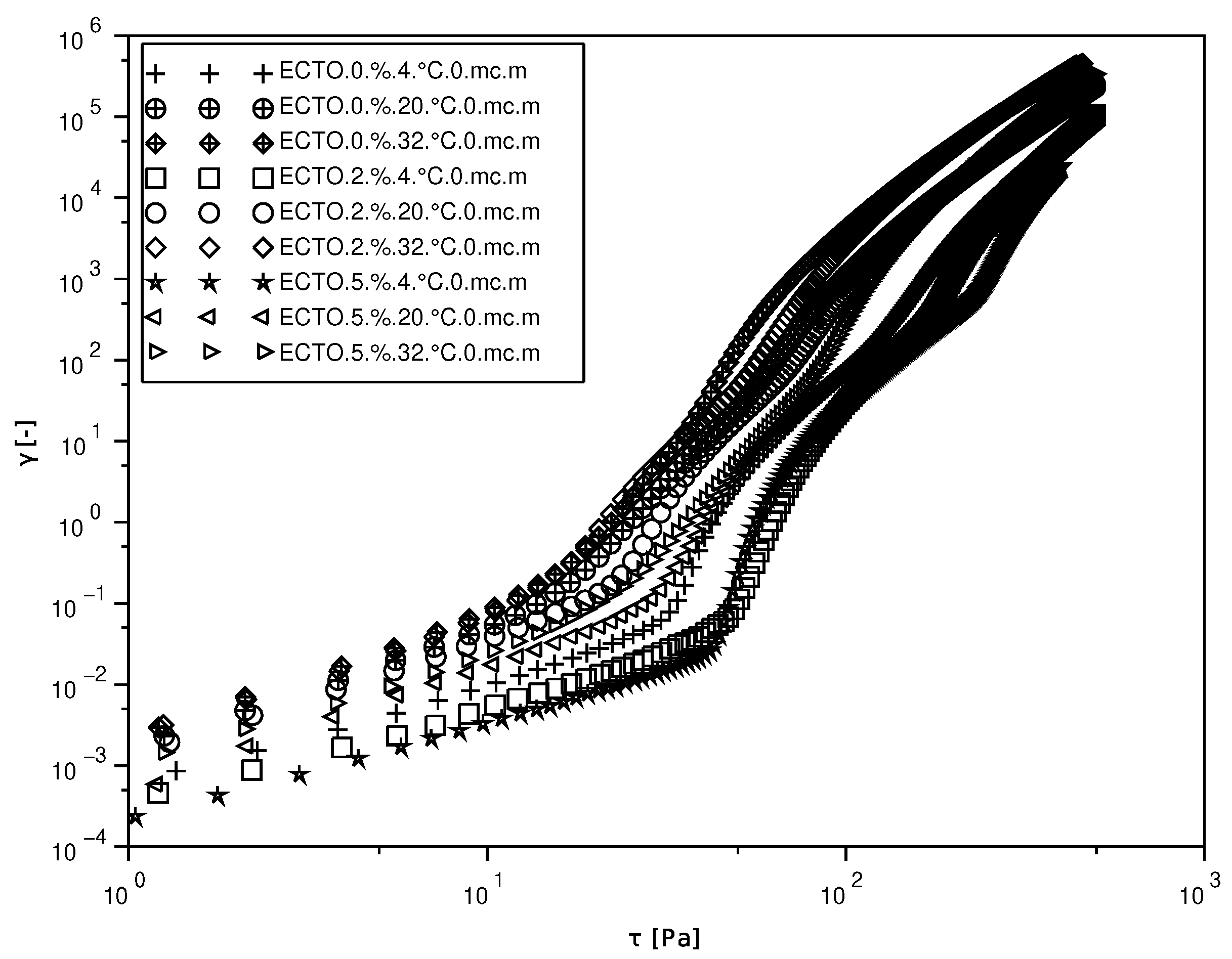
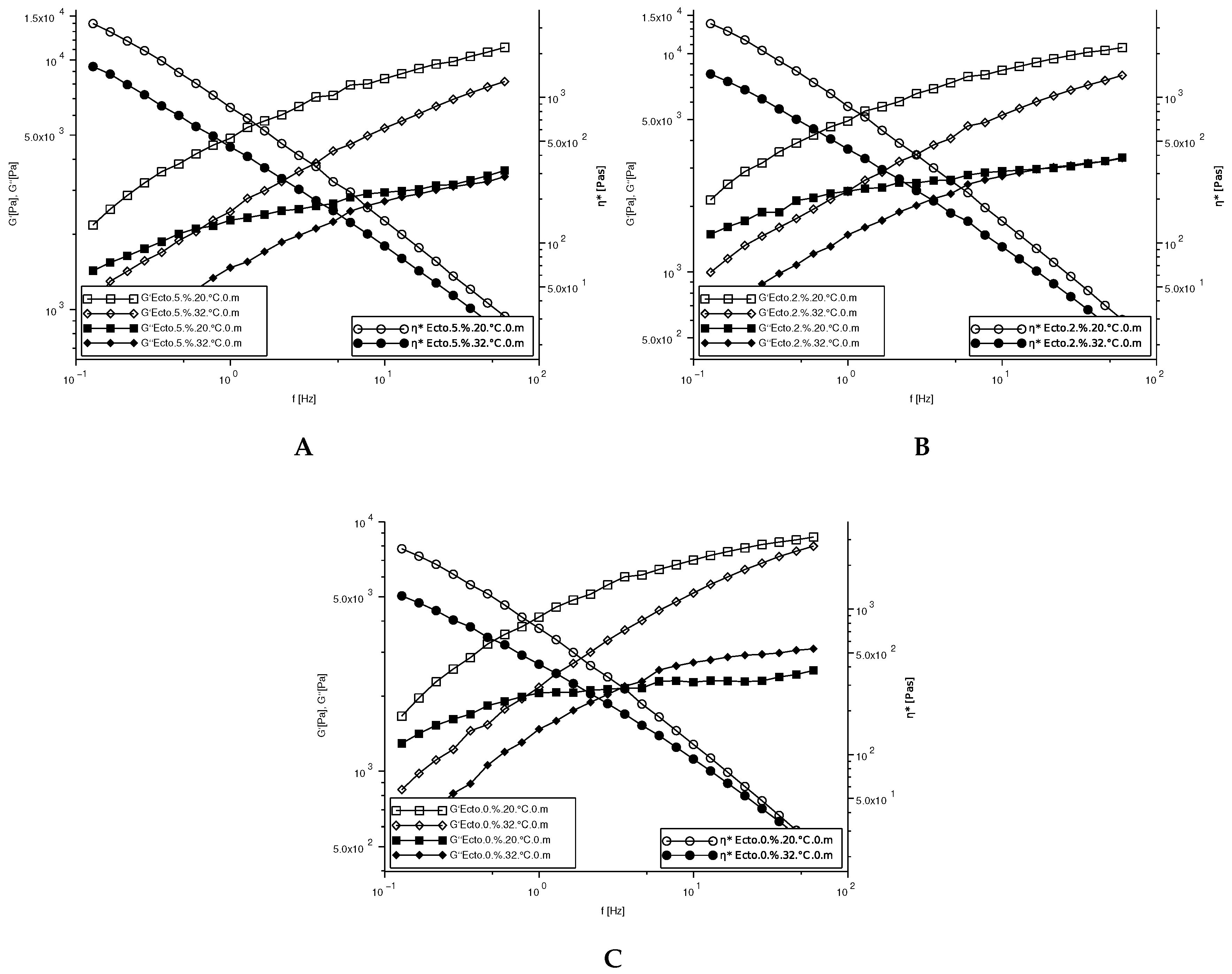
1. Introduction
The environment and lifestyle have a direct impact on the shape and condition of the human skin [
1]. Diet, environmental pollution, lack of exercise, a sedentary lifestyle, especially in dry and air-conditioned rooms, living in a hurry, stress, and sleep deprivation, as well as frequent exposure of the skin to UV radiation, trigger the generation of Reactive Oxygen Species (ROS), which negatively affect the state of our cells [
2]. The human body tries to counteract the negative effects of ROS; healthy skin cells activate defense and repair mechanisms. When cells become insufficient, it is necessary to use substances that prevent and eliminate the negative effects of ROS. Promising raw materials that support skin regeneration are compounds synthesized by extremophilic microorganisms, used by them to protect against the adverse conditions of the environment in which they live [
3]. Ectoine is a compound with such properties and it has recently been used in cosmetic formulations. Ectoine is an amino acid, 1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid (
Figure 1), naturally produced by many types of microorganisms to protect cells and their organelles from the adverse effects of the external environment [
4,
5,
6]. Microorganisms living, for example, in geysers, salt lakes, and deserts produce ectoine—which protects them from harmful factors such as high temperature, very low humidity or UV radiation. It appears commonly in aerobic, chemoheterotrophic and halophilic organisms and enables them to survive in extreme conditions [
3].
Ectoine was isolated for the first time from extremophilic, halophilic and phototrophic bacteria
Ectothiorhodospira halochloris and described by Galiński in 1985 [
7]. Ectoine belongs to osmoprotectants, which are produced when the osmotic pressure inside the cell is too high, preventing the loss of water from the cell, while not affecting the metabolism of the microorganism even at high concentrations in the cytoplasm. Ectoine not only maintains osmotic activity but also has the ability to protect biomolecules in cells, such as proteins, enzymes and membranes, against dehydration, drying, heating and freezing [
8]. The growing demand for ectoine has resulted in increased production of ectoine from microbiological sources. Many microbiological sources of ectoine and its derivatives, as well as microbiological production and fermentation methods, have been characterized. Traditional methods and new technologies for improved production and recovery of ectoine from microbial fermentation are known and used [
9]. Ectoine synthesis is initiated by microorganisms in extreme environmental conditions and is inhibited when the stress factor disappears. Ectoine is a kind of defense system for these microorganisms. Ectoine is synthesized in a similar way to other cyclic amino acids. On an industrial scale, ectoine is produced in a complex high-salt process [
10]. In [
11], a new strain of
Corynebacterium glutamicum for use in the low-salt fermentation process using well-known raw materials for the production of ectoine with industrial efficiency was proposed. In the next report [
12], an ectoine-producing
Escherichia coli variant, ET08, was constructed by introducing the ectABC gene cluster and eliminating the metabolic pathways involving lysine and pyruvate. Increasing the ectoine production of
E. coli has great industrial prospects. Ectoine has cosmotropic properties, i.e., it has the ability to bind and incorporate water molecules into complexes. Thanks to this, it helps in the reconstruction of the skin cell membrane [
13], moisturizes the skin [
14,
15], protects the lipid layers of cell membranes, and acts preventively as a filter protecting the skin against UV rays [
3,
16]. Ectoine prevents various skin diseases such as photocarcinogenesis, photodermatoses, and photoaging by protecting skin cells with its singlet oxygen-quenching properties and delaying the skin aging process, which is often associated with skin exposure to UVA radiation causing the production of singlet oxygen [
17]. Ectoine also protects DNA against ionizing radiation [
18] and has an antioxidant effect, delaying premature skin aging [
16]. Moreover, ectoine has been shown to be useful in the treatment of atopic dermatitis [
19,
20] and preventing the negative effects of radiotherapy and chemotherapy on the human body [
21], and it can also be used as a skin-whitening substance [
22]. Ectoine has an immunostimulating effect, and also has anti-inflammatory and anti-cancer properties [
23]. Ectoine has found widespread application in the cosmetics industry due to its protective and stabilizing effects on human skin cell membranes against harmful external factors such as ultraviolet (UV) radiation, wind, humidity, and drastic temperatures [
24]. In [
25] the adverse effects of stress on the skin (damage to keratinocyte proteins, loss of basement membrane proteins, and collagen degradation) were studied. These studies confirm the effectiveness of ectoine in skin regeneration and anti-aging.
Ectoine was also found to improve the dispersion and hydration of keratin bundles in corneocytes to a greater extent than hydration with water alone [
26]. The results obtained in [
27] show that ectoine can also be a good ingredient for improving the safety of cleansing cosmetics. The C
laser causes micro-damage to the skin, initiating healing processes such as inflammation, re-epithelialization, and collagen remodeling. This treatment temporarily damages the skin barrier, increases transepidermal water loss (TEWL), and induces local inflammation and oxidative stress, which may lead to discomfort, prolonged redness, and the risk of post-inflammatory hyperpigmentation (PIH). That is why we are looking for various solutions and methods to support skin regeneration processes after laser treatments. Previous methods of supporting skin regeneration include, among others, testing the effects of skincare products with a copper tripeptide complex, platelet-rich plasma, betulin-based emulsions, preparations with
Centella asiatica extracts, panthenol, and recombinant human epidermal growth factor (rhEGF). The study by [
28] assessed redness, overall improvement of wrinkles, and overall improvement of skin appearance after 12 weeks of treatment with a copper tripeptide complex (glycyl-L-histidyl-L-lysine-C
). The use of skincare products with a copper tripeptide complex after skin resurfacing treatment with a C
laser did not result in a significant reduction or elimination of post-treatment erythema. The use of platelet-rich plasma on laser-ablated skin to deliver concentrated growth factors to accelerate healing and rejuvenation, as well as to shorten patient recovery time, has also been studied with good results [
29]. Laser treatment of skin lesions using an emulsion preparation with betulin addition as an active ingredient has also been studied. In comparison to the standard treatment (dressing alone, hydrocolloid dressing) betulin-based emulsions lead to rapid regeneration of the aesthetic aspects of the skin [
30]. For skin regeneration after laser resurfacing, preparations with the
Centella asiatica extract addition are also used. Their use results in a reduction in skin redness and an improvement in the appearance of the wound. Skin moisture, TEWL, and pH did not differ between the study groups, and their values were, respectively, 34.9 × 0.02 mg/c
, 11.7 g/(h·
) and 5.4 [
31]. Another active ingredient that increases the expression of genes key to the healing processes is panthenol. Clinical studies confirm that topical application of panthenol accelerates wound healing, causing rapid re-epithelialization and restoration of skin barrier function after skin injuries [
32]. The results of comparative studies of an ointment containing 5% panthenol with petrolatum in wound treatment after laser skin treatment indicate that the ointment with panthenol addition causes faster wound healing and a higher rate of re-epithelialization. Similar results can be achieved with the use of hyaluronic acid [
33]. In [
34], the effect of a fractional C
laser in combination with recombinant human epidermal growth factor (rhEGF) on the skin was investigated. The use of rhEGF after C
laser treatment significantly improves the effectiveness of acne scar treatment, strengthens skin barrier function, and reduces inflammation. An increased water content was observed in the control group from 31 × 0.02 mg/c
to 34 × 0.02 mg/c
and in the treatment from 30 × 0.02 mg/c
to 44 × 0.02 mg/c
in the stratum corneum, and reduced pH (control group from 5.8 to 5.6, treatment from 5.8 to 5.0) and TEWL (control group from 21 to 19 g/(h·
) treatment from 22 to 16 g/(h·
)) was also observed [
34]. Unlike the above ingredients, ectoine has unique properties. As a natural extremolite, ectoine stabilizes biological membranes, protects macromolecules from oxidative stress, and modulates the inflammatory response by reducing the levels of proinflammatory cytokines such as IL-6 and TNF-
. The osmoregulatory and membrane-stabilizing effects of ectoine are particularly beneficial in restoring the integrity of the skin barrier under conditions of extreme stress, such as that induced by ablative fractional laser treatment. While other ingredients mainly focus on hydration or the stimulation of cell proliferation, ectoine offers cytoprotection, preventing structural damage to keratinocytes and maintaining homeostasis in the presence of ROS. All these properties predispose preparations containing ectoine to be used after skin fractionation treatments with a C
laser. Previous research on ectoine focused mainly on its protective effects in the context of inflammatory skin diseases, atopy, sun damage, and environmental stress. The following paper describes the effect of ectoine on skin parameters, focusing on redness, hydration, and transepidermal water loss (TEWL) after the application of a C
laser.