Microalgae: A Promising Source of Bioactive Polysaccharides for Biotechnological Applications
Abstract
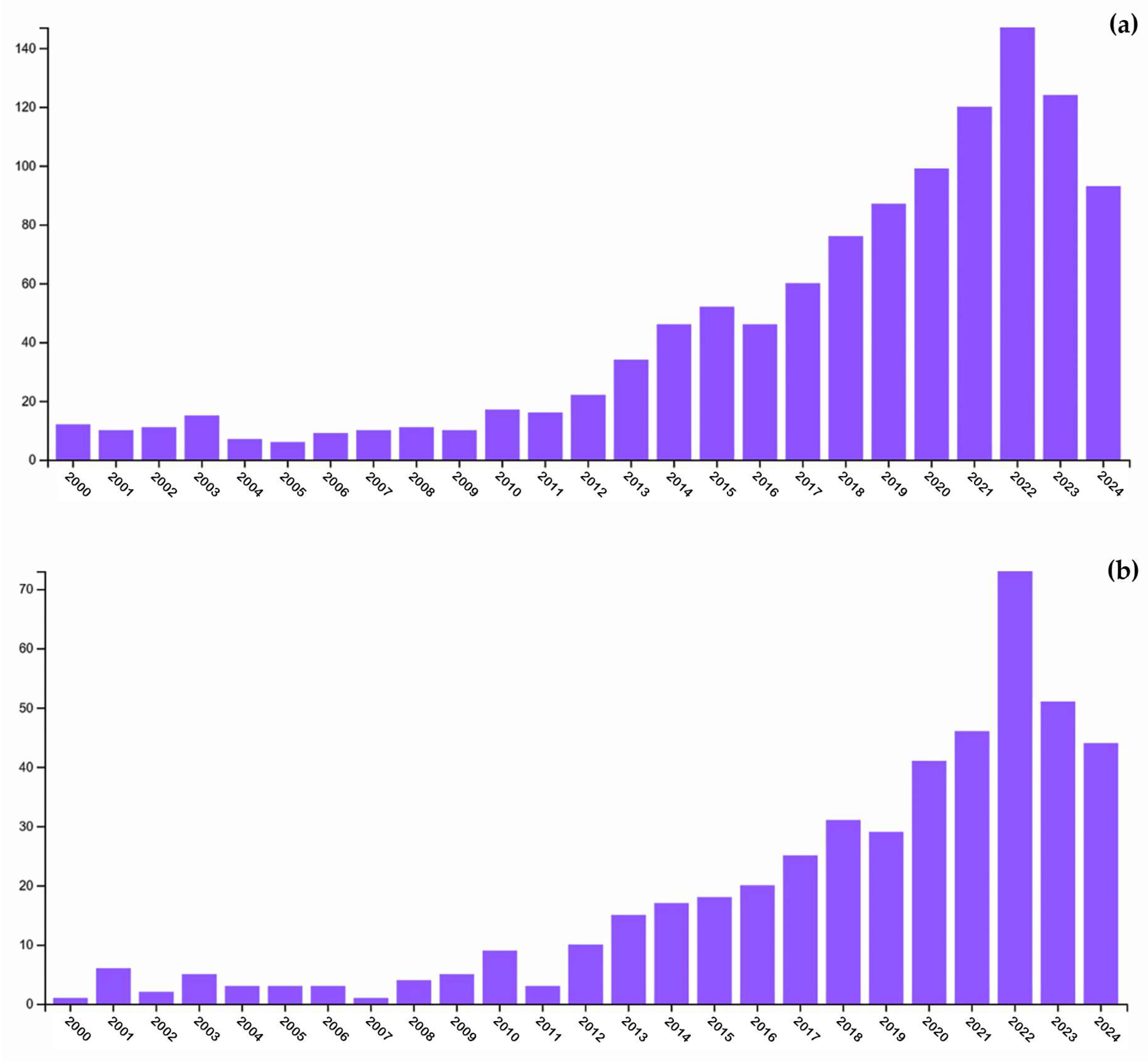
1. Introduction
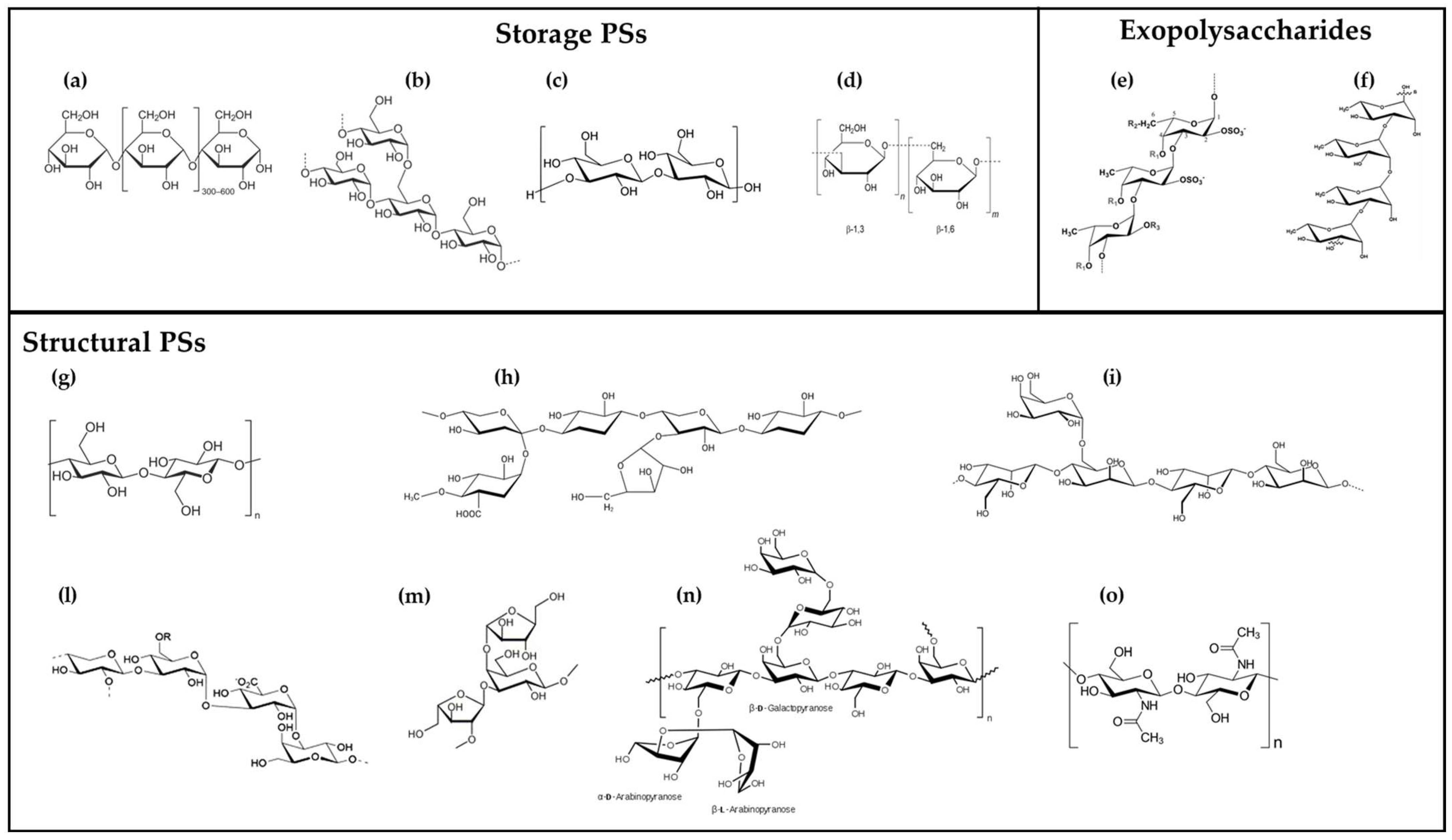
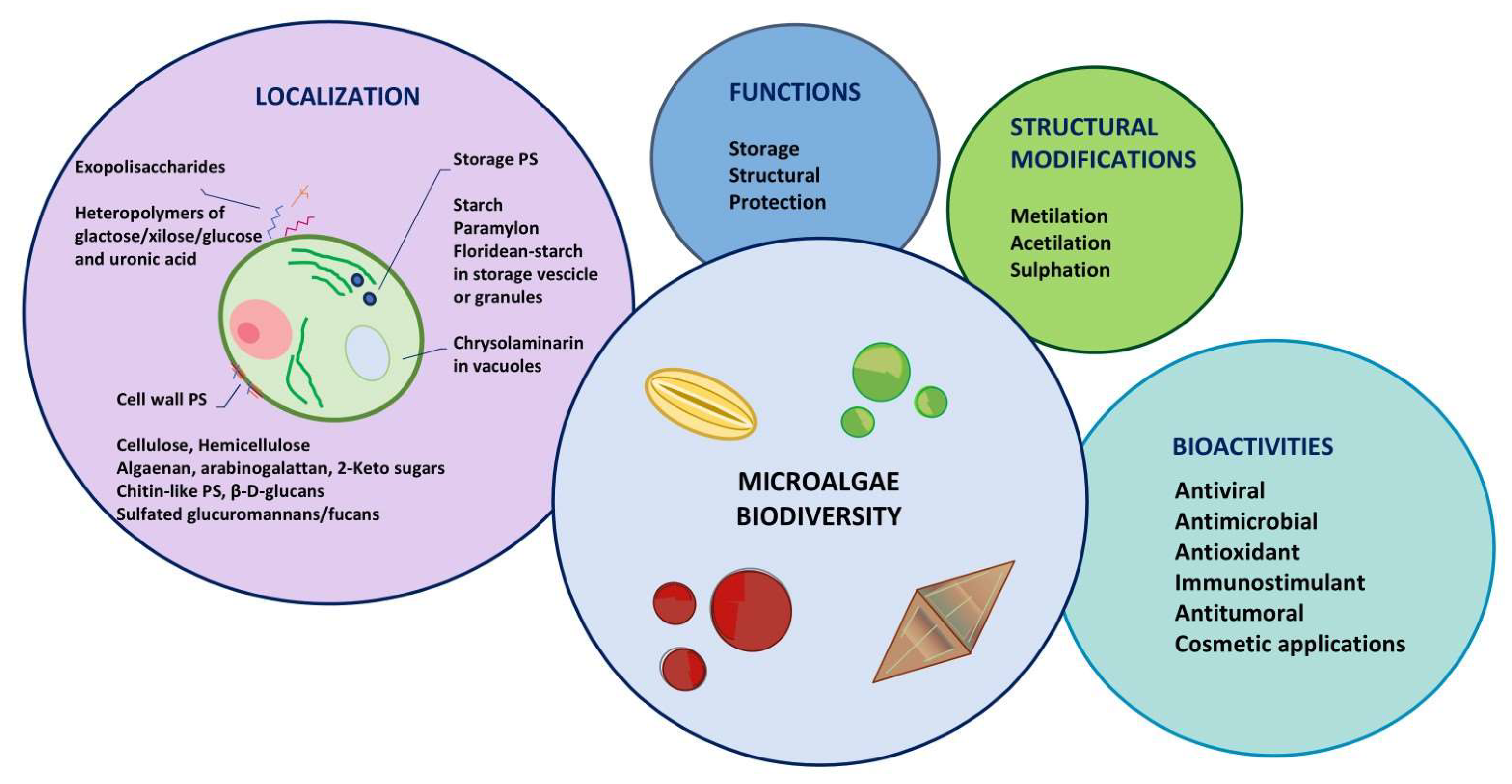
2. Microalgal Polysaccharides: Diversity and Structure
3. Factors Influencing Polysaccharide Production in Microalgae
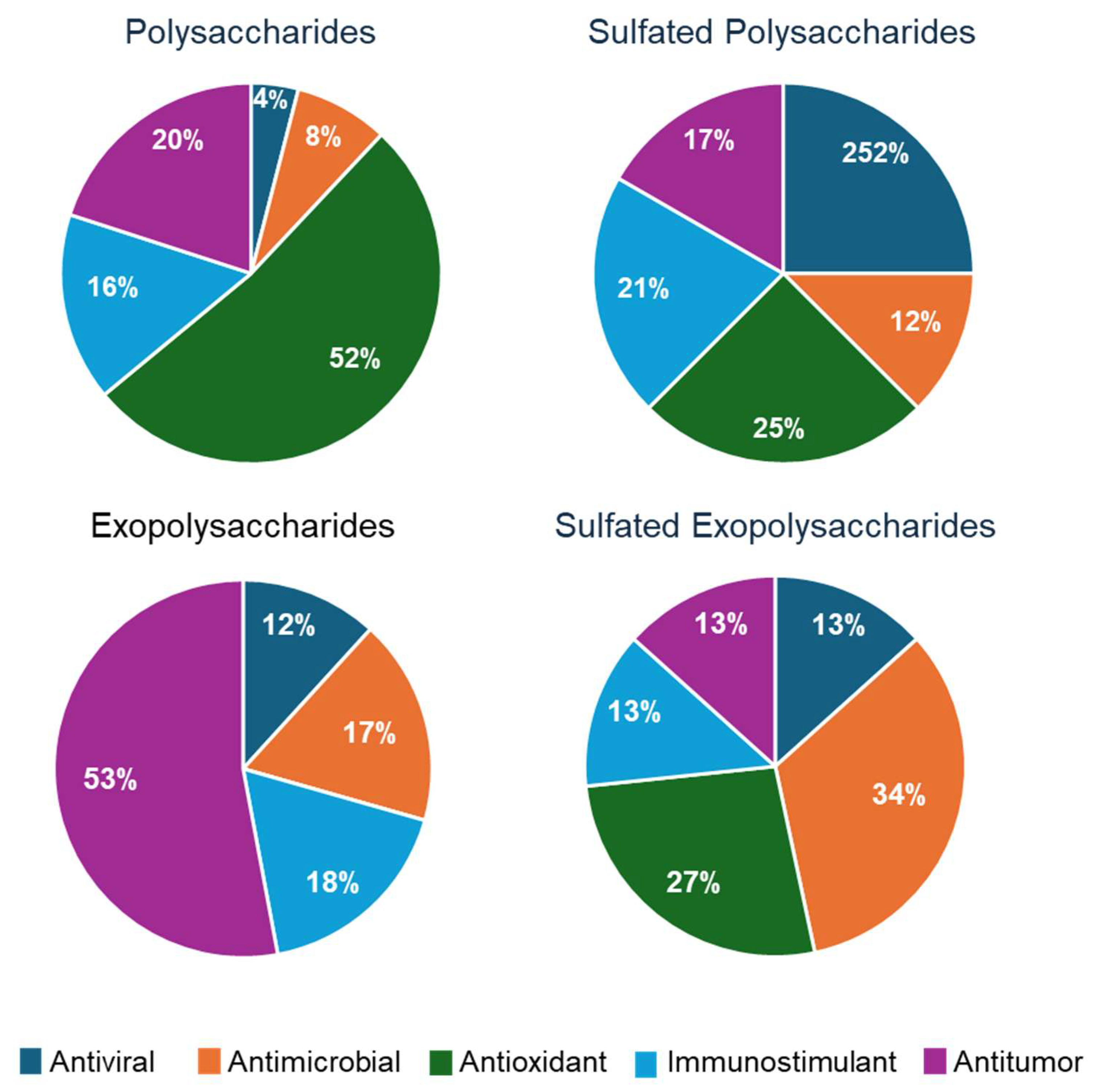
4. Biological Activities of Microalgal Polysaccharides
4.1. Antiviral Activity
4.2. Antimicrobial Activity
4.3. Antioxidant Activity
4.4. Immunomodulatory Activity
4.5. Antitumor Activity
4.6. Applications for Cosmesis
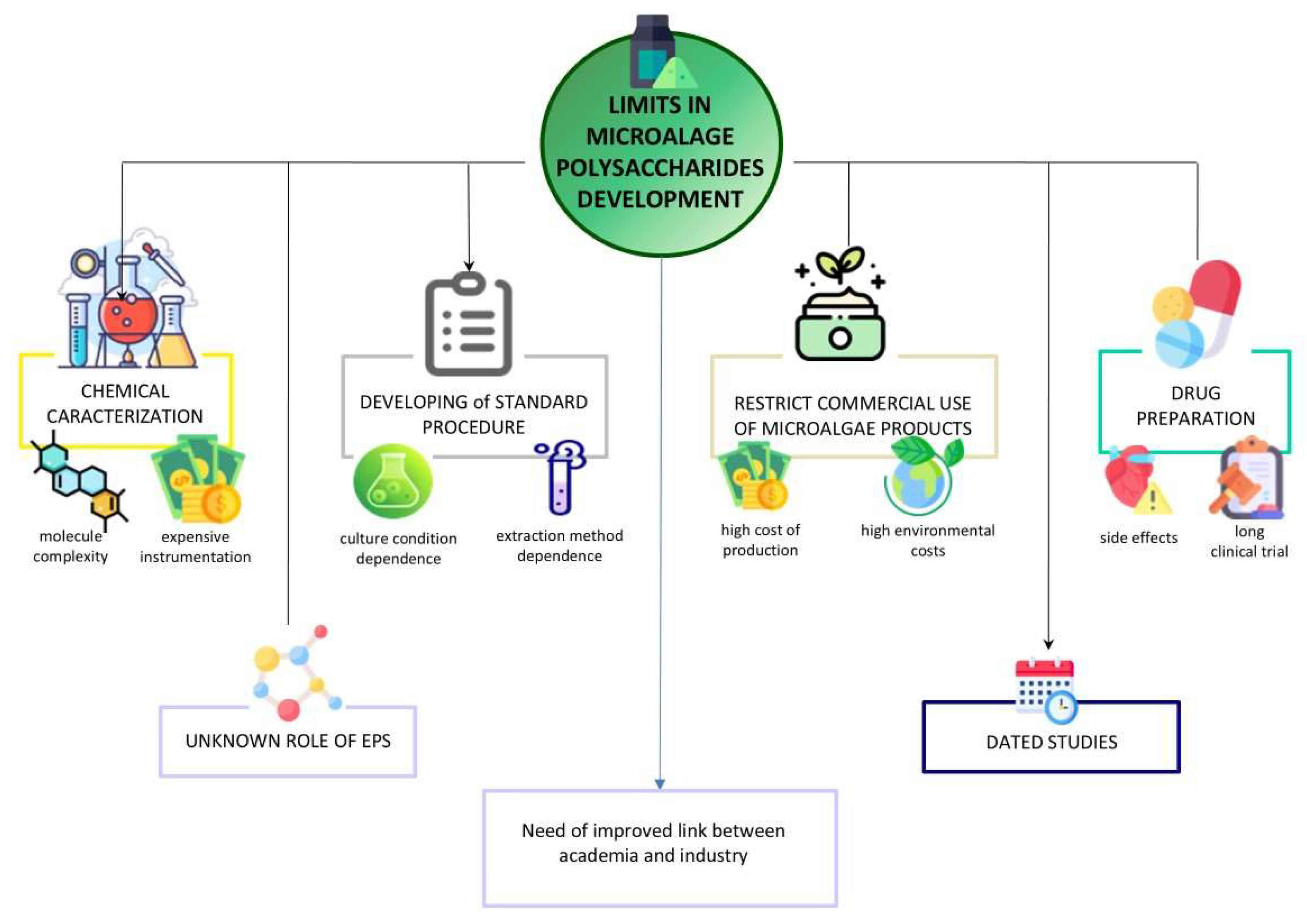
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | exopolysaccharide |
| ESI | electrospray ionization |
| DEAE | diethylaminoethanol |
| GC-MS | gas chromatography-mass spectrometry |
| HIV | human immunodeficiency virus |
| HSV | herpes simplex virus |
| LC-MS | liquid chromatography-mass spectrometry |
| MALDI | matrix-assisted laser desorption/ionization |
| MLSV | measles virus |
| PS | polysaccharide |
| RSVA | respiratory syncytial virus type A |
| SELDI | surface-enhanced laser desorption/ionization |
| SP | sulfated polysaccharide |
| S-EPS | sulfated exopolysaccharide |
| TOF | time of flight |
| VHSV | haemorrhagic septicaemia virus |
References
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, A.K.; Maldener, I. Cell–Cell Communication through Septal Junctions in Filamentous Cyanobacteria. Curr. Opin. Microbiol. 2021, 61, 35–41. [Google Scholar] [CrossRef]
- Zimmermann, S.; Lepenies, B. Glycans as Vaccine Antigens and Adjuvants: Immunological Considerations. Methods Mol. Biol. 2015, 1331, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Xu, B.W.; Li, S.S.; Ding, W.L.; Zhang, C.; Rehman, M.U.; Tareen, M.F.; Wang, L.; Huang, S.C. From Structure to Function: A Comprehensive Overview of Polysaccharide Roles and Applications. Food Front. 2025, 6, 15–39. [Google Scholar] [CrossRef]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine Fucoidans: Structural, Extraction, Biological Activities and Their Applications in the Food Industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Giji, S.; Arumugam, M. Isolation and Characterization of Hyaluronic Acid from Marine Organisms. Adv. Food Nutr. Res. 2014, 72, 61–77. [Google Scholar]
- Varki, A. Biological Roles of Glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, X.; Ma, H.; Yao, Y.; Zhang, B.; Zhao, L. Recent Progress in Marine Chondroitin Sulfate, Dermatan Sulfate, and Chondroitin Sulfate/Dermatan Sulfate Hybrid Chains as Potential Functional Foods and Therapeutic Agents. Int. J. Biol. Macromol. 2024, 262, 129969. [Google Scholar] [CrossRef]
- Das, S.; Roy, D.; Sen, R. Utilization of Chitinaceous Wastes for the Production of Chitinase. Adv. Food Nutr. Res. 2016, 78, 27–46. [Google Scholar]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10. [Google Scholar]
- Wan, M.C.; Qin, W.; Lei, C.; Li, Q.H.; Meng, M.; Fang, M.; Song, W.; Chen, J.H.; Tay, F.; Niu, L.N. Biomaterials from the Sea: Future Building Blocks for Biomedical Applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef]
- Kabir, S.F.; Rahman, A.; Yeasmin, F.; Sultana, S.; Masud, R.A.; Kanak, N.A.; Haque, P. Occurrence, Distribution, and Structure of Natural Polysaccharides. In Radiation-Processed Polysaccharides: Emerging Roles in Agriculture; Academic Press: Cambridge, MA, USA, 2021; pp. 21–27. [Google Scholar]
- Groult, H.; Cousin, R.; Chot-Plassot, C.; Maura, M.; Bridiau, N.; Piot, J.M.; Maugard, T.; Fruitier-Arnaudin, I. Λ-Carrageenan Oligosaccharides of Distinct Anti-Heparanase and Anticoagulant Activities Inhibit MDA-MB-231 Breast Cancer Cell Migration. Mar. Drugs 2019, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jiao, H.; Sun, J.; Okoye, C.O.; Zhang, H.; Li, Y.; Lu, X.; Wang, Q.; Liu, J. Structure-Activity Relationships of Bioactive Polysaccharides Extracted from Macroalgae towards Biomedical Application: A Review. Carbohydr. Polym. 2024, 324, 121533. [Google Scholar] [CrossRef]
- Mazepa, E.; Biscaia, S.M.; Bellan, D.D.; Trindade, E.D.; Simas, F.F. Structural Characteristics of Native and Chemically Sulfated Polysaccharides from Seaweed and Their Antimelanoma Effects. Carbohydr. Polym. 2022, 289, 119436. [Google Scholar] [CrossRef]
- Vega-Gómez, L.M.; Molina-Gilarranz, I.; Fontes-Candia, C.; Cebrián-Lloret, V.; Recio, I.; Martínez-Sanz, M. Production of Hybrid Protein-Polysaccharide Extracts from Ulva spp. Seaweed with Potential as Food Ingredients. Food Hydrocoll. 2024, 153, 110046. [Google Scholar] [CrossRef]
- Franco-Morgado, M.; Amador-Espejo, G.G.; Pérez-Cortés, M.; Gutiérrez-Uribe, J.A. Microalgae and Cyanobacteria Polysaccharides: Important Link for Nutrient Recycling and Revalorization of Agro-Industrial Wastewater. Appl. Food Res. 2023, 3, 100296. [Google Scholar] [CrossRef]
- Chanda, M.J.; Merghoub, N.; EL Arroussi, H. Microalgae Polysaccharides: The New Sustainable Bioactive Products for the Development of Plant Bio-Stimulants? World J. Microbiol. Biotechnol. 2019, 35, 177. [Google Scholar] [CrossRef]
- Moreira, J.B.; Vaz, B.d.S.; Cardias, B.B.; Cruz, C.G.; Almeida, A.C.A.d.; Costa, J.A.V.; de Morais, M.G. Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture. Polysaccharides 2022, 3, 441–457. [Google Scholar] [CrossRef]
- Tiwari, A.; Melchor-Martínez, E.M.; Saxena, A.; Kapoor, N.; Singh, K.J.; Saldarriaga-Hernández, S.; Parra-Saldívar, R.; Iqbal, H.M.N. Therapeutic Attributes and Applied Aspects of Biological Macromolecules (Polypeptides, Fucoxanthin, Sterols, Fatty Acids, Polysaccharides, and Polyphenols) from Diatoms—A Review. Int. J. Biol. Macromol. 2021, 171, 398–413. [Google Scholar] [CrossRef]
- Tounsi, L.; Hentati, F.; Ben Hlima, H.; Barkallah, M.; Smaoui, S.; Fendri, I.; Michaud, P.; Abdelkafi, S. Microalgae as Feedstock for Bioactive Polysaccharides. Int. J. Biol. Macromol. 2022, 221, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Deprá, M.C.; Severo, I.A.; dos Santos, A.M.; Zepka, L.Q.; Jacob-Lopes, E. Environmental Impacts on Commercial Microalgae-Based Products: Sustainability Metrics and Indicators. Algal Res. 2020, 51, 102056. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.P.; Probert, I.; Michaud, P. What Is in Store for EPS Microalgae in the next Decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The Potential of Microalgae and Their Biopolymers as Structuring Ingredients in Food: A Review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef] [PubMed]
- Le Costaouëc, T.; Unamunzaga, C.; Mantecon, L.; Helbert, W. New Structural Insights into the Cell-Wall Polysaccharide of the Diatom Phaeodactylum tricornutum. Algal Res. 2017, 26, 172–179. [Google Scholar] [CrossRef]
- Zhang, Z.; Volkman, J.K. Algaenan Structure in the Microalga Nannochloropsis oculata Characterized from Stepwise Pyrolysis. Org. Geochem. 2017, 104, 1–7. [Google Scholar] [CrossRef]
- Baudelet, P.H.; Ricochon, G.; Linder, M.; Muniglia, L. A New Insight into Cell Walls of Chlorophyta. Algal Res. 2017, 25, 333–371. [Google Scholar] [CrossRef]
- Severo, I.A.; Dias, R.R.; do Nascimento, T.C.; Deprá, M.C.; Maroneze, M.M.; Zepka, L.Q.; Jacob-Lopes, E. Microalgae-Derived Polysaccharides: Potential Building Blocks for Biomedical Applications. World J. Microbiol. Biotechnol. 2022, 38, 150. [Google Scholar] [CrossRef]
- Colusse, G.A.; Carneiro, J.; Duarte, M.E.R.; Carvalho, J.C.d.; Noseda, M.D. Advances in Microalgal Cell Wall Polysaccharides: A Review Focused on Structure, Production, and Biological Application. Crit. Rev. Biotechnol. 2022, 42, 562–577. [Google Scholar] [CrossRef]
- Koller, M.; Sarkar, N.; Mahajan, A.A.; Pathak, S.; Seth, P.; Chowdhury, A.; Ghose, I.; Das, S.; Chowdhury, R.; Bera, A.; et al. Beta-Glucans in Biotechnology: A Holistic Review with a Special Focus on Yeast. Bioengineering 2025, 12, 365. [Google Scholar] [CrossRef]
- Shao, Z.; Thomas, Y.; Hembach, L.; Xing, X.; Duan, D.; Moerschbacher, B.M.; Bulone, V.; Tirichine, L.; Bowler, C. Comparative Characterization of Putative Chitin Deacetylases from Phaeodactylum tricornutum and Thalassiosira pseudonana Highlights the Potential for Distinct Chitin-Based Metabolic Processes in Diatoms. New Phytol. 2019, 221, 1890–1905. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Vidal-Melgosa, S.; Sichert, A.; Becker, S.; Fang, Y.; Niggemann, J.; Iversen, M.H.; Cao, Y.; Hehemann, J.H. Secretion of Sulfated Fucans by Diatoms May Contribute to Marine Aggregate Formation. Limnol. Ocean. Oceanogr. 2021, 66, 3768–3782. [Google Scholar] [CrossRef]
- Miranda-Arizmendi, V.; Fimbres-Olivarria, D.; Miranda-Baeza, A.; Rascón-Chu, A.; Marquez-Escalante, J.; Lizardi-Mendoza, J.; Méndez-Encinas, M.A.; Carvajal-Millan, E. Sulfated Polysaccharides from Marine Diatoms: Insight into Molecular Characteristics and Biological Activity. AIMS Bioeng. 2024, 11, 110–129. [Google Scholar] [CrossRef]
- Synytsya, A.; Sushytskyi, L.; Saloň, I.; Babayeva, T.; Čopíková, J. Intracellular and Extracellular Carbohydrates in Microalgae. In Handbook of Food and Feed from Microalgae Production, Application, Regulation, and Sustainability; Academic Press: Cambridge, MA, USA, 2023; pp. 87–102. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Xu, B.; Xiang, W.; Li, A.; Li, T. Extraction Optimization of Polysaccharides from Wet Red Microalga Porphyridium purpureum Using Response Surface Methodology. Mar. Drugs 2024, 22, 498. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Mendonça, I.; Póvoa, I.; Carvalho, H.; Correia, A.; Vilanova, M.; Silva, T.H.; Coimbra, M.A.; Nunes, C. Impact of Growth Medium Salinity on Galactoxylan Exopolysaccharides of Porphyridium purpureum. Algal Res. 2021, 59, 102439. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wang, W.; Chen, Z.; Li, C.; Wu, H.; Wu, H.; Xiang, W. A Novel Three-Step Extraction Strategy for High-Value Products from Red Algae Porphyridium purpureum. Foods 2021, 10, 2164. [Google Scholar] [CrossRef] [PubMed]
- Casas-Arrojo, V.; Decara, J.; Arrojo-Agudo, M.d.l.Á.; Pérez-Manríquez, C.; Abdala-Díaz, R.T. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (s.f.Gray) Nägeli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef]
- Alam, M.A.; Xu, J.-L.; Wang, Z. (Eds.) Microalgae Biotechnology for Food, Health and High Value Products; Springer Singapore: Singapore, 2020; ISBN 978-981-15-0168-5. [Google Scholar]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Lind, J.L.; Heimann, K.; Miller, E.A.; Van Vliet, C.; Hoogenraad, N.J.; Wetherbee, R. Substratum Adhesion and Gliding in a Diatom Are Mediated by Extracellular Proteoglycans. Planta 1997, 203, 213–221. [Google Scholar] [CrossRef]
- Lee, R.E. Phycology, 5th ed.; Cambridge University Press: Cambridge, UK, 2018; pp. 1–535. [Google Scholar] [CrossRef]
- Borjas Esqueda, A.; Gardarin, C.; Laroche, C. Exploring the Diversity of Red Microalgae for Exopolysaccharide Production. Mar. Drugs 2022, 20, 246. [Google Scholar] [CrossRef]
- Medina-Cabrera, E.V.; Rühmann, B.; Schmid, J.; Sieber, V. Characterization and Comparison of Porphyridium sordidum and Porphyridium purpureum Concerning Growth Characteristics and Polysaccharide Production. Algal Res. 2020, 49, 101931. [Google Scholar] [CrossRef]
- Yi, Z.; Su, Y.; Brynjolfsson, S.; Olafsdóttir, K.; Fu, W. Bioactive Polysaccharides and Their Derivatives from Microalgae: Biosynthesis, Applications, and Challenges. Stud. Nat. Prod. Chem. 2021, 71, 67–85. [Google Scholar]
- Zayed, A.; Cao, H.T.T.; Trang, V.T.D.; Ulber, R. Structural Tailoring of Fucoidan Backbones for Maximizing Their Benefits: Enzymatic, Chemical, and Physical Strategies. J. Appl. Phycol. 2023, 35, 2445–2462. [Google Scholar] [CrossRef]
- Geresh, S.; Arad, S.M.; Levy-Ontman, O.; Zhang, W.; Tekoah, Y.; Glaser, R. Isolation and Characterization of Poly- and Oligosaccharides from the Red Microalga Porphyridium sp. Carbohydr. Res. 2009, 344, 343–349. [Google Scholar] [CrossRef]
- Suárez, E.R.; Kralovec, J.A.; Noseda, M.D.; Ewart, H.S.; Barrow, C.J.; Lumsden, M.D.; Grindley, T.B. Isolation, Characterization and Structural Determination of a Unique Type of Arabinogalactan from an Immunostimulatory Extract of Chlorella pyrenoidosa. Carbohydr. Res. 2005, 340, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Prybylski, N.; Toucheteau, C.; El Alaoui, H.; Bridiau, N.; Maugard, T.; Abdelkafi, S.; Fendri, I.; Delattre, C.; Dubessay, P.; Pierre, G.; et al. Bioactive Polysaccharides from Microalgae. In Handbook of Microalgae-Based Processes and Products: Fundamentals and Advances in Energy, Food, Feed, Fertilizer, and Bioactive Compounds; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; de Morais, M.G. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Stengel, D.B. Towards the Biorefinery Concept: Interaction of Light, Temperature and Nitrogen for Optimizing the Co-Production of High-Value Compounds in Porphyridium purpureum. Algal Res. 2015, 10, 152–163. [Google Scholar] [CrossRef]
- Ognistaia, A.V.; Markina, Z.V.; Orlova, T.Y. Antimicrobial Activity of Marine Microalgae. Russ. J. Mar. Biol. 2022, 48, 217–230. [Google Scholar] [CrossRef]
- You, T.; Barnett, S.M. Effect of Light Quality on Production of Extracellular Polysaccharides and Growth Rate of Porphyridium cruentum. Biochem. Eng. J. 2004, 19, 251–258. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Lei, S. Effects of Light Regime on Extracellular Polysaccharide Production by Porphyridium cruentum Cultured in Flat Plate Photobioreactors. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering, iCBBE 2008, Shanghai, China, 16–18 May 2008; pp. 1488–1491. [Google Scholar] [CrossRef]
- Kronusová, O.; Kaštánek, P.; Koyun, G.; Kaštánek, F.; Brányik, T. Factors Influencing the Production of Extracellular Polysaccharides by the Green Algae Dictyosphaerium chlorelloides and Their Isolation, Purification, and Composition. Microorganisms 2022, 10, 1473. [Google Scholar] [CrossRef]
- Nagappan, S.; Kumar, G. Investigation of Four Microalgae in Nitrogen Deficient Synthetic Wastewater for Biorefinery Based Biofuel Production. Environ. Technol. Innov. 2021, 23, 101572. [Google Scholar] [CrossRef]
- Dong, L.; Wang, F.; Chen, L.; Zhang, W. Metabolomic Analysis Reveals the Responses of Docosahexaenoic-Acid-Producing Schizochytrium under Hyposalinity Conditions. Algal Res. 2023, 70, 102987. [Google Scholar] [CrossRef]
- Shivakumar, S.; Serlini, N.; Esteves, S.M.; Miros, S.; Halim, R. Cell Walls of Lipid-Rich Microalgae: A Comprehensive Review on Characterisation, Ultrastructure, and Enzymatic Disruption. Fermentation 2024, 10, 608. [Google Scholar] [CrossRef]
- Mishra, A.; Jha, B. Isolation and Characterization of Extracellular Polymeric Substances from Micro-Algae Dunaliella salina under Salt Stress. Bioresour. Technol. 2009, 100, 3382–3386. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Moreira, J.B.; Fanka, L.S.; Kosinski, R.d.C.; de Morais, M.G. Microalgal Biotechnology Applied in Biomedicine. In Handbook of Algal Science, Technology and Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 429–439. [Google Scholar]
- Zhao, T.; Han, X.; Cao, H. Effect of Temperature on Biological Macromolecules of Three Microalgae and Application of FT-IR for Evaluating Microalgal Lipid Characterization. ACS Omega 2020, 5, 33262–33268. [Google Scholar] [CrossRef]
- Ekelhof, A.; Melkonian, M. Enhanced Extracellular Polysaccharide Production and Growth by Microalga Netrium digitus in a Porous Substrate Bioreactor. Algal Res. 2017, 28, 184–191. [Google Scholar] [CrossRef]
- Eze, C.N.; Onyejiaka, C.K.; Ihim, S.A.; Ayoka, T.O.; Aduba, C.C.; Ndukwe, J.K.; Nwaiwu, O.; Onyeaka, H. Bioactive Compounds by Microalgae and Potentials for the Management of Some Human Disease Conditions. AIMS Microbiol. 2023, 9, 55. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, W.; Fan, X.; Wen, D.; Wu, D.; Wang, H.; Liu, Z.; Li, B. Beyond Cellulose: Pharmaceutical Potential for Bioactive Plant Polysaccharides in Treating Disease and Gut Dysbiosis. Front. Microbiol. 2023, 14, 1183130. [Google Scholar] [CrossRef]
- Caetano, P.A.; do Nascimento, T.C.; Fernandes, A.S.; Nass, P.P.; Vieira, K.R.; Maróstica Junior, M.R.; Jacob-Lopes, E.; Zepka, L.Q. Microalgae-Based Polysaccharides: Insights on Production, Applications, Analysis, and Future Challenges. Biocatal. Agric. Biotechnol. 2022, 45, 102491. [Google Scholar] [CrossRef]
- Figueroa, F.A.; Abdala-Díaz, R.T.; Pérez, C.; Casas-Arrojo, V.; Nesic, A.; Tapia, C.; Durán, C.; Valdes, O.; Parra, C.; Bravo-Arrepol, G.; et al. Sulfated Polysaccharide Extracted from the Green Algae Codium bernabei: Physicochemical Characterization and Antioxidant, Anticoagulant and Antitumor Activity. Mar. Drugs 2022, 20, 458. [Google Scholar] [CrossRef]
- Kim, M.; Yim, J.H.; Kim, S.Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.K. In Vitro Inhibition of Influenza A Virus Infection by Marine Microalga-Derived Sulfated Polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Parra-Saldivar, R.; Bilal, M.; Afroze, C.A.; Ahmed, M.N.; Iqbal, H.M.N.; Xu, J. Algae-Derived Bioactive Molecules for the Potential Treatment of Sars-Cov-2. Molecules 2021, 26, 2134. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Amaro, H.; Guedes, A.; Malcata, F. Antimicrobial Activities of Microalgae: An Invited Review. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 2, 1272–1284. [Google Scholar]
- Carbone, D.A.; Pellone, P.; Lubritto, C.; Ciniglia, C. Evaluation of Microalgae Antiviral Activity and Their Bioactive Compounds. Antibiotics 2021, 10, 746. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S. Antiviral Effect of Red Microalgal Polysaccharides on Herpes simplex and Varicella zoster Viruses. J. Appl. Phycol. 2001, 13, 127–134. [Google Scholar] [CrossRef]
- Radonić, A.; Thulke, S.; Achenbach, J.; Kurth, A.; Vreemann, A.; König, T.; Walter, C.; Possinger, K.; Nitsche, A. Anionic Polysaccharides from Phototrophic Microorganisms Exhibit Antiviral Activities to Vaccinia Virus. J. Antivir. Antiretrovir. 2010, 2, 51–55. [Google Scholar] [CrossRef]
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S. Activity of Porphyridium sp. Polysaccharide against Herpes Simplex Viruses In Vitro and In Vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200. [Google Scholar] [CrossRef]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Anti-Viral Activity of Red Microalgal Polysaccharides against Retroviruses. Cancer Cell Int. 2002, 2, 8. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hirata, M.; Kuroda, E.; Suzuki, E.; Kubo, Y.; Hayashi, T. Antiviral Sulfated Polysaccharide from Navicula directa, a Diatom Collected from Deep-Sea Water in Toyama Bay. Biol. Pharm. Bull. 2006, 29, 2135–2139. [Google Scholar] [CrossRef]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In Vitro Antiviral Activities of Sulfated Polysaccharides from a Marine Microalga (Cochlodinium polykrikoides) against Human Immunodeficiency Virus and Other Enveloped Viruses. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar] [CrossRef]
- Parra-Riofrio, G.; Moreno, P.; García-Rosado, E.; Alonso, M.C.; Uribe-Tapia, E.; Abdala-Diaz, R.T.; Bejar, J. Tetraselmis suecica and Porphyridium cruentum exopolysaccharides show Anti-VHSV Activity on RTG-2 Cells. Aquac. Int. 2023, 31, 3145–3157. [Google Scholar] [CrossRef]
- Mustopa, A.Z.; Lages, A.C.; Ridwan, M.; Sukmarini, L.; Susilaningsih, D.; Hasim, H.; Delicia, D. Purification and characterization of polysaccharide from microalgae BTM 11 as inhibitor of Hepatitis C virus RNA helicase. Indones. J. Pharm. 2015, 25, 134. [Google Scholar] [CrossRef]
- Fabregas, J.; García, D.; Fernandez-Alonso, M.; Rocha, A.I.; Gómez-Puertas, P.; Escribano, J.M.; Otero, A.; Coll, J.M. In Vitro Inhibition of the Replication of Haemorrhagic Septicaemia Virus (VHSV) and African Swine Fever Virus (ASFV) by Extracts from Marine Microalgae. Antivir. Res. 1999, 44, 67–73. [Google Scholar] [CrossRef]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, C.K.; Rhie, K.T.; Lee, H.K. Antiviral Effects of Sulfated Exopolysaccharide from the Marine Microalga Gyrodinium impudicum Strain KG03. Mar. Biotechnol. 2004, 6, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; Gutiérrez-Del-río, I.; López, Y.; Redondo-Blanco, S.; Gabasa, Y.; Iglesias, M.J.; Soengas, R.; Fernández-Lorenzo, A.; López-Ibáñez, S.; Villar, C.J.; et al. Microalgae and Cyanobacteria Strains as Producers of Lipids with Antibacterial and Antibiofilm Activity. Mar. Drugs 2021, 19, 675. [Google Scholar] [CrossRef] [PubMed]
- Pina-Pérez, M.C.; Rivas, A.; Martínez, A.; Rodrigo, D. Antimicrobial Potential of Macro and Microalgae against Pathogenic and Spoilage Microorganisms in Food. Food Chem. 2017, 235, 34–44. [Google Scholar] [CrossRef] [PubMed]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 March 2025).
- Hafsa, M.B.; Ismail, M.B.; Garrab, M.; Aly, R.; Gagnon, J.; Naghmouchi, K. Antimicrobial, Antioxidant, Cytotoxic and Anticholinesterase Activities of Water-Soluble Polysaccharides Extracted from Microalgae Isochrysis Galbana and Nannochloropsis Oculata. J. Serbian Chem. Soc. 2017, 82, 509–522. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Influence of Sulphate on the Composition and Antibacterial and Antiviral Properties of the Exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and Antifungal Activities of Selected Microalgae and Cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Murillo, M.A.; Ascencio, F. Anti-Adhesive Activity of Sulphated Exopolysaccharides of Microalgae on Attachment of Red Sore Disease-Associated Bacteria and Helicobacter Pylori to Tissue Culture Cells. Lett. Appl. Microbiol. 2000, 30, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Netanel Liberman, G.; Ochbaum, G.; Bitton, R.; Arad, S.M. Antimicrobial Hydrogels Composed of Chitosan and Sulfated Polysaccharides of Red Microalgae. Polymer 2021, 215, 123353. [Google Scholar] [CrossRef]
- Kralovec, J.A. Fractions of Chlorella Extract Containing Polysaccharide Having Immunomodulating Properties 2003. Patent n. US09925953. Available online: https://patents.google.com/patent/US6551596B2/en (accessed on 26 April 2025).
- Navarro Gallón, S.M.; Alpaslan, E.; Wang, M.; Larese-Casanova, P.; Londoño, M.E.; Atehortúa, L.; Pavón, J.J.; Webster, T.J. Characterization and Study of the Antibacterial Mechanisms of Silver Nanoparticles Prepared with Microalgal Exopolysaccharides. Mater. Sci. Eng. C 2019, 99, 685–695. [Google Scholar] [CrossRef]
- Borase, H.P.; Patil, C.D.; Suryawanshi, R.K.; Koli, S.H.; Mohite, B.V.; Benelli, G.; Patil, S.V. Mechanistic Approach for Fabrication of Gold Nanoparticles by Nitzschia Diatom and Their Antibacterial Activity. Bioprocess. Biosyst. Eng. 2017, 40, 1437–1446. [Google Scholar] [CrossRef]
- Amna Kashif, S.; Hwang, Y.J.; Park, J.K. Potent Biomedical Applications of Isolated Polysaccharides from Marine Microalgae Tetraselmis Species. Bioprocess. Biosyst. Eng. 2018, 41, 1611–1620. [Google Scholar] [CrossRef]
- Pointcheval, M.; Massé, A.; Floc’hlay, D.; Chanonat, F.; Estival, J.; Durand, M.-J. Antimicrobial Properties of Selected Microalgae Exopolysaccharide-Enriched Extracts: Influence of Antimicrobial Assays and Targeted Microorganisms. Front. Microbiol. 2025, 16, 1536185. [Google Scholar] [CrossRef]
- Vishwakarma, J.; Vavilala, S.L. Evaluating the Antibacterial and Antibiofilm Potential of Sulphated Polysaccharides Extracted from Green Algae Chlamydomonas reinhardtii. J. Appl. Microbiol. 2019, 127, 1004–1017. [Google Scholar] [CrossRef]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; El Alaoui, H.; Diogon, M. Antimicrosporidian Activity of Sulphated Polysaccharides from Algae and Their Potential to Control Honeybee Nosemosis. Carbohydr. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef]
- Manlusoc, J.K.T.; Hsieh, C.L.; Hsieh, C.Y.; Salac, E.S.N.; Lee, Y.T.; Tsai, P.W. Pharmacologic Application Potentials of Sulfated Polysaccharide from Marine Algae. Polymers 2019, 11, 1163. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Pierre, G.; Michaud, P.; Delattre, C. Marine Bacteria versus Microalgae: Who Is the Best for Biotechnological Production of Bioactive Compounds with Antioxidant Properties and Other Biological Applications? Mar. Drugs 2020, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Mousavian, Z.; Safavi, M.; Azizmohseni, F.; Hadizadeh, M.; Mirdamadi, S. Characterization, Antioxidant and Anticoagulant Properties of Exopolysaccharide from Marine Microalgae. AMB Express 2022, 12, 27. [Google Scholar] [CrossRef]
- Fimbres-Olivarria, D.; Carvajal-Millan, E.; Lopez-Elias, J.A.; Martinez-Robinson, K.G.; Miranda-Baeza, A.; Martinez-Cordova, L.R.; Enriquez-Ocaña, F.; Valdez-Holguin, J.E. Chemical Characterization and Antioxidant Activity of Sulfated Polysaccharides from Navicula Sp. Food Hydrocoll. 2018, 75, e15189. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, C.; Wu, Y.; Sun, Q.; Ma, M.; Xie, Z.; Sun, R.; Pei, H. Extraction, Structural Characterization, and Antioxidant Activity of Polysaccharides from Three Microalgae. Sci. Total Environ. 2024, 931, 172567. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; Van-Moppes, D.; Grossman, S.; Arad, S. Antioxidant Activity of the Polysaccharide of the Red Microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- González, M.E.; Carrasco, L. Animal Viruses Promote the Entry of Polysaccharides with Antiviral Activity into Cells. Biochem. Biophys. Res. Commun. 1987, 146, 1303–1310. [Google Scholar] [CrossRef]
- Affan, A.; Karawita, R.; Jeon, Y.J.; Lee, J.B. Growth Characteristics and Antioxidant Properties of the Benthic Diatom Navicula Incerta (Bacillariophyceae) from Jeju Island, Korea. J. Phycol. 2007, 43, 823–832. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Li, A.; Xiong, J.; Ao, Z.; Zhang, C. Preliminary Characterization, Antioxidant Properties and Production of Chrysolaminarin from Marine Diatom Odontella Aurita. Mar. Drugs 2014, 12, 4883. [Google Scholar] [CrossRef]
- Yi, R.; Deng, L.; Mu, J.; Li, C.; Tan, F.; Zhao, X. The Impact of Antarctic Ice Microalgae Polysaccharides on D-Galactose-Induced Oxidative Damage in Mice. Front. Nutr. 2021, 8, 651088. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Li, X.; Liu, D.; Gao, X.; Chen, Y.; Chen, Z.; Fu, C.; Lin, L.; Liu, B.; Zhao, C. Physicochemical Characterization and Antioxidant Effects of Green Microalga Chlorella Pyrenoidosa Polysaccharide by Regulation of MicroRNAs and Gut Microbiota in Caenorhabditis elegans. Int. J. Biol. Macromol. 2021, 168, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Liu, X.Y.; Xiao, Z.; Huang, Y.F.; Liu, B. Antioxidant Activities of Polysaccharides Obtained from Chlorella Pyrenoidosa via Different Ethanol Concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar] [CrossRef]
- Song, H.; He, M.; Gu, C.; Wei, D.; Liang, Y.; Yan, J.; Wang, C. Extraction Optimization, Purification, Antioxidant Activity, and Preliminary Structural Characterization of Crude Polysaccharide from an Arctic Chlorella sp. Polymers 2018, 10, 292. [Google Scholar] [CrossRef]
- Gui, J.; Tong, W.; Huang, S.; Liang, X.; Fang, Z.; Wang, W.; Zhang, Y. Effects of Chlorella Vulgaris Polysaccharides Accumulation on Growth Characteristics of Trachemys Scripta Elegans. Int. J. Biol. Macromol. 2019, 141, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, Z.; Ma, S.; Rasool, M.A.; Wang, L.; Zhang, J. Optimization of Ultrasonic-Assisted Extraction, Refinement and Characterization of Water-Soluble Polysaccharide from Dictyosphaerium sp. and Evaluation of Antioxidant Activity In Vitro. J. Food Meas. Charact. 2020, 14, 963–977. [Google Scholar] [CrossRef]
- Singab, A.N.; Ibrahim, N.; Elsayed, A.E.; El-Senousy, W.; Aly, H.; Abd Elsamiae, A.; Matloub, A. Antiviral, Cytotoxic, Antioxidant and Anti-Cholinesterase Activities of Polysaccharides Isolated from Microalgae Spirulina platensis, Scenedesmus obliquus and Dunaliella salina. Arch. Pharm. Sci. Ain Shams Univ. 2018, 2, 121–137. [Google Scholar] [CrossRef]
- Balavigneswaran, C.K.; Sujin Jeba Kumar, T.; Moses Packiaraj, R.; Veeraraj, A.; Prakash, S. Anti-Oxidant Activity of Polysaccharides Extracted from Isochrysis galbana Using RSM Optimized Conditions. Int. J. Biol. Macromol. 2013, 60, 100–108. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The Isolation and Antioxidant Activity of Polysaccharides from the Marine Microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Li, J.; Liu, H. Characterization and Antioxidant Activities of Degraded Polysaccharides from Two Marine Chrysophyta. Food Chem. 2014, 160, 1–7. [Google Scholar] [CrossRef]
- Chen, B.; You, W.; Huang, J.; Yu, Y.; Chen, W. Isolation and Antioxidant Property of the Extracellular Polysaccharide from Rhodella reticulata. World J. Microbiol. Biotechnol. 2010, 26, 833–840. [Google Scholar] [CrossRef]
- Kamble, P.; Cheriyamundath, S.; Lopus, M.; Sirisha, V.L. Chemical Characteristics, Antioxidant and Anticancer Potential of Sulfated Polysaccharides from Chlamydomonas reinhardtii. J. Appl. Phycol. 2018, 30, 1641–1653. [Google Scholar] [CrossRef]
- Li, S.; Guo, W.; Zhang, M.; Zeng, M.; Wu, H. Microalgae Polysaccharides Exert Antioxidant and Anti-Inflammatory Protective Effects on Human Intestinal Epithelial Cells in Vitro and Dextran Sodium Sulfate-Induced Mouse Colitis in Vivo. Int. J. Biol. Macromol. 2024, 254, 127811. [Google Scholar] [CrossRef]
- Yu, M.; Chen, M.; Gui, J.; Huang, S.; Liu, Y.; Shentu, H.; He, J.; Fang, Z.; Wang, W.; Zhang, Y. Preparation of Chlorella vulgaris Polysaccharides and Their Antioxidant Activity In Vitro and In Vivo. Int. J. Biol. Macromol. 2019, 137, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dai, L.; Chen, Z.; Li, T.; Wu, J.; Wu, H.; Wu, H.; Xiang, W. Extraction Optimization, Physicochemical Characterization, and Antioxidant Activity of Polysaccharides from Rhodosorus sp. SCSIO-45730. J. Appl. Phycol. 2022, 34, 285–299. [Google Scholar] [CrossRef]
- Huo, S.; Wang, H.; Chen, J.; Hu, X.; Zan, X.; Zhang, C.; Qian, J.; Zhu, F.; Ma, H.; Elshobary, M. A Preliminary Study on Polysaccharide Extraction, Purification, and Antioxidant Properties of Sugar-Rich Filamentous Microalgae Tribonema minus. J. Appl. Phycol. 2022, 34, 2755–2767. [Google Scholar] [CrossRef]
- Wang, W.N.; Li, T.; Li, Y.; Zhang, Y.; Wu, H.L.; Xiang, W.Z.; Li, A.F. Exopolysaccharides from the Energy Microalga Strain Botryococcus braunii: Purification, Characterization, and Antioxidant Activity. Foods 2022, 11, 110. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2020, 18, 2. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and Antitumor Activities of Different-Molecular-Weight Polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Joung, H.Y.; Son, E.; Pyo, S.; Hong, K.L. Novel Sulfated Polysaccharide Derived from Red-Tide Microalga Gyrodinium impudicum Strain KG03 with Immunostimulating Activity In Vivo. Mar. Biotechnol. 2005, 7, 331–338. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, F.; He, P.L.; Zhou, W.L.; Wu, Q.L.; Xu, J.Y.; Zhou, Y.; Tang, W.; Li, X.Y.; Yang, Y.F.; et al. (5R)-5-Hydroxytriptolide (LLDT-8), a Novel Triptolide Analog Mediates Immunosuppressive Effects In Vitro and In Vivo. Int. Immunopharmacol. 2005, 5, 1895–1903. [Google Scholar] [CrossRef]
- Chen, X.; Song, L.; Wang, H.; Liu, S.; Yu, H.; Wang, X.; Li, R.; Liu, T.; Li, P. Partial Characterization, the Immune Modulation and Anticancer Activities of Sulfated Polysaccharides from Filamentous Microalgae Tribonema sp. Molecules 2019, 24, 322. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-Inflammatory and Immunomodulatory Activities of Polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Carballo, C.; Chronopoulou, E.G.; Letsiou, S.; Maya, C.; Labrou, N.E.; Infante, C.; Power, D.M.; Manchado, M. Antioxidant Capacity and Immunomodulatory Effects of a Chrysolaminarin-Enriched Extract in Senegalese sole. Fish. Shellfish Immunol. 2018, 82, 1–8. [Google Scholar] [CrossRef]
- Yang, F.; Shi, Y.; Sheng, J.; Hu, Q. In Vivo Immunomodulatory Activity of Polysaccharides Derived from Chlorella pyrenoidosa. Eur. Food Res. Technol. 2006, 224, 225–228. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Li, S.; Sun, H.; He, X.; Huang, Y.; Long, H. Transcriptome Analysis Reveals Possible Immunomodulatory Activity Mechanism of Chlorella sp. Exopolysaccharides on RAW264.7 Macrophages. Mar. Drugs 2021, 19, 217. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Liu, H.; Su, J.; Lan, C.Q.; Zhong, M.; Hu, X. Production, Isolation and Bioactive Estimation of Extracellular Polysaccharides of Green Microalga Neochloris oleoabundans. Algal Res. 2020, 48, 101883. [Google Scholar] [CrossRef]
- Park, G.T.; Go, R.E.; Lee, H.M.; Lee, G.A.; Kim, C.W.; Seo, J.W.; Hong, W.K.; Choi, K.C.; Hwang, K.A. Potential Anti-Proliferative and Immunomodulatory Effects of Marine Microalgal Exopolysaccharide on Various Human Cancer Cells and Lymphocytes In Vitro. Mar. Biotechnol. 2017, 19, 136–146. [Google Scholar] [CrossRef]
- Qi, J.; Kim, S.M. Characterization and Immunomodulatory Activities of Polysaccharides Extracted from Green Alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. [Google Scholar] [CrossRef]
- Sushytskyi, L.; Synytsya, A.; Lukáč, P.; Rajsiglová, L.; Capek, P.; Pohl, R.; Bleha, R.; Vannucci, L.E.; Smrz, D.; Čopíková, J.; et al. Immunologically Active Cell Wall Polysaccharides of Green Microalga Dictyosphaerium chlorelloides (Chlorellacea). Carbohydr. Polym. 2025, 353, 123242. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, Z.H.; Lee, C.G.; Synytsya, A.; Jo, H.S.; Kim, S.O.; Park, J.W.; Park, Y. Il Characterization and Immunostimulating Activity of a Water-Soluble Polysaccharide Isolated from Haematococcus lacustris. Biotechnol. Bioprocess. Eng. 2011, 16, 1090–1098. [Google Scholar] [CrossRef]
- Pandeirada, C.O.; Maricato, É.; Ferreira, S.S.; Correia, V.G.; Pinheiro, B.A.; Evtuguin, D.V.; Palma, A.S.; Correia, A.; Vilanova, M.; Coimbra, M.A.; et al. Structural Analysis and Potential Immunostimulatory Activity of Nannochloropsis oculata Polysaccharides. Carbohydr. Polym. 2019, 222, 114962. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating Intrinsic and Non-Intrinsic Cancer Risk Factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 28 March 2024).
- Shaikh, R.; Rizvi, A.; Pandit, S.; Desai, N.; Patil, R. Microalgae: Classification, Bioactives, Medicinal Properties, Industrial Applications, and Future Prospectives. In An Integration of Phycoremediation Processes in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 451–486. [Google Scholar] [CrossRef]
- Santaniello, G.; Nebbioso, A.; Altucci, L.; Conte, M. Recent Advancement in Anticancer Compounds from Marine Organisms: Approval, Use and Bioinformatic Approaches to Predict New Targets. Mar. Drugs 2023, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.S.; Stonik, I. Low-Molecular-Weight Metabolites from Diatoms: Structures, Biological Roles and Biosynthesis. Mar. Drugs 2015, 13, 3672–3709. [Google Scholar] [CrossRef]
- Geresh, S.; Mamontov, A.; Weinstein, J. Sulfation of Extracellular Polysaccharides of Red Microalgae: Preparation, Characterization and Properties. J. Biochem. Biophys. Methods 2002, 50, 179–187. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Minkova, K.; Gigova, L. Cancer Protective Action of Polysaccharide, Derived from Red Microalga Porphyridium cruentum—A Biological Background. Biotechnol. Biotechnol. Equip. 2009, 23, 783–787. [Google Scholar] [CrossRef]
- Yang, S.; Wan, H.; Wang, R.; Hao, D. Sulfated Polysaccharides from Phaeodactylum tricornutum: Isolation, Structural Characteristics, and Inhibiting HepG2 Growth Activity In Vitro. PeerJ 2019, 2019, e6409. [Google Scholar] [CrossRef]
- Sun, L.; Chu, J.; Sun, Z.; Chen, L. Physicochemical Properties, Immunomodulation and Antitumor Activities of Polysaccharide from Pavlova viridis. Life Sci. 2016, 144, 156–161. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Li, J.; Liu, H. Immunomodulation and Antitumor Activities of Degraded Polysaccharide from Marine Microalgae Sarcinochrysis marina Geitler. Int. Bioautomation 2013, 17, 107. [Google Scholar]
- Ishiguro, S.; Uppalapati, D.; Goldsmith, Z.; Robertson, D.; Hodge, J.; Holt, H.; Nakashima, A.; Turner, K.; Tamura, M. Exopolysaccharides Extracted from Parachlorella kessleri Inhibit Colon Carcinoma Growth in Mice via Stimulation of Host Antitumor Immune Responses. PLoS ONE 2017, 12, e0175064. [Google Scholar] [CrossRef] [PubMed]
- Sanniyasi, E.; Patrick, A.P.R.; Rajagopalan, K.; Gopal, R.K.; Damodharan, R. Characterization and in Vitro Anticancer Potential of Exopolysaccharide Extracted from a Freshwater Diatom Nitzschia palea (Kütz.) W.Sm. 1856. Sci. Rep. 2022, 12, 22114. [Google Scholar] [CrossRef]
- Umemura, K.; Yanase, K.; Suzuki, M.; Okutani, K.; Yamori, T.; Andoh, T. Inhibition of DNA Topoisomerases I and II, and Growth Inhibition of Human Cancer Cell Lines by a Marine Microalgal Polysaccharide. Biochem. Pharmacol. 2003, 66, 481–487. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Ren, Y.; Chen, F. Characterization of Exopolysaccharides Produced by Microalgae with Antitumor Activity on Human Colon Cancer Cells. Int. J. Biol. Macromol. 2019, 128, 761–767. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Chen, F. Production and Characterization of Exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with Anti-Colorectal Cancer Activity. Int. J. Biol. Macromol. 2019, 134, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xiong, L.; Zang, Y.; Tang, Z.; Shang, Z.; Zhang, J.; Jia, Z.; Huang, Y.; Ye, X.; Liu, H. Extraction Optimization and Anti-Tumor Activity of Polysaccharides from Chlamydomonas reinhardtii. Mar. Drugs 2024, 22, 356. [Google Scholar] [CrossRef]
- Sheng, J.; Yu, F.; Xin, Z.; Zhao, L.; Zhu, X.; Hu, Q. Preparation, Identification and Their Antitumor Activities in Vitro of Polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007, 105, 533–539. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grard, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, P.S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical Structure and Biological Activity of a Highly Branched (1 → 3,1 → 6)-β-D-Glucan from Isochrysis galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef]
- Castro, V.; Oliveira, R.; Dias, A.C.P. Microalgae and Cyanobacteria as Sources of Bioactive Compounds for Cosmetic Applications: A Systematic Review. Algal Res. 2023, 76, 103287. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Kim, S.K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the Cosmeceuticals Derived from Marine Organisms. Biotechnol. Bioprocess. Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria and Microalgae Bioactive Compounds in Skin-Ageing: Potential to Restore Extracellular Matrix Filling and Overcome Hyperpigmentation. J. Enzym. Inhib. Med. Chem. 2021, 36, 1829–1838. [Google Scholar] [CrossRef]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine Algae as Attractive Source to Skin Care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Bayona, K.; Navarro, G.S.M.; Lara, A.D.; Colorado, J.; Atehortúa, L.; Martínez, A. Activity of Sulfated Polysaccharides from Microalgae Porphyridium cruentum over Degenerative Mechanisms of the Skin. Int. J. Sci. Adv. Technol. 2012, 2, 85–92. [Google Scholar]
- Potter, A.; Ghibaudo, M.; Baltenneck, C. Association de Polysaccharides Sulfatés et de C-Glycoside et Leurs Utilisations. WO Patent 2014174188A1, 30 October 2014. [Google Scholar]
- Yanhui, C. Cosmetic Composition with Functions of Repairing and Strengthening Skin Barrier and Application. CN Patent 107412042A, 12 August 2017. [Google Scholar]
- Potter, A.; Thibaut, S.; Ribaut, C. Utilisation De Polysaccharides Sulfatés Comme Agent Antipelliculaire. WO Patent 2013093307A1, 27 June 2013. [Google Scholar]
- Tamaru, Y.; Takani, Y.; Yoshida, T.; Sakamoto, T. Crucial Role of Extracellular Polysaccharides in Desiccation and Freezing Tolerance in the Terrestrial Cyanobacterium Nostoc commune. Appl. Environ. Microbiol. 2005, 71, 7327–7333. [Google Scholar] [CrossRef]
- INCIDecoder. Available online: https://incidecoder.com/ingredients/porphyridium-polysaccharide (accessed on 4 September 2024).
- Skin Active Scientific. Available online: https://skinactives.com/ (accessed on 4 September 2024).
- Algologie. Available online: https://www.algologie.com/gb/ (accessed on 4 February 2025).
- COSMILE Europe. Available online: https://cosmileeurope.eu/inci/detail/12584/porphyridium-polysaccharide/; (accessed on 4 September 2024).
- Algenist. Available online: https://www.algenist.com/pages/alguronic-acid/ (accessed on 4 September 2024).
- Coragliotti, A.; Franklin, S.; Day, A.G.; Decker, S.M. Microalgal Polysaccharide Compositions. U.S. Patent 2012/0202768, 9 August 2012. [Google Scholar]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as Versatile Cellular Factories for Valued Products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Du, B.; Bian, Z.; Xu, B. Skin Health Promotion Effects of Natural Beta-Glucan Derived from Cereals and Microorganisms: A Review. Phytother. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef]
- Wang, Y.; Viennet, C.; Jeudy, A.; Fanian, F.; He, L.; Humbert, P. Assessment of the Efficacy of a New Complex Antisensitive Skin Cream. J. Cosmet. Dermatol. 2018, 17, 1101–1107. [Google Scholar] [CrossRef]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin Effects, a β-(1,3)-Glucan, on Skin Cell Inflammation and Oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Granum, E.; Kirkvold, S.; Myklestad, S.M. Cellular and Extracellular Production of Carbohydrates and Amino Acids by the Marine Diatom Skeletonema costatum: Diel Variations and Effects of N Depletion. Mar. Ecol. Prog. Ser. 2002, 242, 83–94. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Ta, Q.V.; Kim, S.K. Marine Organisms as a Therapeutic Source against Herpes simplex Virus Infection. J. Pharm. Sci. 2011, 44, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef]
- Sivasankar, P.; Seedevi, P.; Poongodi, S.; Sivakumar, M.; Murugan, T.; Sivakumar, L.; Sivakumar, K.; Balasubramanian, T. Characterization, Antimicrobial and Antioxidant Property of Exopolysaccharide Mediated Silver Nanoparticles Synthesized by Streptomyces violaceus MM72. Carbohydr. Polym. 2018, 181, 752–759. [Google Scholar] [CrossRef]
- Kang, J.; Jia, X.; Wang, N.; Xiao, M.; Song, S.; Wu, S.; Li, Z.; Wang, S.; Cui, S.W.; Guo, Q. Insights into the Structure-Bioactivity Relationships of Marine Sulfated Polysaccharides: A Review. Food Hydrocoll. 2022, 123, 107049. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Andreu, S.; Ruiz-Carpio, V.; Ripa, I.; López-Guerrero, J.A. Extracellular Polymeric Substances: Still Promising Antivirals. Viruses 2022, 14, 1337. [Google Scholar] [CrossRef]
- Kumar, D.; Kaštánek, P.; Adhikary, S.P. Exopolysaccharides from Cyanobacteria and Microalgae and Their Commercial Application. Curr. Sci. 2018, 115, 234–241. [Google Scholar] [CrossRef]
- Martínez-Ruiz, M.; Martínez-González, C.A.; Kim, D.H.; Santiesteban-Romero, B.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; Meléndez-Sánchez, E.R.; Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Sosa-Hernández, J.E.; et al. Microalgae Bioactive Compounds to Topical Applications Products—A Review. Molecules 2022, 27, 3512. [Google Scholar] [CrossRef]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic Potentials and Applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- Martínez Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.J.; Chen, C.W.; Dong, C. Di Algal Polysaccharides: Current Status and Future Prospects. Phytochem. Rev. 2023, 22, 1167–1196. [Google Scholar] [CrossRef]
- Anyaoha, K.E.; Krujatz, F.; Hodgkinson, I.; Maletz, R.; Dornack, C. Microalgae Contribution in Enhancing the Circular Economy Drive of Biochemical Conversion Systems–A Review. Carbon. Resour. Convers. 2024, 7, 100203. [Google Scholar] [CrossRef]
- Ezhumalai, G.; Arun, M.; Manavalan, A.; Rajkumar, R.; Heese, K. A Holistic Approach to Circular Bioeconomy Through the Sustainable Utilization of Microalgal Biomass for Biofuel and Other Value-Added Products. Microb. Ecol. 2024, 87, 61. [Google Scholar] [CrossRef] [PubMed]
- Ova Ozcan, D.; Ovez, B. Phaeodactylum tricornutum as a Potential Feedstock for an Integrated Biorefinery Process under Varying Cultivation Conditions. Biocatal. Agric. Biotechnol. 2022, 45, 102508. [Google Scholar] [CrossRef]
- Rumin, J.; de Oliveira Junior, R.G.; Bérard, J.B.; Picot, L. Improving Microalgae Research and Marketing in the European Atlantic Area: Analysis of Major Gaps and Barriers Limiting Sector Development. Mar. Drugs 2021, 19, 319. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Chen, C.W.; Tseng, Y.S.; Kuo, C.H.; Wu, C.H.; Dong, C. Di Advances in Micro- and Nano Bubbles Technology for Application in Biochemical Processes. Environ. Technol. Innov. 2021, 23, 101729. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, Y.F.; Prasetya, S.J.; Yu, F.Y.; Lai, S.Y.; Wang, M.Y. An Integrated Process for Enhanced Production and Purification of Fucoxanthin and Sulfated Polysaccharides in Diatom Hyalosynedra toxoneides Cultures. J. Taiwan Inst. Chem. Eng. 2024, 155, 105308. [Google Scholar] [CrossRef]
- Maliki, I.M.; Misson, M.; Teoh, P.L.; Rodrigues, K.F.; Yong, W.T.L. Production of Lectins from Marine Algae: Current Status, Challenges, and Opportunities for Non-Destructive Extraction. Mar. Drugs 2022, 20, 102. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.G.; Lembo, D.; Cravotto, G. Effect of Different Non-Conventional Extraction Methods on the Antibacterial and Antiviral Activity of Fucoidans Extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef]
- Babich, O.; Ivanova, S.; Tupitsyn, A.; Vladimirov, A.; Nikolaeva, E.; Tiwari, A.; Budenkova, E.; Kashirskikh, E.; Anokhova, V.; Michaud, P.; et al. Study of the Polysaccharide Production by the Microalgae C-1509 Nannochloris sp. Naumann. Biotechnol. Rep. 2023, 40, e00818. [Google Scholar] [CrossRef]
- Valeriano González, M.T.; Orta Ledesma, M.T.; Velasquez-Orta, S.B.; Monje Ramírez, I. Harvesting Microalgae Using Ozone-Air Flotation for Recovery of Biomass, Lipids, Carbohydrates, and Proteins. Environ. Technol. 2021, 42, 3267–3277. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Kim, Y.S.; Jeon, Y.J.; Kim, S.K. Enzyme-Assisted Extraction of Bioactive Compounds from Seaweeds and Microalgae. TrAC Trends Anal. Chem. 2023, 167, 117266. [Google Scholar] [CrossRef]
- Gerken, H.G.; Donohoe, B.; Knoshaug, E.P. Enzymatic Cell Wall Degradation of Chlorella vulgaris and Other Microalgae for Biofuels Production. Planta 2013, 237, 239–253. [Google Scholar] [CrossRef]
- Maffei, G.; Bracciale, M.P.; Broggi, A.; Zuorro, A.; Santarelli, M.L.; Lavecchia, R. Effect of an Enzymatic Treatment with Cellulase and Mannanase on the Structural Properties of Nannochloropsis Microalgae. Bioresour. Technol. 2018, 249, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.; Wilken, L.R. Green Microalgae Biomolecule Separations and Recovery. Bioresour. Bioprocess. 2018, 5, 14. [Google Scholar] [CrossRef]
- Sierra, L.S.; Dixon, C.K.; Wilken, L.R. Enzymatic Cell Disruption of the Microalgae Chlamydomonas reinhardtii for Lipid and Protein Extraction. Algal Res. 2017, 25, 149–159. [Google Scholar] [CrossRef]
- Peng, H.; Xv, X.; Cui, X.; Fu, Y.; Zhang, S.; Wang, G.; Chen, X.; Song, W. Physicochemical Characterization and Antioxidant Activity of Polysaccharides from Chlorella sp. by Microwave-Assisted Enzymatic Extraction. Front. Bioeng. Biotechnol. 2023, 11, 1264641. [Google Scholar] [CrossRef]
- Gurpilhares, D.d.B.; Cinelli, L.P.; Simas, N.K.; Pessoa, A.; Sette, L.D. Marine Prebiotics: Polysaccharides and Oligosaccharides Obtained by Using Microbial Enzymes. Food Chem. 2019, 280, 175–186. [Google Scholar] [CrossRef]
- Leal, B.E.S.; Prado, M.R.; Grzybowski, A.; Tiboni, M.; Koop, H.S.; Scremin, L.B.; Sakuma, A.C.; Takamatsu, A.A.; Santos, A.F.d.; Cavalcanti, V.F.; et al. Potential Prebiotic Oligosaccharides from Aqueous Thermopressurized Phosphoric Acid Hydrolysates of Microalgae Used in Treatment of Gaseous Steakhouse Waste. Algal Res. 2017, 24, 138–147. [Google Scholar] [CrossRef]
- Kwon, O.M.; Kim, D.H.; Kim, S.K.; Jeong, G.T. Production of Sugars from Macro-Algae Gracilaria verrucosa Using Combined Process of Citric Acid-Catalyzed Pretreatment and Enzymatic Hydrolysis. Algal Res. 2016, 13, 293–297. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Lens, P.N.L. Green Extraction and Esterification of Marine Polysaccharide (Ulvan) from Green Macroalgae Ulva sp. Using Citric Acid for Hydrogel Preparation. J. Clean. Prod. 2022, 366, 132952. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed Electric Field (PEF): Avant-Garde Extraction Escalation Technology in Food Industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Yang, P.; Lu, M.; Zhao, J.; Rohani, E.R.; Han, R.; Yu, N. Efficient Separation of Proteins and Polysaccharides from Dendrobium huoshanense Using Aqueous Two-Phase System with Ionic Liquids. Molecules 2022, 27, 5284. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.; Tadda, M.A.; Zhao, Y.; Farmanullah, F.; Chu, B.; Li, X.; He, Y. Microalgae Bioactive Carbohydrates as a Novel Sustainable and Eco-Friendly Source of Prebiotics: Emerging Health Functionality and Recent Technologies for Extraction and Detection. Front. Nutr. 2022, 9, 806692. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Nie, S.; Xie, M.; Li, S. Rapid Profiling Strategy for Oligosaccharides and Polysaccharides by MALDI TOF Mass Spectrometry. Food Hydrocoll. 2022, 124, 107237. [Google Scholar] [CrossRef]
- Wei, X.; Jie, D.; Cuello, J.J.; Johnson, D.J.; Qiu, Z.; He, Y. Microalgal Detection by Raman Microspectroscopy. TrAC Trends Anal. Chem. 2014, 53, 33–40. [Google Scholar] [CrossRef]
- Li, X.; Sha, J.; Chu, B.; Wei, Y.; Huang, W.; Zhou, H.; Xu, N.; He, Y. Quantitative Visualization of Intracellular Lipids Concentration in a Microalgae Cell Based on Raman Micro-Spectroscopy Coupled with Chemometrics. Sens. Actuators B Chem. 2019, 292, 7–15. [Google Scholar] [CrossRef]
- Spain, O.; Funk, C. Detailed Characterization of the Cell Wall Structure and Composition of Nordic Green Microalgae. J. Agric. Food Chem. 2022, 70, 9711–9721. [Google Scholar] [CrossRef]
- Van Norman, G.A. Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices. JACC Basic Transl. Sci. 2016, 1, 277–287. [Google Scholar] [CrossRef]
- Kelterborn, S.; Boehning, F.; Sizova, I.; Baidukova, O.; Evers, H.; Hegemann, P. Gene Editing in Green Alga Chlamydomonas reinhardtii via CRISPR-Cas9 Ribonucleoproteins. Methods Mol. Biol. 2022, 2379, 45–65. [Google Scholar] [CrossRef]
- Masi, A.; Leonelli, F.; Scognamiglio, V.; Gasperuzzo, G.; Antonacci, A.; Terzidis, M.A. Chlamydomonas Reinhardtii: A Factory of Nutraceutical and Food Supplements for Human Health. Molecules 2023, 28, 1185. [Google Scholar] [CrossRef]
- Wei, L.; Jiang, Z.; Liu, B. A CRISPR/DCas9-Based Transcription Activated System Developed in Marine Microalga Nannochloropsis oceanica. Aquaculture 2022, 546, 737064. [Google Scholar] [CrossRef]
- Kurita, T.; Iwai, M.; Moroi, K.; Okazaki, K.; Nomura, S.; Saito, F.; Maeda, S.; Takami, A.; Sakamoto, A.; Ohta, H.; et al. Genome Editing with Removable TALEN Vectors Harboring a Yeast Centromere and Autonomous Replication Sequence in Oleaginous Microalga. Sci. Rep. 2022, 12, 2480. [Google Scholar] [CrossRef] [PubMed]
- Thevarajah, B.; Piyathilleke, S.; Sahu, A.; Nimarshana, P.H.V.; Malik, A.; Ariyadasa, T.U. Microalgae-Based Bioproducts and Biomaterials Towards a Sustainable Circular Bioeconomy. In Bioeconomy for Sustainability; Springer Nature Singapore: Singapore, 2024; pp. 125–162. [Google Scholar] [CrossRef]
| Species | PS | Main Sugars | Target Virus | Active Concentration | Reference |
|---|---|---|---|---|---|
| Porphiridium cruentum Tetraselmis suecica | EPS | - | Viral Hemorrhagic Septicaemia virus (VHS) | 100 µg/mL 100 µg/mL | [80] |
| BTM 11 | PS | - | Hepatitis C-related virus | - | [81] |
| Chlorella autotrophica | SP | - | VHS African swine fever virus | 47% of inhibition of virus replication at 2–20 µg/mL 67.4% of inhibition of virus replication at 20 µg/mL | [82] |
| Cochlodinium polykrikoides | SP | mannose, galactose, glucose | HIV1, HSV, RSVA-long Parainfluenza virus | 1.7 µg/mL 4.5 µg/mL 5 2–3 µg/mL 40–26.1 µg/mL | [79] |
| Navicula directa | SP | fucose, xylose, galactose, mannose, rhamnose | Herpes simplex 1 virus Herpes simplex 2 virus Influenza A virus | CC50/IC50 1 = 270 CC50/IC50 = 510 CC50/IC50 = 32 | [78] |
| Porphyridium aerugineum | SP | - | Herpes simplex 1 virus Herpes simplex 2 virus Varicella zoster virus Moloney murine sarcoma virus Moloney murine leukemia virus | CPE50 = 100 µg/mL CPE50 = 200 µg/mL CPE50 = 100 µg/mL Ffu50 3 = 500 µg/mL RT50 4 = 200 µg/mL | [74,77] |
| Porphyridium sp. | SP | xylose, glucose, galactose | Herpes simplex 1 virus Herpes simplex 2 virus Varicella zoster virus Herpes simplex 1 virus murine and rabbit model Moloney murine sarcoma virus Moloney murine leukemia virus | CPE50 2 = 1 µg/mL CPE50 = 5 µg/mL CPE50 = 0.7 µg/mL 100 µg/mL 55% of recovered virus transforming capacity at 100 µg/mL 20% of recovered virus transforming capacity at 100 µg/mL | [74,76,77] |
| Rhodella reticulata | SP | - | Herpes simplex 1 virus Herpes simplex 2 virus Varicella zoster virus Moloney murine sarcoma virus Moloney murine leukemia virus | CPE50 = 10 µg/mL CPE50 = 20 µg/mL CPE50 = 8 µg/mL Ffu50 = 150 µg/mL RT50 = 50 µg/mL | [74,77] |
| Gyrodinium impudicum | S-EPS S-EPS | galactose galactose and uronic acid | Influenza virus type A Encephalomyocarditis virus | IC50 = 0.19–1.48 µg/mL IC50 = 26.9 µg/mL | [68,83] |
| Species | PS | Main Sugars | Microorganism Targeted | Active Concentration | Reference |
|---|---|---|---|---|---|
| Botryococcus braunii Chlorella pyrenoidosa | EPS | – | Escherichia coli Staphylococcus aureus | MIC = 7.5 μg/mL MIC = 30 μg/mL | [94] |
| Microchloropsis gaditana | EPS | – | Cladosporium cladosporioides | 500 mgGlcEq/L | [97] |
| Rhodella reticulata | EPS | – | Staphylococcus aureus | MIC = 250 μg/mL | [89] |
| Chlorella pyreionodosa | PS | glucose, galactose, rhamnose, mannose and arabinose, N-acetyl glucosamine, N-acetyl galactosamine | Candida albicans Listeria monocytogenes | 0.1 mg–4 mg orally administered in mouse model | [93] |
| Tetraselmis spp. | PS | – | Candida albicans Penicillium italic | - | [96] |
| Chlamydomonas reinhardtii | SP | - | Neisseria mucosa Escherichia coli Streptococcus sp. Bacillus subtilis | MIC = 480 μg/mL MIC = 420 μg/mL MIC = 480 μg/mL MIC = 420 μg/mL | [98] |
| Isochrysis galbana Nannochloropsis oculata | SP | xylose, mannose, galactose, glucose, mannitol | Pseudomonas aeruginosa Escherichia coli Candida krusei | 1870 µg/mL 1250 µg/mL and 2500 µg/mL 60 µg/mL and 80 µg/mL | [87] |
| Porphyridium cruentum | S-EPS | galactose, glucose | Eschierichia coli Staphylococcus aureus Salmonella enteritidis | 1% of S-EPS aqueous solution | [88] |
| Porphyridium marinum | S-EPS | – | Nosema ceranae | 100 μg/mL inhibit 90% of growth | [99] |
| Neochloris oleobundan Phaeodactylum tricornutum Tetraselmis sp. | S-EPS | – | Helicobacter pylori | 50% of bacterial adhesion inhibition | [91] |
| Species | PS | Main Sugars | Assay/Activity | Reference |
|---|---|---|---|---|
| Chlamydomonas sp. | PS | mannitol, ribose, anhydrous glucose, xylose, fucose | In vivo protection against oxidative damage in mice model | [110] |
| Chlorella pyrenoidosa | PS | mannose, rhamnose, glucuronic acid, galacturonic acid, glucose, galactose, arabinose. | In vivo evaluation of antioxidant enzymes in Caenorhabditis elegans | [111] |
| Chlorella pyrenoidosa | PS | d-arabinose, d-glucose, d-xylose, d-galactose, d-mannose, rhamnose | DPPH 1, hydroxyl and superoxide anion radicals scavenging | [112] |
| Chlorella sp. | PS | galactose, arabinose, rhamnose, glucose | DPPH | [113] |
| Chlorella vulgaris | PS | rhamnose, ribose, arabinose, xylose, 2-deoxy-D-glucose, mannose, glucose, galactose, and glucosamine | In vivo evaluation of antioxidant enzymes in Caenorhabditis elegans | [114] |
| Dictyosphaerium sp. | PS | - | DPPH scavenging activities | [115] |
| Dunaliella salina Scenedesmus obliquus | PS | galactose, mannose, glucose, rhamnose | DPPH scavenging activities | [116] |
| Isocrysis galbana | PS (lambda-carrageenan) PS | galactose mannose, glucose, galactose, rhamnose | DPPH Hydroxyl and superoxide radicals scavenging ROS scavenging activity | [117,118] |
| Odontella aurita | PS (chrysolaminarin) | glucose | hydroxyl radicals scavenging | [109] |
| Pavlova viridis Sarcinochrysis marina | PS | uronic acid sulphate groups | Radical scavenging (LPO) 2 inhibition Mouse red blood cell hemolysis assay | [119] |
| Rhodella reticulata | PS | - | scavenging activities | [120] |
| Chlamydomonas reinhardtii | SP | - | DPPH, hydroxyl radicals scavenging | [121] |
| Chlorella pyreinodosa | SP | rhamnose, glucose, glucosamine, glucuronic acid, mannose, fucose, xylose, galactose, sulphate | In vitro hydroxyl and superoxide radicals scavenging activities in human intestinal Caco-2 cells | [122] |
| Chlorella vulgaris | SP | rhamnose, arabinose, fucose, xylose, mannose, glucose, galactose, glucosamine | In vitro in DPPH-, superoxide-, hydroxyl radical-scavenging activities, metal chelating ability; In vivo in C. elegans model, oxidative stress resistance, increasing of SOD and CAT activities | [123] |
| Navicula sp. | SP | glucose, rhamnose, galactose, mannose, xylose | DPPH free radical scavenging | [104] |
| Rhodosorus sp. | SP | glucose, galactose, xylose, galacturonic acid | DPPH and ABTS 3 radical scavenging activities | [124] |
| Tribonema minus | SP | rhamnose, arabinose, xylose, mannose, glucose galactose | DPPH, superoxide, hydroxyl radicals scavenging activities | [125] |
| Botryococcus braunii | S-EPS | arabinose, fucose, glucose, galactose | ABTS, hydroxyl, superoxidea nion, DPPH radical scavenging axtivities in vitro | [126] |
| Chlorella sp. Chlorella sorokiniana Picochlorum sp. | S-EPS | sucrose, glucose glucosamine | DPPH and ABTS radical scavenging activities | [103] |
| Species | PS | Main Sugars | Immunomodulant Activity | Reference |
|---|---|---|---|---|
| Chlorella sp. | EPS | glucosamine hydrochloride glucuronic acid | production of nitric oxide (NO), TNF-α and IL-6 | [135] |
| Neochloris oleoabundans | EPS | glucose, mannose, galactose, xylose, ribose, arabinose, rhamnose | Reverse phagocytosis inhibition Enhancing lymphocyte proliferation | [136] |
| Thraustochytriidae sp. | EPS | - | Induction of B cell proliferation. Inhibition of IL-6 and INF-γ formation | [137] |
| Chlorella ellipsoidea | PS | rhamnose, mannose, galactose | Stimulation of NO and cytokine production in macrophages | [138] |
| Dictyosphaerium chlorelloides | PS | galactose, 2-O-methyl-galactse, rhamnose, mannose | Activation of NK and Tc, increase in IFN-γ expression | [139] |
| Haematococcus lacustris | PS | galactose, glucose, mannose | Stimulation of macrophage secretion of pro-inflammatory cytokine, TNF-α; enhance the expression of COX-2 and iNOS | [140] |
| Phaeodactylum tricornutum | PS | glucose (chrysolaminarin) | Increase in IL-1β, cytokine production, macrophage migration and phagocytosis activation | [133] |
| Chlorella stigmatophora Phaeodactylum tricornutum | SP | Enhancement of phagocytosis process | [132] | |
| Nannochloropsis oculata | SP | glucose, mannose, rhamnose | Stimulation of murine ß-lymphocytes | [141] |
| Tribonema sp. | SP | glucose, galactose, xylose | Increase in macrophage cell viability and the production of IL-6, IL-10, TNF-α | [131] |
| Porphyridium cruentum | SP | ribose, fucose, xylose, arabinose, mannose, galactose, glucose, glucuronic acid | Stimulation of mice white blood cell differentiation and peritoneal macrophage profile modification | [39] |
| Porphyridium cruentum | S-EPS | - | Stimulation of macrophage proliferation and nitric oxide (NO) production | [128] |
| Gyrodinium impudicum | S-EPS | galactose | In vivo stimulation of macrophages and natural killer cells; production of IL-1b, TNF-a, and NO | [129] |
| Species | PS | Main Sugars | Activity | Reference |
|---|---|---|---|---|
| Chlorella pyrenoidosa Chlorococcum sp. Scenedesmus sp. | EPS | 10 different monosaccharides | cytotoxic activity against colon cancer cell lines HCT116 and HCT8 | [155] |
| Chlorella vulgaris Chlorella zofingiensis | EPS | 10 different monosaccharides | cytotoxic activity against colon cancer cell line HCT8 | [156] |
| Gymnodinium sp. | EPS | D-galactan sulphate, associated with L-(+)-lactic acid | DNA topoisomerase I and II inhibition in K562 cells | [154] |
| Nitzschia palea | EPS | – | apoptosis induction in A549 cells | [153] |
| Parachlorella kessleri | EPS | galactose, mannose, xylose, rhamnose, arabinose | in vivo antiproliferative activity against CT26 colon carcinoma in mouse model | [152] |
| Thraustochytriidae sp. | EPS | - | anti-proliferative activity against BG-1 ovarian, MCF-7 breast, and SW-620 colon cancer cell lines | [137] |
| Chlamydomonas reinhardtii | PS | α- and β-pyranoses containing furoic acid | anti-proliferative activity against colon cancer HCT-116, liver cancer HepG-2, and cervical cancer HeLa cells | [157] |
| Chlorella pyrenoidosa | PS | rhamnose, mannose, glucose, galactose | antitumor activity against A549 | [158] |
| Dunaliella salina | PS | galactose, mannose, glucose, rhamnose | cytotoxic activity against HCT 116 cell line | [116] |
| Isochrysis galbana | PS | β-D-glucose | anti-proliferative on U937 human leukemic monocyte lymphoma cells | [159] |
| P. tricornutum | PS | xylose, fucose, glucose, glucuronic acid | blocking cell cycle and mitosis in HepG2 cells | [149] |
| Chlamydomonas reinhardtii | SP | - | anti-proliferative activity against breast cancer MDA-MB-231 cells | [121] |
| Pavlova viridis | SP | fructose, glucose, mannose, uronic acid | in vivo test in transplanted S180 mice | [150] |
| Sarcinochrysis marina | SP | arabinose, D-fructose and glucose | in vivo test in transplanted S180 mice | [151] |
| Tribonema sp. | SP | glucose, galactose, xylose | apoptosis induction, block of cell cycle and mitosis in HepG2 cells | [131] |
| Porphyridium cruentum | S-EPS | - | in vivo in transplanted S180 mice | [128] |
| Porphyridium sp. | S-EPS | - | inhibition of T-cell lymphoma line 24-1 proliferation Apoptosis induction in Graffi myeloid tumour | [147,148] |
| Species | PS | Main Sugars | Cosmetic Application | Reference |
|---|---|---|---|---|
| C. vulgaris | ESP | – | collagen repair mechanisms | [22] |
| Porphyridium sp. | ESP | – | inhibitor of hyaluronidase anti-allergic | [42] |
| Chlorella sp. | PS | – | anti-ageing | [175] |
| Porphyridium sp. | PS | β-1,3-glucans | moisturizing properties | [181,182] |
| Skeletonema costatum | PS | β-1,3-glucans | texturizing agents moisturizing properties | [180] |
| P.cruentum | SP | xylose, galactose and glucose | inhibitor of hyaluronidase and elastase | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnabosco, C.; Santaniello, G.; Romano, G. Microalgae: A Promising Source of Bioactive Polysaccharides for Biotechnological Applications. Molecules 2025, 30, 2055. https://doi.org/10.3390/molecules30092055
Magnabosco C, Santaniello G, Romano G. Microalgae: A Promising Source of Bioactive Polysaccharides for Biotechnological Applications. Molecules. 2025; 30(9):2055. https://doi.org/10.3390/molecules30092055
Chicago/Turabian StyleMagnabosco, Chiara, Giovanna Santaniello, and Giovanna Romano. 2025. "Microalgae: A Promising Source of Bioactive Polysaccharides for Biotechnological Applications" Molecules 30, no. 9: 2055. https://doi.org/10.3390/molecules30092055
APA StyleMagnabosco, C., Santaniello, G., & Romano, G. (2025). Microalgae: A Promising Source of Bioactive Polysaccharides for Biotechnological Applications. Molecules, 30(9), 2055. https://doi.org/10.3390/molecules30092055






