A Targeted Mass Spectrometric Approach to Evaluate the Anti-Inflammatory Activity of the Major Metabolites of Foeniculum vulgare Mill. Waste in Human Bronchial Epithelium
Abstract
:1. Introduction
2. Results
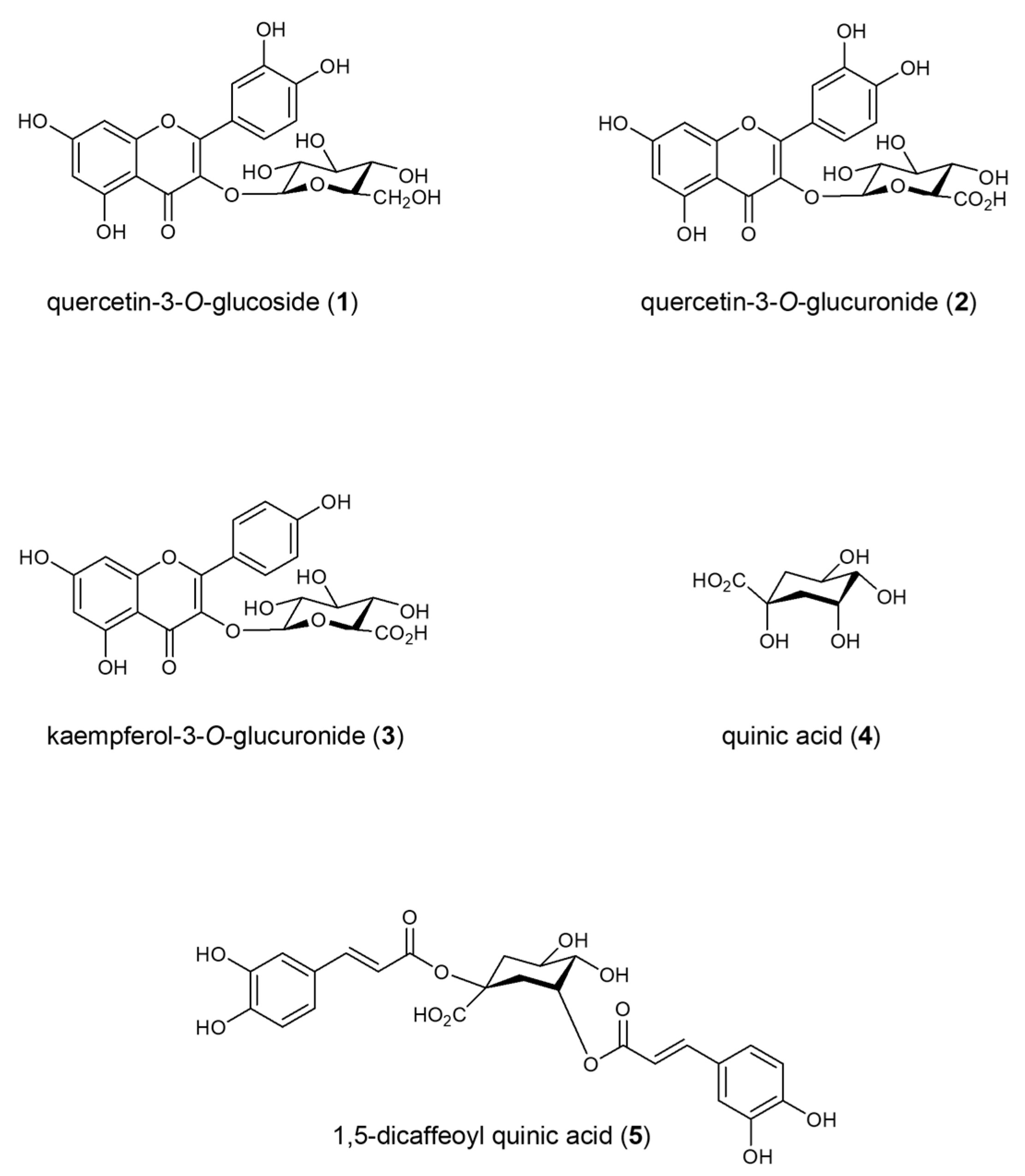
2.1. Isolation
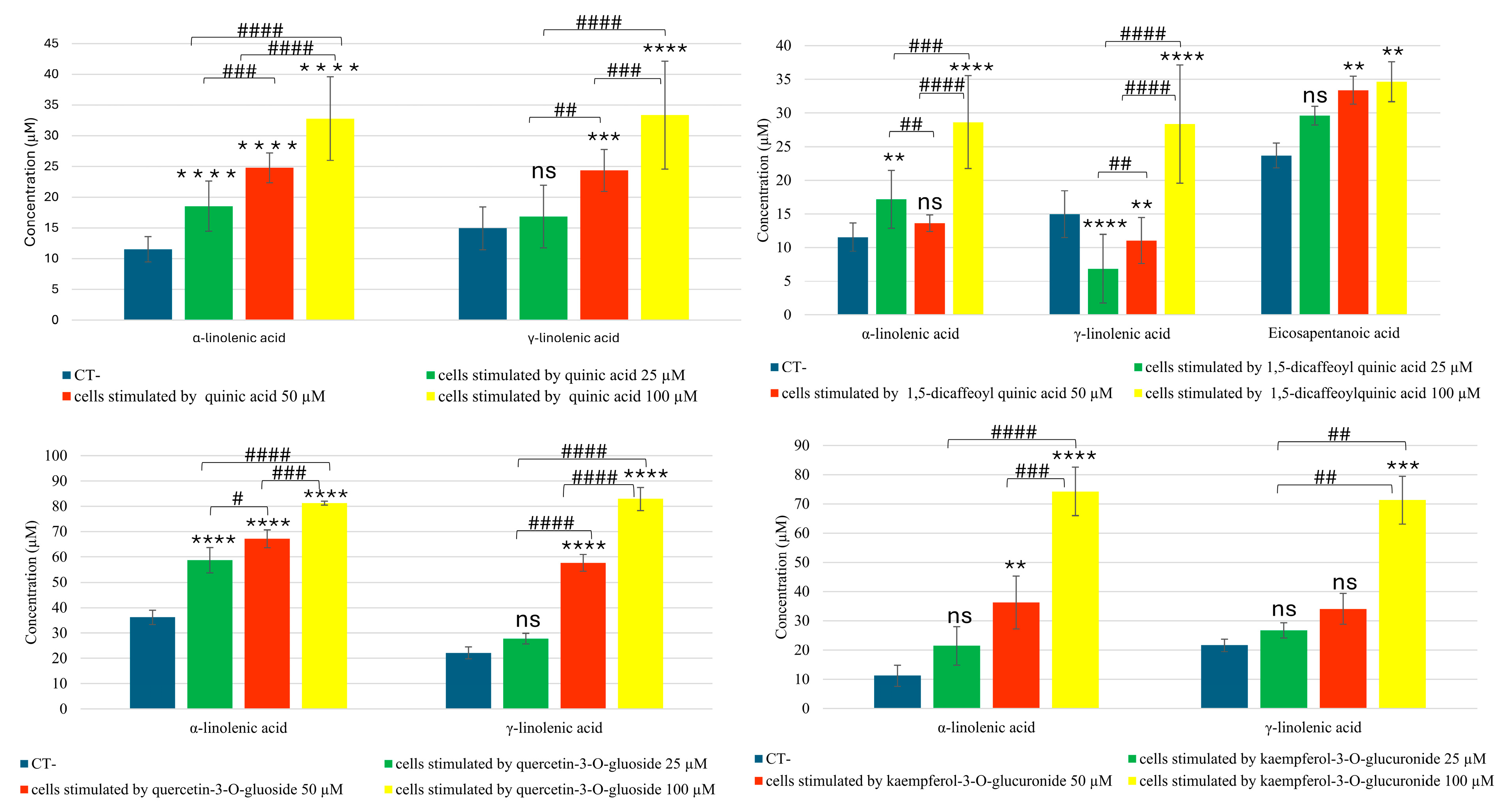
2.2. UPLC–ESI-QTRAP-MS/MS Analyses of Eicosanoids and Fatty Acids in MRM (Multiple Reaction Monitoring) Mode
2.3. UPLC–ESI-QTRAP-MS Analyses of Sphingolipids in MRM (Multiple Reaction Monitoring) Modality
3. Discussion
4. Materials and Methods
4.1. Plant Material and Isolation Procedure
4.2. Cell Experiments
4.3. Eicosanoids and Fatty Acids Extraction
4.4. UPLC–ESI-QTRAP-MS Analyses of Eicosanoids and Fatty Acids in MRM (Multiple Reaction Monitoring) Modality
4.5. Sphingolipids Extraction
4.6. UPLC–ESI-QTRAP-MS Analyses of Sphingolipids in MRM (Multiple Reaction Monitoring) Modality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catalán, Ú.; Barrubés, L.; Valls, R.M.; Solà, R.; Rubió, L. In vitro Metabolomic Approaches to Investigating the Potential Biological Effects of Phenolic Compounds: An Update. Genom. Proteom. Bioinform. 2017, 15, 236–245. [Google Scholar] [CrossRef]
- Papaioannou, K.G.; Kadi, F.; Nilsson, A. Consumption of Vegetables Is Associated with Systemic Inflammation in Older Adults. Nutrients 2022, 14, 1765. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO: Geneva, Switzerland, 2002; p. 149. [Google Scholar]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Aidhen, I.; Sudarshan, K. Convenient Synthesis of 3-Glycosylated Isocoumarins. Eur. J. Org. Chem. 2017, 2017, 34–38. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Fotoohabadi, L.; Gerasimova, Y.; Nanduri, R.; Lama Tamang, P.; Kandala, M.; Kelesidis, T. The Role of Inflammation in the Pathogenesis of Viral Respiratory Infections. Microorganisms 2024, 12, 2526. [Google Scholar] [CrossRef]
- Nicolaou, A.; Kendall, A.C. Current insights into skin lipids and their roles in cutaneous health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2022, 26, 83–90. [Google Scholar] [CrossRef]
- Khanapure, S.P.; Garvey, D.S.; Janero, D.R.; Gordon Letts, L. Eicosanoids in inflammation: Biosynthesis, pharmacology, and therapeutic frontiers. Curr. Top. Med. Chem. 2007, 7, 311–340. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. UPLC-ESI-QTRAP-MS/MS Analysis to Quantify Bioactive Compounds in Fennel (Foeniculum vulgare Mill.) Waste with Potential Anti-Inflammatory Activity. Metabolites 2022, 12, 701. [Google Scholar] [CrossRef]
- Li, X.; Jin, Q.; Yao, Q.; Xu, B.; Li, L.; Zhang, S.; Tu, C. The Flavonoid Quercetin Ameliorates Liver Inflammation and Fibrosis by Regulating Hepatic Macrophages Activation and Polarization in Mice. Front. Pharmacol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Kwon, S.H.; Nam, J.I.; Kim, S.H.; Kim, J.H.; Yoon, J.H.; Kim, K.S. Kaempferol and quercetin, essential ingredients in Ginkgo biloba extract, inhibit interleukin-1beta-induced MUC5AC gene expression in human airway epithelial cells. Phytother. Res. 2009, 23, 1708–1712. [Google Scholar] [CrossRef]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. LC-ESI/LTQOrbitrap/MS Metabolomic Analysis of Fennel Waste (Foeniculum vulgare Mill.) as a Byproduct Rich in Bioactive Compounds. Foods 2021, 10, 1891. [Google Scholar] [CrossRef]
- Pratima Tatke, S.D.a.S.Y.G. Isolation of Quercetin-3-O-β-D-Glucoside from Azadirachta indica. Am. J. Phtyomedicine Clin. Ther. 2014, 2, 870–876. [Google Scholar]
- Severino, P.; Brigida, D.A.; Monica, S.; Marialuisa, G.; Silvia, G.; Pietro, M.; Antonio, F. Antioxidant Polyphenolic Constituents of cv. ‘Isabella’ Leaves. Nat. Prod. J. 2013, 6, 5–11. [Google Scholar] [CrossRef]
- Hameed, R.; van Mourik, T.; Khan, A. 13C-1H coupling constants as a conformational tool for structural assignment of quinic and octulosonic acid. J. Mol. Model. 2018, 24, 324. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, Y.S. Identification of new dicaffeoylquinic acids from Chrysanthemum morifolium and their antioxidant activities. Planta Med. 2005, 71, 871–876. [Google Scholar] [CrossRef]
- Agency, E.M. ICH Guideline Q2(R2) on Validation of Analytical Procedures; European Medicines Agency: Amsterdam, The Netherlands, 2024; Available online: https://europass.europa.eu/en (accessed on 11 March 2022).
- Kutzner, L.; Rund, K.M.; Ostermann, A.I.; Hartung, N.M.; Galano, J.M.; Balas, L.; Durand, T.; Balzer, M.S.; David, S.; Schebb, N.H. Development of an Optimized LC-MS Method for the Detection of Specialized Pro-Resolving Mediators in Biological Samples. Front. Pharmacol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Panthi, S.; Chen, J.; Wilson, L.; Nichols, J.J. Detection of Lipid Mediators of Inflammation in the Human Tear Film. Eye Contact Lens 2019, 45, 171–181. [Google Scholar] [CrossRef]
- Johnson, D.W. Contemporary clinical usage of LC/MS: Analysis of biologically important carboxylic acids. Clin. Biochem. 2005, 38, 351–361. [Google Scholar] [CrossRef]
- Yum, H.W.; Na, H.K.; Surh, Y.J. Anti-inflammatory effects of docosahexaenoic acid: Implications for its cancer chemopreventive potential. Semin. Cancer Biol. 2016, 40–41, 141–159. [Google Scholar] [CrossRef]
- Archambault, A.S.; Poirier, S.; Lefebvre, J.S.; Robichaud, P.P.; Larose, M.C.; Turcotte, C.; Martin, C.; Provost, V.; Boudreau, L.H.; McDonald, P.P.; et al. 20-Hydroxy- and 20-carboxy-leukotriene (LT)B4 downregulate LTB4-mediated responses of human neutrophils and eosinophils. J. Leukoc. Biol. 2019, 105, 1131–1142. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ito, Y.; Kawai-Yamada, M. Molecular characterization and targeted quantitative profiling of the sphingolipidome in rice. Plant J. 2016, 88, 681–693. [Google Scholar] [CrossRef]
- Shaner, R.L.; Allegood, J.C.; Park, H.; Wang, E.; Kelly, S.; Haynes, C.A.; Sullards, M.C.; Merrill, A.H. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J. Lipid Res. 2009, 50, 1692–1707. [Google Scholar] [CrossRef]
- Reifen, R.; Karlinsky, A.; Stark, A.H.; Berkovich, Z.; Nyska, A. α-Linolenic acid (ALA) is an anti-inflammatory agent in inflammatory bowel disease. J. Nutr. Biochem. 2015, 26, 1632–1640. [Google Scholar] [CrossRef]
- Mulligan, C.M.; Le, C.H.; deMooy, A.B.; Nelson, C.B.; Chicco, A.J. Inhibition of delta-6 desaturase reverses cardiolipin remodeling and prevents contractile dysfunction in the aged mouse heart without altering mitochondrial respiratory function. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 799–809. [Google Scholar] [CrossRef]
- Li, S.; Yuan, H.; Li, L.; Li, Q.; Lin, P.; Li, K. Oxidative Stress and Reprogramming of Lipid Metabolism in Cancers. Antioxidants 2025, 14, 201. [Google Scholar] [CrossRef]
- Tahir, A.; Bileck, A.; Muqaku, B.; Niederstaetter, L.; Kreutz, D.; Mayer, R.L.; Wolrab, D.; Meier, S.M.; Slany, A.; Gerner, C. Combined Proteome and Eicosanoid Profiling Approach for Revealing Implications of Human Fibroblasts in Chronic Inflammation. Anal. Chem. 2017, 89, 1945–1954. [Google Scholar] [CrossRef]
- Checa, A.; Khademi, M.; Sar, D.G.; Haeggström, J.Z.; Lundberg, J.O.; Piehl, F.; Olsson, T.; Wheelock, C.E. Hexosylceramides as intrathecal markers of worsening disability in multiple sclerosis. Mult. Scler. 2015, 21, 1271–1279. [Google Scholar] [CrossRef]


| Cells Stimulated by Kaempferol 3-O-glucuronide | |||
| CT- | 25 µM | 50 µM | 100 µM |
| 8.6 ± 0.5 | 58 ± 7 (**) | 75.3 ± 6.0 (***) | 106 ± 9 (***) |
| Cells stimulated by quercetin 3-O-glucuronide | |||
| CT- | 25 µM | 50 µM | 100 µM |
| 8.6 ± 0.5 | 49.4 ± 2.6 (ns) | 55.6 ± 1.7 (*) | 71.8 ± 4.9 (**) |
| Cells stimulated by quercetin 3-O-glucoside | |||
| CT- | 25 µM | 50 µM | 100 µM |
| 12.8 ± 3.4 | 69.5 ± 2.8 (****) | 83.2 ± 5.8 (****) | 107.4 ± 2.5 (****) |
| Cells stimulated by 1,5-dicaffeoylquinic acid | |||
| CT- | 25 µM | 50 µM | 100 µM |
| 7.2 ± 0.7 | 51.91 ± 0.56 (**) | 114.0 ± 1.5 (****) | 140.9 ± 1.3 (****) |
| Cells Stimulated by Quercetin 3-O-glucuronide | |||
| CT- | 25 µM | 50 µM | 100 µM |
| 171.9 ± 8.6 | 208.7 ± 8.1 (**) | 198 ± 18 (*) | 301.2 ± 4.9 (****) |
| Cells stimulated by quercetin 3-O-glucoside | |||
| CT- | 25 µM | 50 µM | 100 µM |
| 56.1 ± 6.9 | 82.4 ± 6.4 (*) | 83 ± 13 (*) | 100 ± 15 (***) |
| Cells stimulated by quinic acid | |||
| CT- | 25 µM | 50 µM | 100 µM |
| 64.6 ± 7.9 | 74.5 ± 6.2 (ns) | 101 ± 18 (**) | 91.86 ± 1.49 (*) |
| Cells stimulated by 1,5-dicaffeoyl quinic acid | |||
| CT- | 25 µM | 50 µM | 100 µM |
| 64.6 ± 7.9 | 344.0 ± 19.8 (****) | 415.7 ± 5.9 (****) | 518 ± 30 (****) |
| Eicosanoids and Fatty Acids | DP | EP | CE | CXP | Precursor | Product |
|---|---|---|---|---|---|---|
| α-Linolenic acid | −30 | −10 | −30 | −10 | 277.2 | 127.1 |
| γ-Linolenic acid | −30 | −10 | −40 | −10 | 277.2 | 191.1 |
| Linolenic acid | −30 | −10 | −20 | −10 | 279.2 | 261.2 |
| Eicosapentanoic acid | −40 | −10 | −16 | −10 | 301.2 | 257.2 |
| Arachidonic acid | −55 | −10 | −20 | −10 | 303.2 | 259.2 |
| Docohexaenoic acid | −40 | −10 | −19 | −10 | 327.3 | 283.2 |
| 5-HETE | −40 | −10 | −20 | −10 | 319.1 | 114.9 |
| 9-HETE | −40 | −10 | −20 | −10 | 319.1 | 179.1 |
| 12-HETE | −50 | −10 | −19 | −10 | 319.0 | 178.8 |
| 15-HETE | −30 | −10 | −17 | −10 | 319.1 | 174.8 |
| 19-HETE | −40 | −10 | −23 | −20 | 319.2 | 275.1 |
| 20-HETE | −50 | −10 | −24 | −20 | 319.2 | 274.9 |
| 9,10,13-TriHOME | −50 | −10 | −29 | −10 | 329.0 | 139.0 |
| 9,12,13-TriHOME | −50 | −10 | −29 | −10 | 329.1 | 211.0 |
| 11-HDoHE | −20 | −10 | −19 | −10 | 343.3 | 149.0 |
| 14-HDoHE | −30 | −10 | −19 | −10 | 343.2 | 281.2 |
| 17-HDoHE | −30 | −10 | −19 | −10 | 343.2 | 281.2 |
| 12,13-DiHOME | −50 | −10 | −29 | −10 | 313.1 | 183.1 |
| 11(12)-EpETrE | −40 | −10 | −20 | −20 | 319.2 | 166.9 |
| 11,12-DiHETrE | −40 | −10 | −25 | −10 | 337.2 | 167.0 |
| 14,15-DiHETrE | −30 | −10 | −24 | −10 | 337.2 | 207.0 |
| LTB4 | −45 | −10 | −23 | −10 | 335.2 | 195.0 |
| 20-COOH-LTB4 | −40 | −10 | −26 | −10 | 365.2 | 195.1 |
| TXB2 | −50 | −10 | −35 | −10 | 369.2 | 168.9 |
| TXB3 | −40 | −10 | −27 | −10 | 367.2 | 195.2 |
| 11-keto TXB3 | −40 | −10 | −26 | −10 | 365.2 | 169.2 |
| tetranor-PGDM | −30 | −10 | −23 | −10 | 327.1 | 155.0 |
| PGB2 | −40 | −10 | −30 | −10 | 333.2 | 175.0 |
| 6-keto-PGF1a | −60 | −10 | −34 | −10 | 369.2 | 162.9 |
| Sphingolipids | DP | EP | CE | CXP | Precursor | Product |
|---|---|---|---|---|---|---|
| GlcCer C16:0 (d18:1/16:0) | 60 | 10 | 43 | 15 | 682.6 | 520.5 |
| GlcCer C24:1 (d18:1/24:1(15Z)) (1) | 60 | 10 | 43 | 15 | 792.7 | 612.6 |
| GlcCer C24:1 (d18:1/24:1(15Z)) (2) | 60 | 10 | 43 | 15 | 792.7 | 264.3 |
| LacCer C24:1 (d18:1/24:1) (1) | 60 | 10 | 43 | 15 | 954.7 | 264.3 |
| LacCer C24:1 (d18:1/24:1) (2) | 60 | 10 | 43 | 15 | 954.7 | 630.6 |
| N-lauroyl-1-deoxysphingosine (1) | 60 | 10 | 43 | 15 | 466.5 | 448.4 |
| N-lauroyl-1-deoxysphingosine (2) | 60 | 10 | 43 | 15 | 466.5 | 266.2 |
| N-nervonoyl-1-deoxysphinganine | 60 | 10 | 43 | 15 | 634.7 | 616.5 |
| SM C12:0 (d18:1/12:0) | 60 | 10 | 43 | 15 | 647.5 | 184.1 |
| SM C16:0 (d18:1/16:0) | 60 | 10 | 43 | 15 | 703.6 | 184.1 |
| SM C18:0 (d18:1/18:0) | 60 | 10 | 43 | 15 | 731.6 | 184.1 |
| SM C18:1 (d18:1/18:1(9Z)) | 60 | 10 | 43 | 15 | 729.6 | 184.1 |
| SM C24:0 (d18:1/24:0) | 60 | 10 | 43 | 15 | 815.7 | 184.1 |
| SM C24:1 (d18:1/24:1(15Z)) | 60 | 10 | 43 | 15 | 813.7 | 184.1 |
| SM C6:0 (d18:1/6:0) | 60 | 10 | 43 | 15 | 563.4 | 184.1 |
| Spa 1P (d18:0) | 60 | 10 | 20 | 15 | 382.3 | 95.1 |
| Sph (d20:1) | 60 | 10 | 43 | 15 | 310.3 | 81.1 |
| 1-deoxysphingosine | 60 | 10 | 43 | 15 | 284.3 | 266.2 |
| C18 Dihydroceramide | 60 | 10 | 43 | 15 | 568.6 | 524.5 |
| C20 Dihydroceramide | 60 | 10 | 43 | 15 | 596.6 | 298.1 |
| C22 Sphingomyelin (1) | 60 | 10 | 43 | 15 | 787.7 | 184.0 |
| C22 Sphingomyelin (2) | 60 | 10 | 43 | 15 | 787.7 | 86.1 |
| Cer C18:1 (d18:1/18:1(9Z) | 60 | 10 | 43 | 15 | 546.5 | 237.1 |
| Cer C2:0 (d18:1/2:0) | 60 | 10 | 43 | 15 | 324.3 | 306.3 |
| 1-desoxymethylsphingosine | 60 | 10 | 43 | 15 | 270.3 | 252.2 |
| Cer C24:0 (d18:1/24:0) | 60 | 10 | 43 | 15 | 632.6 | 264.3 |
| Cer C20:0 (d18:1/20:0) | 60 | 10 | 43 | 15 | 576.6 | 264.3 |
| Cer1P C16:0 (d18:1/16:0) | 60 | 10 | 43 | 15 | 600.5 | 520.5 |
| Cer1P C18:1 (d18:1/18:1(9Z)) | 60 | 10 | 43 | 15 | 644.5 | 264.3 |
| DhCer C24:0 (d18:0/24:0) (1) | 60 | 10 | 43 | 15 | 652.7 | 634.6 |
| DhCer C24:0 (d18:0/24:0) (2) | 60 | 10 | 43 | 15 | 652.7 | 302.3 |
| DhCer C24:0 (d18:0/24:0) (3) | 60 | 10 | 43 | 15 | 652.7 | 284.3 |
| DhCer C24:1 (d18:0/24:1(15Z)) (1) | 60 | 10 | 43 | 15 | 650.6 | 632.6 |
| DhCer C24:1 (d18:0/24:1(15Z)) (2) | 60 | 10 | 43 | 15 | 650.6 | 302.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crescenzi, M.A.; Gallart-Ayala, H.; Stellato, C.; Popolo, A.; Ivanisevic, J.; Piacente, S.; Montoro, P. A Targeted Mass Spectrometric Approach to Evaluate the Anti-Inflammatory Activity of the Major Metabolites of Foeniculum vulgare Mill. Waste in Human Bronchial Epithelium. Molecules 2025, 30, 1407. https://doi.org/10.3390/molecules30071407
Crescenzi MA, Gallart-Ayala H, Stellato C, Popolo A, Ivanisevic J, Piacente S, Montoro P. A Targeted Mass Spectrometric Approach to Evaluate the Anti-Inflammatory Activity of the Major Metabolites of Foeniculum vulgare Mill. Waste in Human Bronchial Epithelium. Molecules. 2025; 30(7):1407. https://doi.org/10.3390/molecules30071407
Chicago/Turabian StyleCrescenzi, Maria Assunta, Hector Gallart-Ayala, Cristiana Stellato, Ada Popolo, Julijana Ivanisevic, Sonia Piacente, and Paola Montoro. 2025. "A Targeted Mass Spectrometric Approach to Evaluate the Anti-Inflammatory Activity of the Major Metabolites of Foeniculum vulgare Mill. Waste in Human Bronchial Epithelium" Molecules 30, no. 7: 1407. https://doi.org/10.3390/molecules30071407
APA StyleCrescenzi, M. A., Gallart-Ayala, H., Stellato, C., Popolo, A., Ivanisevic, J., Piacente, S., & Montoro, P. (2025). A Targeted Mass Spectrometric Approach to Evaluate the Anti-Inflammatory Activity of the Major Metabolites of Foeniculum vulgare Mill. Waste in Human Bronchial Epithelium. Molecules, 30(7), 1407. https://doi.org/10.3390/molecules30071407









