Remediation of Hg-Contaminated Groundwater via Adsorption on Supramolecular Polymers in Batch Process and Column Test
Abstract
1. Introduction
2. Results and Discussion
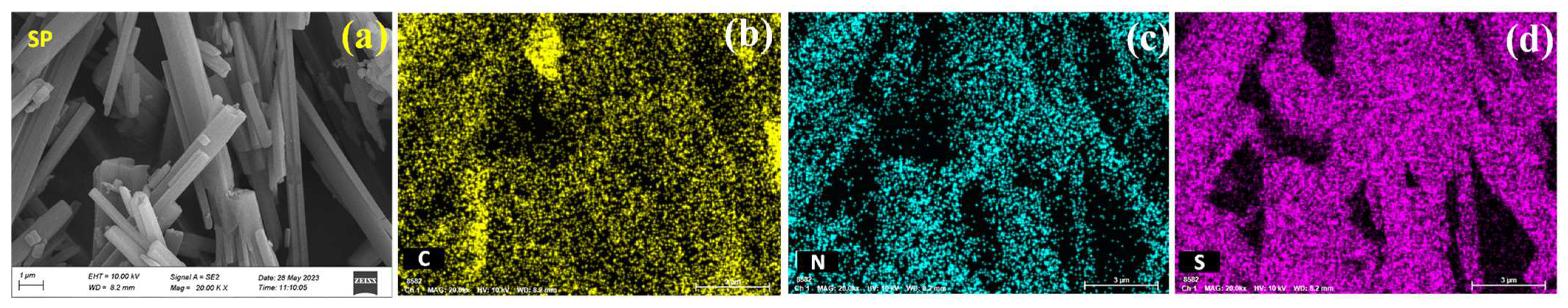
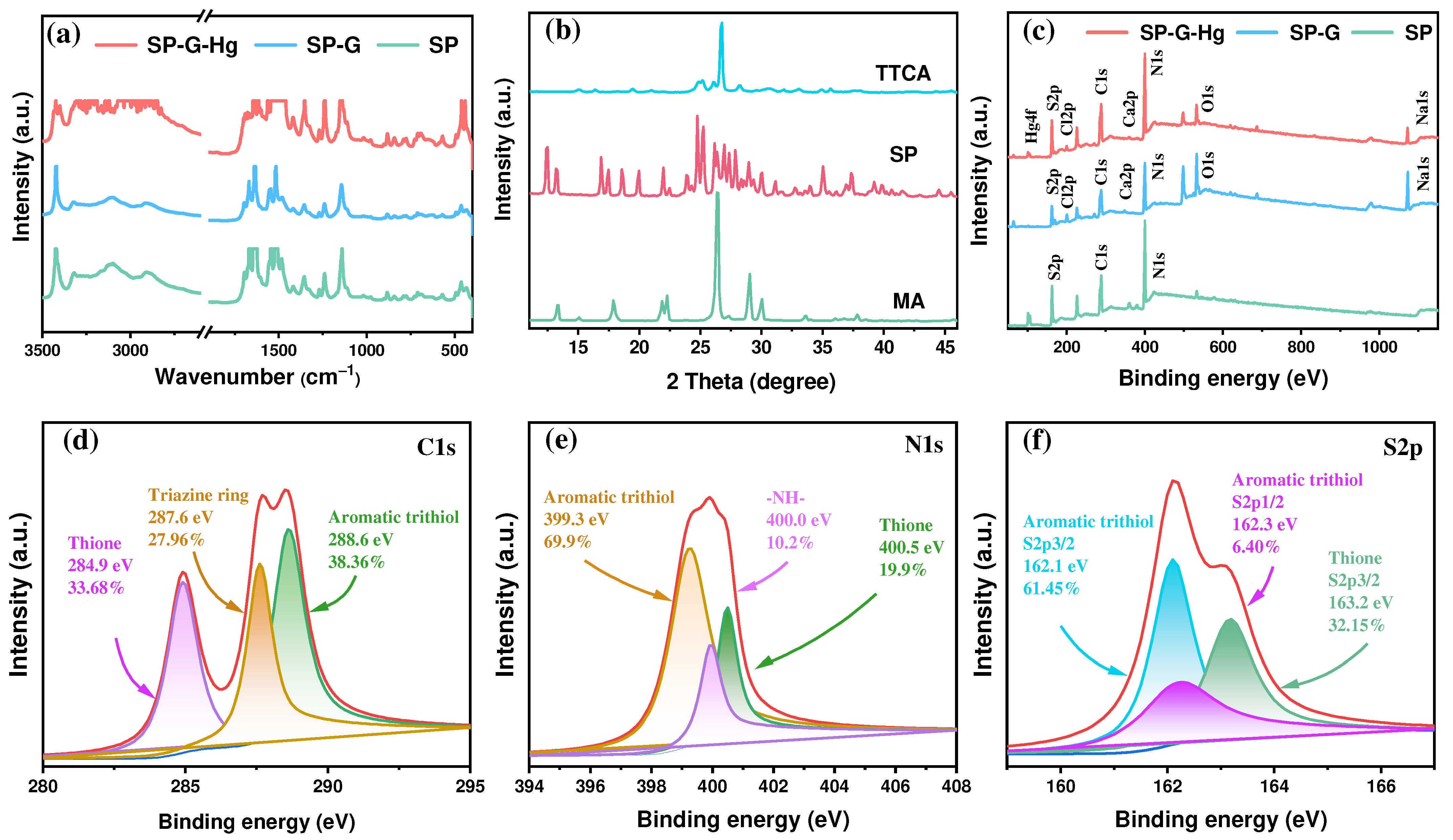
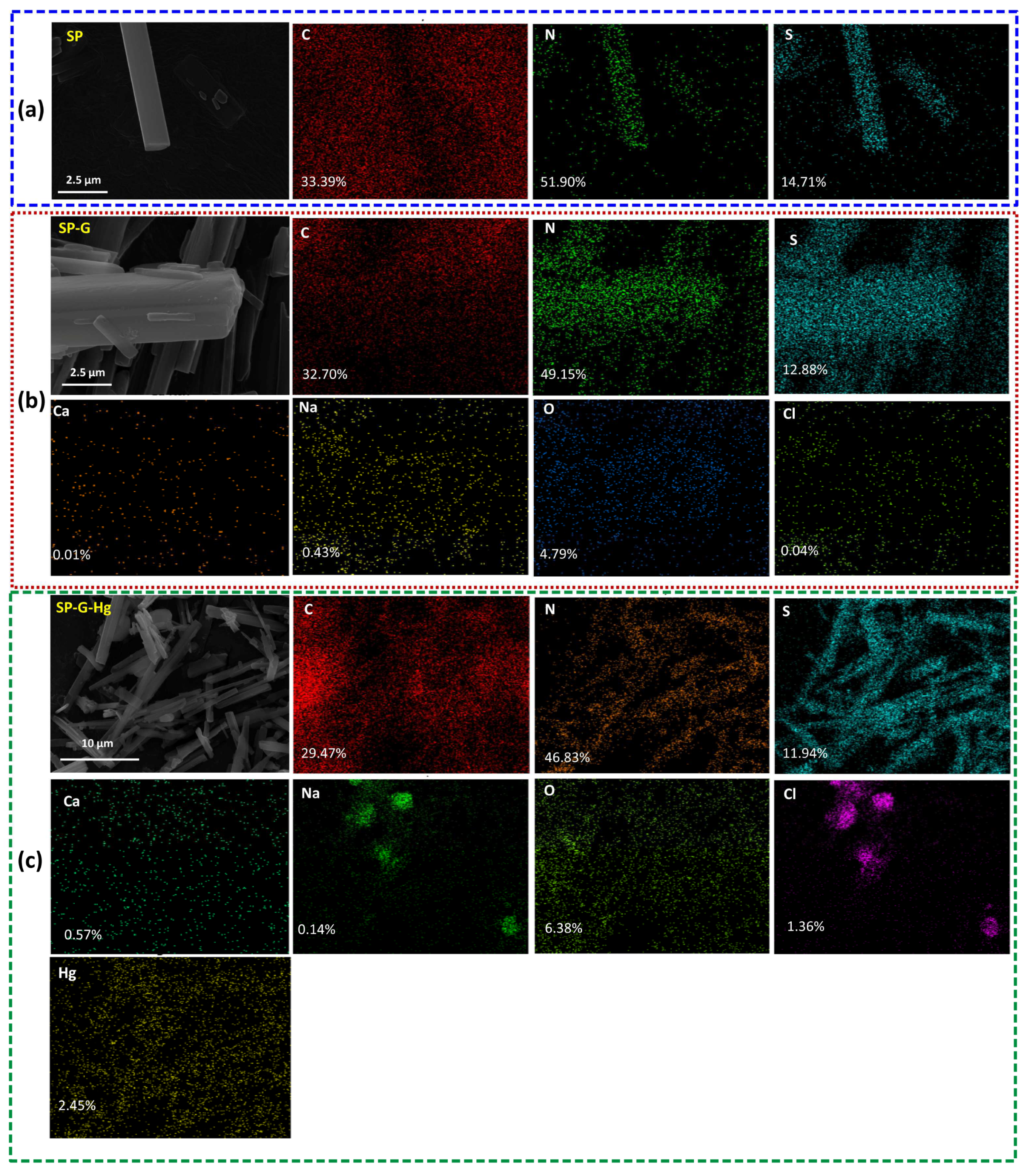
2.1. Material Characterization
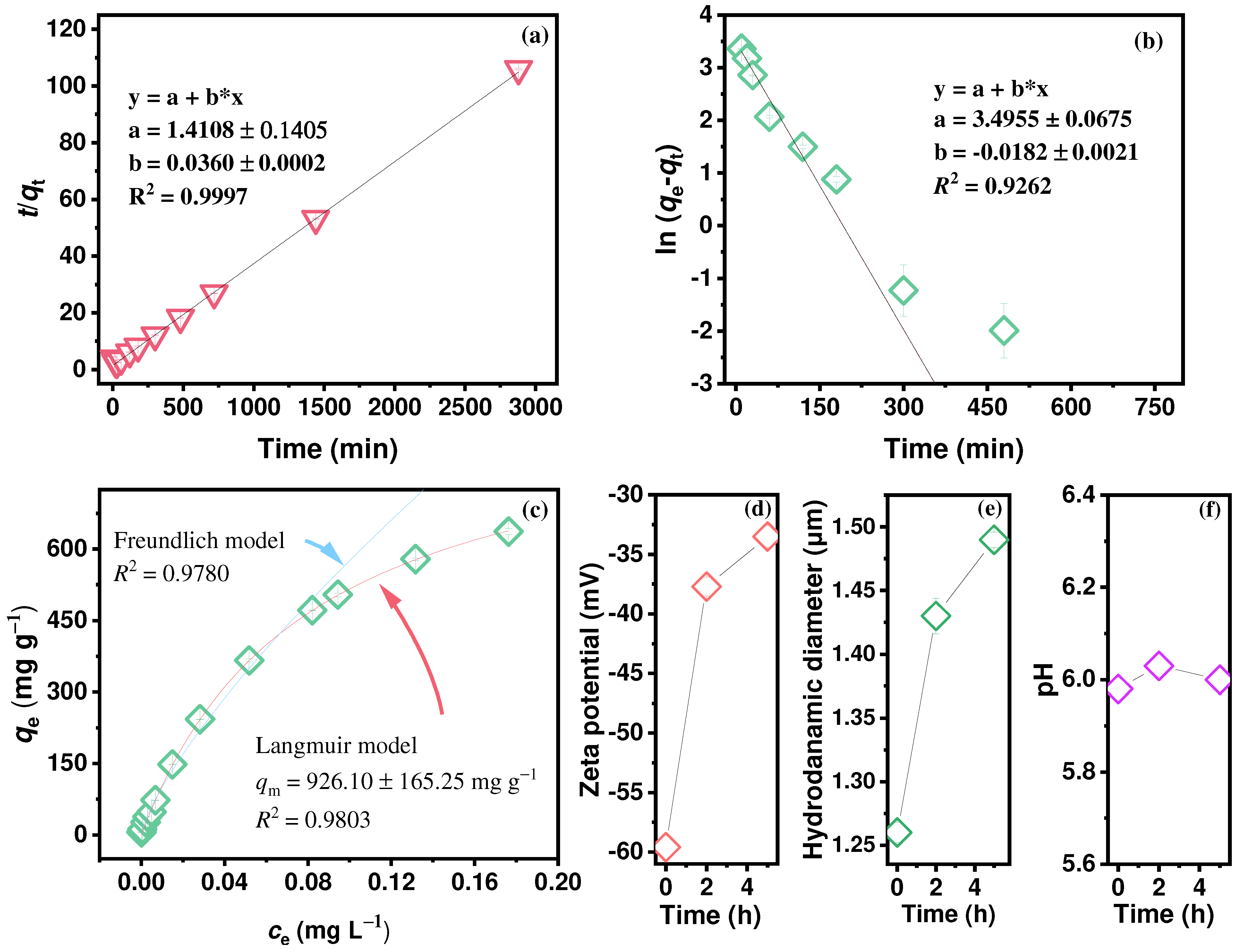
2.2. Interfacial Processes of Hg in Groundwater
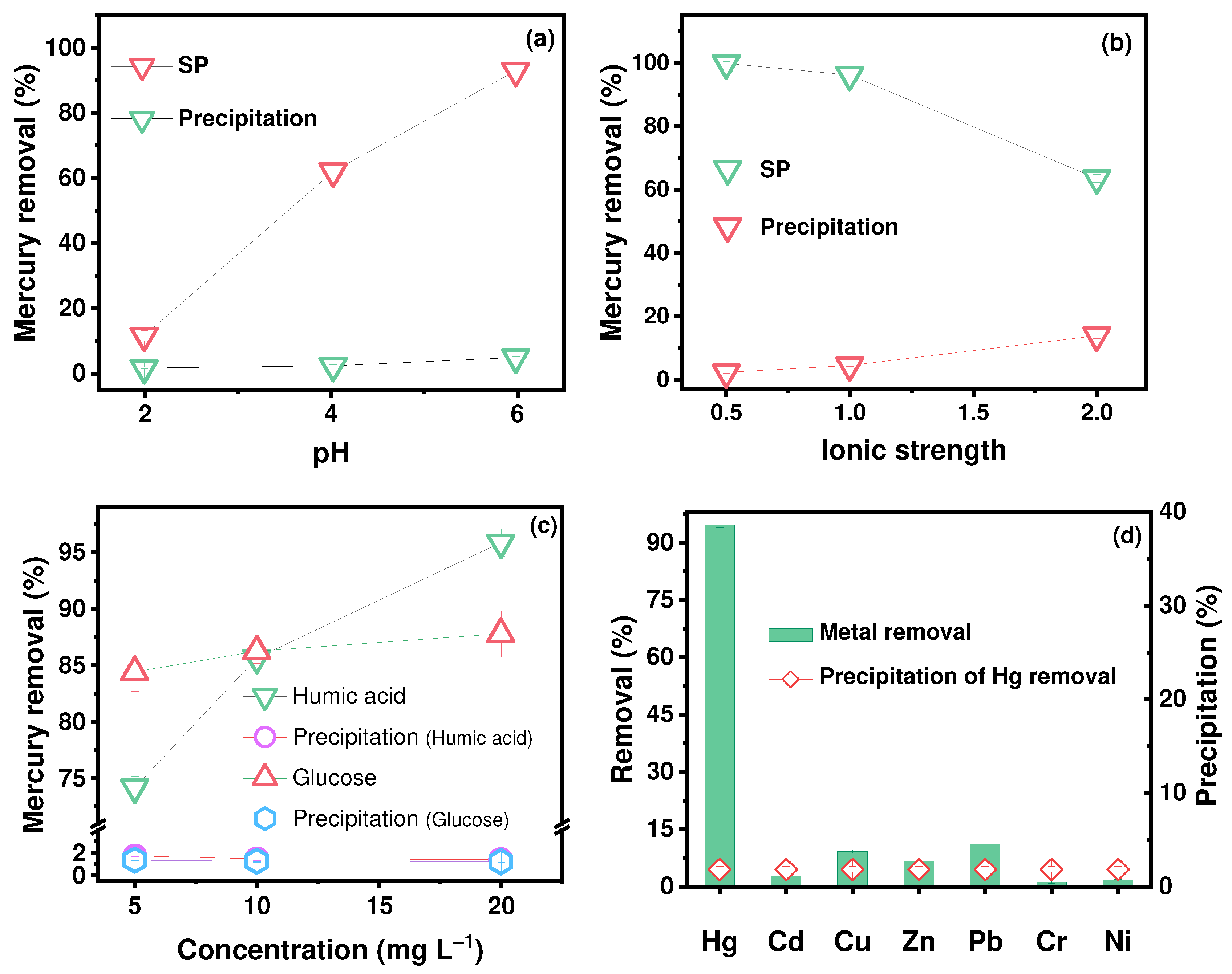
2.3. Effects of pH, Ionic Strength, DOM, and Coexisting Ions
2.4. Regeneration and Reusability
2.5. Mathematical Modeling of Breakthrough Curves
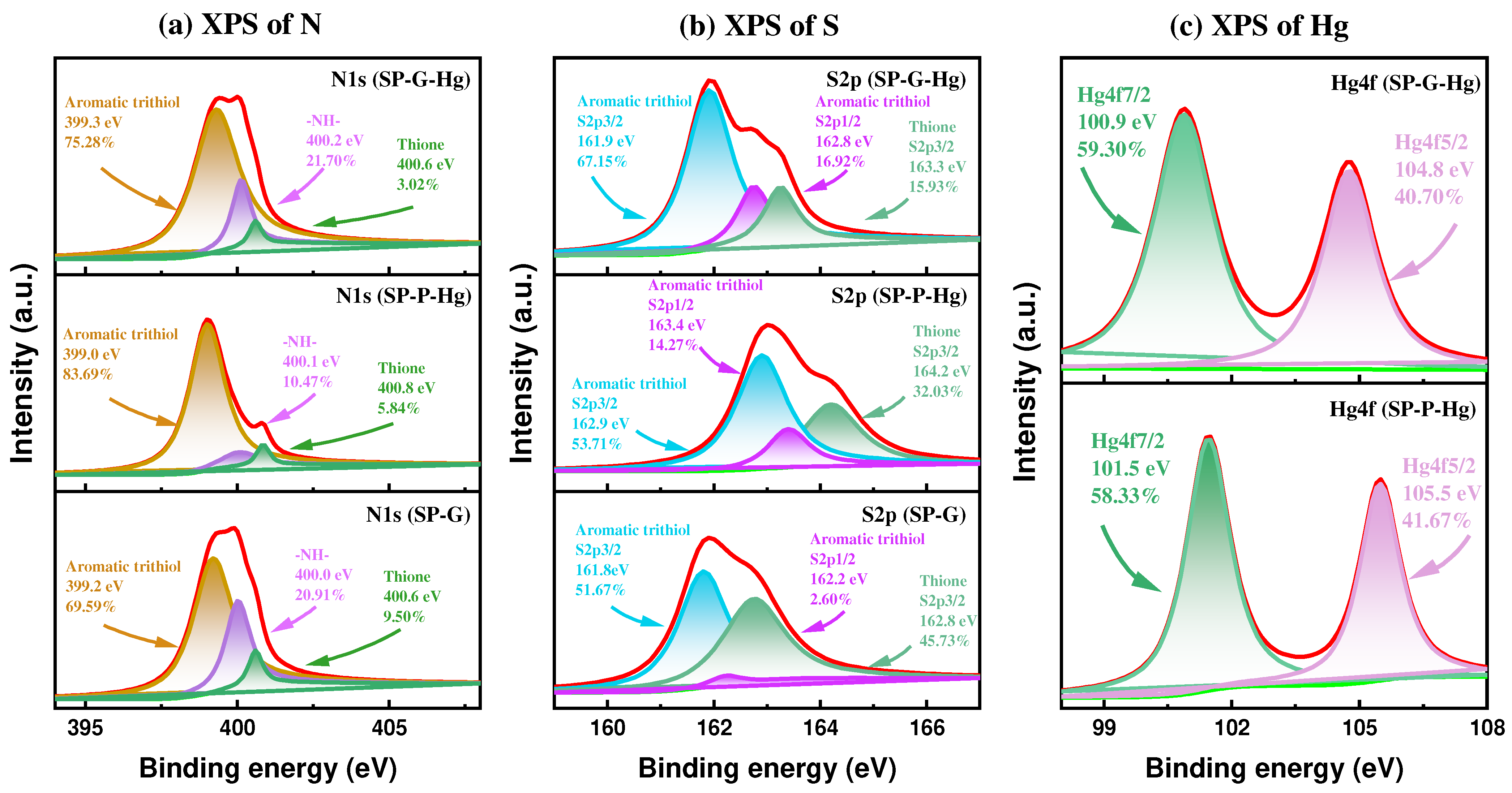
2.6. Plausible Mechanism for Hg(II) Adsorption
3. Materials and Methods
3.1. Materials and Characterization
3.2. Synthesis of SP
3.3. Batch Experiments
3.4. Column Tests
3.5. Mathematical Models for Breakthrough Curves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lourenço, M.A.O.; Figueira, P.; Pereira, E.; Gomes, J.R.B.; Lopes, C.B.; Ferreira, P. Simple, mono and bifunctional periodic mesoporous organosilicas for removal of priority hazardous substances from water: The case of mercury(II). Chem. Eng. J. 2017, 322, 263–274. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, J.; Liu, W.; Huang, S.; Chen, X.; Zhang, N.; Huang, Y. Mercury speciation transformation mediated by thiolated biochar in high salinity groundwater: Interfacial processes, influencing factors, and mechanisms. Chem. Eng. J. 2024, 484, 149443. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Zhao, D.; Zhuang, L.; Yang, G.; Gong, Y. Immobilization of mercury by iron sulfide nanoparticles alters mercury speciation and microbial methylation in contaminated groundwater. Chem. Eng. J. 2020, 381, 122664. [Google Scholar] [CrossRef]
- Bollen, A.; Wenke, A.; Biester, H. Mercury speciation analyses in HgCl2-contaminated soils and groundwater-Implications for risk assessment and remediation strategies. Water Res. 2008, 42, 91–100. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Sigmund, G.; Bone, S.E.; Kumar, N.; Hofmann, T. Mercury removal from contaminated water by wood-based biochar depends on natural organic matter and ionic composition. Environ. Sci. Technol. 2022, 56, 11354–11362. [Google Scholar] [CrossRef]
- Aleku, D.L.; Lazareva, O.; Pichler, T. Mercury in groundwater—Source, transport and remediation. Appl. Geochem. 2024, 170, 106060. [Google Scholar] [CrossRef]
- GB/T 14848-2017; Standard for Groundwater Quality. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2017. Available online: https://www.chinesestandard.net/PDF.aspx/GBT14848-2017 (accessed on 22 December 2024).
- Edmunds, W.M.; Smedley, P.L. Groundwater geochemistry and health: An overview. Geol. Soc. Lond. Spec. Publ. 1996, 113, 91–105. [Google Scholar] [CrossRef]
- Wang, M.; Han, Q.; Shu, Y.; Wang, K.; Wang, L.; Liu, B.; Zucker, I.; Wang, Z. Matrix effects on the performance and mechanism of Hg removal from groundwater by MoS2 nanosheet. Environ. Sci. Adv. 2022, 1, 59–69. [Google Scholar] [CrossRef]
- Chen, M.; Price, R.M.; Yamashita, Y.; Jaffé, R. Comparative study of dissolved organic matter from groundwater and surface water in the Florida coastal Everglades using multi-dimensional spectrofluorometry combined with multivariate statistics. Appl. Geochem. 2010, 25, 872–880. [Google Scholar] [CrossRef]
- Richard, J.-H.; Bischoff, C.; Ahrens, C.G.M.; Biester, H. Mercury (II) reduction and co-precipitation of metallic mercury on hydrous ferric oxide in contaminated groundwater. Sci. Total Environ. 2016, 539, 36–44. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Han, Q.; Shu, Y.; Liu, X.; Chen, B.; Chen, Y.; Liu, B.; Wang, Z. Effect of natural organic matter (NOM) on the removal efficiency of Hg(ii) by MoS2: Dependence on the Hg/MoS2 ratio and NOM properties. Environ. Sci. Nano 2024, 11, 1129–1141. [Google Scholar] [CrossRef]
- Weisener, C.G.; Sale, K.S.; Smyth, D.J.A.; Blowes, D.W. Field column study using zerovalent iron for mercury removal from contaminated groundwater. Environ. Sci. Technol. 2005, 391, 6306–6312. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, F.; Shen, Z.; Wang, F.; Lynch, R.; Al-Tabba, A. Adsorption of methyl tertbutyl ether (MTBE) onto ZSM-5 zeolite: Fixed-bed column tests, breakthrough curve modelling and regeneration. Chemosphere 2019, 220, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Vilensky, M.Y.; Berkowitz, B.; Warshawsky, A. In situ remediation of groundwater contaminated by heavy- and transition-metal ions by selective ion-exchange methods. Environ. Sci. Technol. 2002, 36, 1851–1855. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Cao, B.; Yin, H.; Al-Tabbaa, A. Simultaneous removal of Pb and MTBE by mixed zeolites in fixed-bed column tests. J. Environ. Sci. 2022, 34, 41–49. [Google Scholar] [CrossRef]
- Bleiman, N.; Mishael, Y.G. Selenium removal from drinking water by adsorption to chitosan–clay composites and oxides: Batch and columns tests. J. Hazard. Mater. 2010, 183, 590–595. [Google Scholar] [CrossRef]
- Biterna, M.; Antonoglou, L.; Lazou, E.; Voutsa, D. Arsenite removal from waters by zero valent iron: Batch and column tests. Chemosphere 2010, 78, 7–12. [Google Scholar] [CrossRef]
- Abbasi, M.; Safari, E.; Baghdadi, M.; Janmohammadi, M. Enhanced adsorption of heavy metals in groundwater using sand columns enriched with graphene oxide: Lab-scale experiments and process modeling. J. Water Process Eng. 2021, 40, 101961. [Google Scholar] [CrossRef]
- Blue, L.Y.; Van Aelstyn, M.A.; Matlock, M.; Atwood, D.A. Low-level mercury removal from groundwater using a synthetic chelating ligand. Water Res. 2008, 42, 2025–2028. [Google Scholar] [CrossRef]
- Rahmi; Fathurrahmi; Lelifajri; PurnamaWati, F. Preparation of magnetic chitosan using local iron sand for mercury removal. Heliyon 2019, 5, e01731. [Google Scholar] [CrossRef]
- Yong, S.K.; Bolan, N.S.; Lombi, E.; Skinner, W.; Guibal, E. Sulfur-containing chitin and chitosan derivatives as trace metal adsorbents: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1741–1794. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Jang, S.B.; Wong, K.T.; Kim, H.; Kim, M.J.; Choong, C.E.; Yang, J.-K.; Chang, Y.-Y.; Oh, S.-E.; Yoon, Y.; et al. Sulfur-anchored palm shell waste-based activated carbon for ultrahigh sorption of Hg(II) for in-situ groundwater treatment. J. Hazard. Mater. 2021, 417, 125995. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Chandra, B.P.; Prathibha, C. Highly efficient and simultaneous remediation of heavy metal ions (Pb(II), Hg(II), As(V), As(III) and Cr(VI)) from water using Ce intercalated and ceria decorated titanate nanotubes. Appl. Surf. Sci. 2023, 612, 155841. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Y.; Wei, Y.; Tang, H.; Zhao, J.; Zhang, D.; Yu, L.; Tan, M.; Liu, X.; Shi, J.; et al. Deep and consecutive removal of Hg2+ from water using a novel dopamine-bridged metal organic framework-carbon sponge composite. Sep. Purif. Technol. 2024, 346, 127512. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Yoon, M. Core-shell ferromagnetic nanorod based on amine polymer composite (Fe3O4@DAPF) for fast removal of Pb(II) from aqueous solutions. ACS Appl. Mater. Interfaces 2015, 7, 25362–25372. [Google Scholar] [CrossRef]
- Huang, X.; Yang, J.; Wang, J.; Bi, J.; Xie, C.; Hao, H. Design and synthesis of core-shell Fe3O4@PTMT composite magnetic microspheres for adsorption of heavy metals from high salinity wastewater. Chemosphere 2018, 206, 513–521. [Google Scholar] [CrossRef]
- Fu, W.; Wang, X.; Huang, Z. Remarkable reusability of magnetic Fe3O4-encapsulated C3N3S3 polymer/reduced graphene oxide composite: A highly effective adsorbent for Pb and Hg ions. Sci. Total Environ. 2019, 659, 895–904. [Google Scholar] [CrossRef]
- Xue, J.; Qu, Y.; Chen, Y.; Zhang, C.; Bu, X. Effective sulfide flotation of cerussite by using trithiocyanuric acid as a novel sulfurizing reagent. Miner. Eng. 2023, 198, 108087. [Google Scholar] [CrossRef]
- Wang, W.; Qi, L.; Han, S.; Yuan, H. Synthesis, characterization of chitosan-trithiocyanuric and its removal mechanism of Cr(VI) and Pb(II) from wastewater. Chem. Eng. Sci. 2023, 281, 119144. [Google Scholar] [CrossRef]
- Huang, S.-A.; Teng, H.-J.; Su, Y.-T.; Liu, X.-M.; Li, B. Trithiocyanurate-functionalized hydrochar for effetively removing methylene blue and Pb (II) cationic pollutants. Environ. Pollut. 2023, 337, 122585. [Google Scholar] [CrossRef]
- Gao, X.; Li, Z.; Wen, H.; Zhao, J.; Zhou, M.; Yang, S.; Liu, J. Instant oxidase- and peroxidase-mimic activities of in-situ reductive coordinated Cu(I)-polytrithiocyanuric acid for H2S colorimetric detection and antibacterial. J. Hazard. Mater. 2025, 483, 136722. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Lee, J.S.; Patel, H.A.; Jakobsen, M.H.; Hwang, Y.; Yavuz, C.T.; Hansen, H.C.B.; Andersen, H.R. Selective removal of heavy metal ions by disulfide linked polymer networks. J. Hazard. Mater. 2017, 332, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Mines, P.D.; Jakobsen, M.H.; Yavuz, C.T.; Hansen, H.C.B.; Andersen, H.R. Disulfide polymer grafted porous carbon composites for heavy metal removal from stormwater runoff. Chem. Eng. J. 2018, 348, 685–692. [Google Scholar] [CrossRef]
- Lin, G.; Wang, S.; Zhang, L.; Hu, T.; Cheng, S.; Fu, L.; Xiong, C. Enhanced and selective adsorption of Hg2+ to a trace level using trithiocyanuric acid-functionalized corn bract. Environ. Pollut. 2019, 244, 938–946. [Google Scholar] [CrossRef]
- Li, Q.; Ruan, B.; Yu, Y.; Ye, L.; Dai, A.; You, S.; Zhao, B.; Ren, L. Green and Mild Fabrication of Magnetic Poly(trithiocyanuric acid) Polymers for Rapid and Selective Separation of Mercury(II) Ions in Aqueous Samples. Polymers 2024, 16, 3067. [Google Scholar] [CrossRef]
- Sharahi, F.J.; Shahbazi, A. Melamine-based dendrimer amine-modified magnetic nanoparticles as an efficient Pb(II) adsorbent for wastewater treatment: Adsorption optimization by response surface methodology. Chemosphere 2017, 189, 291–300. [Google Scholar] [CrossRef]
- Al Hamouz, O.C.S.; Adelabu, I.O.; Saleh, T.A. Novel cross-linked melamine based polyamine/CNT composites for lead ions removal. J. Environ. Manag. 2017, 192, 163–170. [Google Scholar] [CrossRef]
- Nagai, D.; Kuribayashi, T.; Tanaka, H.; Morinaga, H.; Uehara, H.; Yamanobea, T. A facile, selective, high recovery system for precious metals based on complexation between melamine and cyanuric acid. RSC Adv. 2015, 5, 30133–30139. [Google Scholar] [CrossRef]
- Nagai, D.; Nagashima, A.; Mori, M. A facile and high-recovery system for palladium(II) ion based on complexation between trithiocyanuric acid and melamine. Chem. Lett. 2016, 45, 1165–1167. [Google Scholar] [CrossRef]
- Ranganathan, A.; Pedireddi, V.R.; Rao, C.N.R. Hydrothermal synthesis of organic channel structures: 1:1 hydrogen-bonded adducts of melamine with cyanuric and trithiocyanuric acids. J. Am. Chem. Soc. 1999, 121, 1752–1753. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. A simple fabrication for sulfur doped graphitic carbon nitride porous rods with excellent photocatalytic activity degrading RhB dye. Appl. Surf. Sci. 2017, 391, 360–368. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Simanek, E.E.; Mathias, J.P.; Seto, C.T.; Chin, D.; Mammen, M.; Gordon, D.M. Noncovalent synthesis:Using physical-organic chemistry to make aggregates. Acc. Chem. Res. 1995, 28, 37–44. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, S.; Wang, W.; Rong, M.; Cai, H.; Xing, H.; Yang, L. Functionalized hydrogen-bonded organic superstructures via molecular self-assembly for enhanced uranium extraction. J. Hazard. Mater. 2024, 464, 133002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Guo, Y.; Xie, H.; Zhang, Y.; Fu, Y.; Ye, C.; Du, Y.; Zhu, M. Green and Controllable synthesis of kelp-like carbon nitride nanosheets via an ultrasound-mediated self-assembly strategy. J. Colloid Interface Sci. 2022, 628, 397–408. [Google Scholar] [CrossRef]
- Li, H.; Zuo, P.; Qu, S.; Qin, F.; Li, N.; Shen, W. Hybrid hierarchically porous carbon micron tubes for trace cadmium and lead ions electrochemical detection. Appl. Surf. Sci. 2023, 615, 156426. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Wu, Q.; Han, X.; Zhang, M.; Liu, W.; Yao, X.; Feng, J.; Dong, S.; Sun, J. Magnetic supramolecular polymer: Ultrahigh and highly selective Pb(II) capture from aqueous solution and battery wastewater. Chemosphere 2020, 248, 126042. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Y.; Fang, L.; Zhao, B.; Zhang, Y.; Zhu, Y.; Wang, Z.; Wang, Q.; Li, F. Interfacial chemistry of mercury on thiol-modified biochar and its implication for adsorbent engineering. Chem. Eng. J. 2023, 454, 140310. [Google Scholar] [CrossRef]
- Shalom, M.; Inal, S.; Fettkenhauer, C.; Neher, D.; Antonietti, M. Improving carbon nitride photocatalysis by supramolecular preorganization of monomers. J. Am. Chem. Soc. 2013, 135, 7118–7121. [Google Scholar] [CrossRef]
- Jun, Y.-S.; Lee, E.Z.; Wang, X.; Hong, W.H.; Stucky, G.D.; Thomas, A. From melamine-cyanuric acid supramolecular aggregates to carbon nitride hollow spheres. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- Fu, W.; Huang, Z. One-pot synthesis of a two-dimensional porous Fe3O4/poly(C3N3S3) network nanocomposite for the selective removal of Pb(II) and Hg(II) from synthetic wastewater. ACS Sustain. Chem. Eng. 2018, 6, 14785–14794. [Google Scholar] [CrossRef]
- Huang, Y.; Xia, S.; Lyu, J.; Tang, J. Highly efficient removal of aqueous Hg2+ and CH3Hg+ by selective modification of biochar with 3-mercaptopropyltrimethoxysilane. Chem. Eng. J. 2019, 360, 1646–1655. [Google Scholar] [CrossRef]
- Dinda, D.; Saha, S.K. Sulfuric acid doped poly diaminopyridine/graphene composite to remove high concentration of toxic Cr(VI). J. Hazard. Mater. 2015, 291, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, M.; Chmiel-Szukiewicz, E. Reactions of trithiocyanuric acid with oxiranes. I. Synthesis of Polyetherols. J. Appl. Polym. Sci. 2000, 76, 439–445. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Ahn, H.; Kim, O.; Park, M.J. Synthesis of three-dimensionally interconnected sulfur-rich polymers for cathode materials of high-rate lithium-sulfur batteries. Nat. Commun. 2015, 6, 7278. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, S.; Zhang, D.; Yu, C.; Lu, J.; Wang, D.; Sun, J. Magnetically recyclable visible-light-responsive MoS2@Fe3O4 photocatalysts targeting efficient wastewater treatment. J. Mater. Sci. 2017, 53, 1135–1147. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Ghodbane, I.; Hamdaoui, O. Removal of mercury(II) from aqueous media using eucalyptus bark: Kinetic and equilibrium studies. J. Hazard. Mater. 2008, 160, 301–309. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, Nickel and Lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Wen, T.; Liu, X.; Wang, Y.; Yang, H.; Sun, J.; Feng, J.; Dong, S.; Sun, J. Highly effective remediation of Pb(II) and Hg(II) contaminated wastewater and soil by flower-like magnetic MoS2 nanohybrid. Sci. Total Environ. 2020, 699, 134341. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, M.; Gong, Y.; Zeng, E.Y. Efficient removal of mercury from simulated groundwater using thiol-modified graphene oxide/Fe–Mn composite in fixed-bed columns: Experimental performance and mathematical modeling. Sci. Total Environ. 2020, 714, 136636. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Polo, M.; Rivera-Utrilla, J. Adsorbent-adsorbate interactions in the adsorption of Cd(II) and Hg(II) on ozonized activated carbons. Environ. Sci. Technol. 2002, 36, 3850–3854. [Google Scholar] [CrossRef]
- Chandra, V.; Kim, K.S. Highly selective adsorption of Hg2+ by a polypyrrole-reduced graphene oxide composite. Chem. Commun. 2011, 47, 3942–3944. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Y.; Tang, J.; Xia, S. Effective removal of inorganic mercury and methylmercury from aqueous solution using novel thiol-functionalized graphene oxide/Fe-Mn composite. J. Hazard. Mater. 2019, 366, 130–139. [Google Scholar] [CrossRef]
- El-Bayaa, A.A.; Badawy, N.A.; AlKhalik, E.A. Effect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineral. J. Hazard. Mater. 2009, 170, 1204–1209. [Google Scholar] [CrossRef]
- Wang, J.; Deng, B.; Chen, H.; Wang, X.; Zheng, J. Removal of aqueous Hg(II) by polyaniline: Sorption characteristics and mechanisms. Environ. Sci. Technol. 2009, 43, 5223–5228. [Google Scholar] [CrossRef]
- Cui, H.; Qian, Y.; Li, Q.; Zhang, Q.; Zhai, J. Adsorption of aqueous Hg(II) by a polyaniline/attapulgite composite. Chem. Eng. J. 2012, 211–212, 216–223. [Google Scholar] [CrossRef]
- Nascimento, F.H.D.; Masini, J.C. Influence of humic acid on adsorption of Hg(II) by vermiculite. J. Environ. Manag. 2014, 143, 1–7. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Hussein, F.B.; Mayer, B.K. Fixed-bed column study of phosphate adsorption using immobilized phosphate-binding protein. Chemosphere 2022, 295, 133908. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Niu, C.-G.; Li, X.-T.; Liang, C.; Guo, H.; Lin, L.-S.; Zheng, C.-W.; Zeng, G.-M. Efficient removal of Cd2+ and Pb2+ from aqueous solution with amino- and thiol-functionalized activated carbon: Isotherm and kinetics modeling. Sci. Total Environ. 2018, 635, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Frankl. Inst. Eng. Appl. Math. 1920, 189, 669. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of gas adsorption kinetics. I. A theoretical model for respirator cartridge service life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A new model for heavy metal removal in a biosorption column. Adsorpt. Sci. Technol. 2001, 19, 25–43. [Google Scholar] [CrossRef]

| Adsorbents | qmax (mg g−1) | Ref. |
|---|---|---|
| rGO-p(C3N3S3)/Fe3O4 | 400.0 | [28] |
| MoS2/Fe3O4 | 425.5 | [63] |
| SGO/Fe–Mn | 112.03 | [64] |
| PSAC-S | 136 | [23] |
| CCTNT | 199.80 | [24] |
| BCS | 4.30 | [2] |
| MPTAPs) | 211 | [36] |
| Zr-MSA/DCS | 312.4 | [25] |
| SP | 926 ± 165 | Present work |
| Variables | Yan Model | Adams–Bohart Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KY | qY | R2 | SS | ARE | tb | pτ | KAB(×10−3) | N0 | R2 | SS | ARE | |
| (mg g−1) | (×10−5) | (%) | (Year) | (L min−1 mg−1) | (mg L−1) | (×10−5) | (%) | |||||
| C0 (mg L−1) a | ||||||||||||
| 0.080 | 0.485 | 3187 | 0.962 | 11.8 | 8.29 | 11.8 | 3646 | 7.50 | 2.75 | 0.957 | 5.38 | 13.8 |
| 0.056 | 0.498 | 2344 | 0.983 | 3.17 | 14.8 | 24.5 | 6142 | 10.6 | 2.18 | 0.916 | 6.03 | 62.6 |
| 0.024 | 0.520 | 1870 | 0.982 | 1.42 | 6.08 | 42.7 | 8350 | 22.8 | 1.11 | 0.926 | 1.87 | 16.5 |
| νp (cm min−1) b | ||||||||||||
| 0.253 | 0.383 | 1764 | 0.989 | 4.03 | 7.94 | 7.16 | 3927 | 17.9 | 2.22 | 0.922 | 5.67 | 17.4 |
| 0.139 | 0.498 | 2344 | 0.983 | 3.17 | 14.8 | 24.5 | 6142 | 10.6 | 2.18 | 0.916 | 6.03 | 62.6 |
| 0.080 | 0.603 | 2984 | 0.977 | 7.62 | 29.0 | 69.4 | 11,531 | 6.01 | 2.22 | 0.920 | 4.76 | 23.3 |
| m (mg) c | ||||||||||||
| 0.5 | 0.485 | 3187 | 0.962 | 11.8 | 8.29 | 11.8 | 3646 | 7.50 | 2.75 | 0.957 | 5.38 | 13.8 |
| 1.0 | 0.504 | 2474 | 0.960 | 8.46 | 56.4 | 26.35 | 8062 | 7.33 | 2.82 | 0.963 | 1.44 | 13.32 |
| 1.5 | 0.576 | 1759 | 0.967 | 1.03 | 16.9 | 47.92 | 9705 | 7.37 | 2.80 | 0.964 | 5.56 | 13.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, W.; Sun, X.; Zhang, Q.; Ji, J.; Yan, Y.; Sun, J. Remediation of Hg-Contaminated Groundwater via Adsorption on Supramolecular Polymers in Batch Process and Column Test. Molecules 2025, 30, 1406. https://doi.org/10.3390/molecules30071406
Wang Z, Liu W, Sun X, Zhang Q, Ji J, Yan Y, Sun J. Remediation of Hg-Contaminated Groundwater via Adsorption on Supramolecular Polymers in Batch Process and Column Test. Molecules. 2025; 30(7):1406. https://doi.org/10.3390/molecules30071406
Chicago/Turabian StyleWang, Zongwu, Wei Liu, Xiaoyan Sun, Qing Zhang, Jiapu Ji, Yimeng Yan, and Jianhui Sun. 2025. "Remediation of Hg-Contaminated Groundwater via Adsorption on Supramolecular Polymers in Batch Process and Column Test" Molecules 30, no. 7: 1406. https://doi.org/10.3390/molecules30071406
APA StyleWang, Z., Liu, W., Sun, X., Zhang, Q., Ji, J., Yan, Y., & Sun, J. (2025). Remediation of Hg-Contaminated Groundwater via Adsorption on Supramolecular Polymers in Batch Process and Column Test. Molecules, 30(7), 1406. https://doi.org/10.3390/molecules30071406






