Yeast Viability in HLD–NAC-Designed Fully Dilutable Lecithin-Linker Microemulsions
Abstract
1. Introduction
2. Results and Discussions
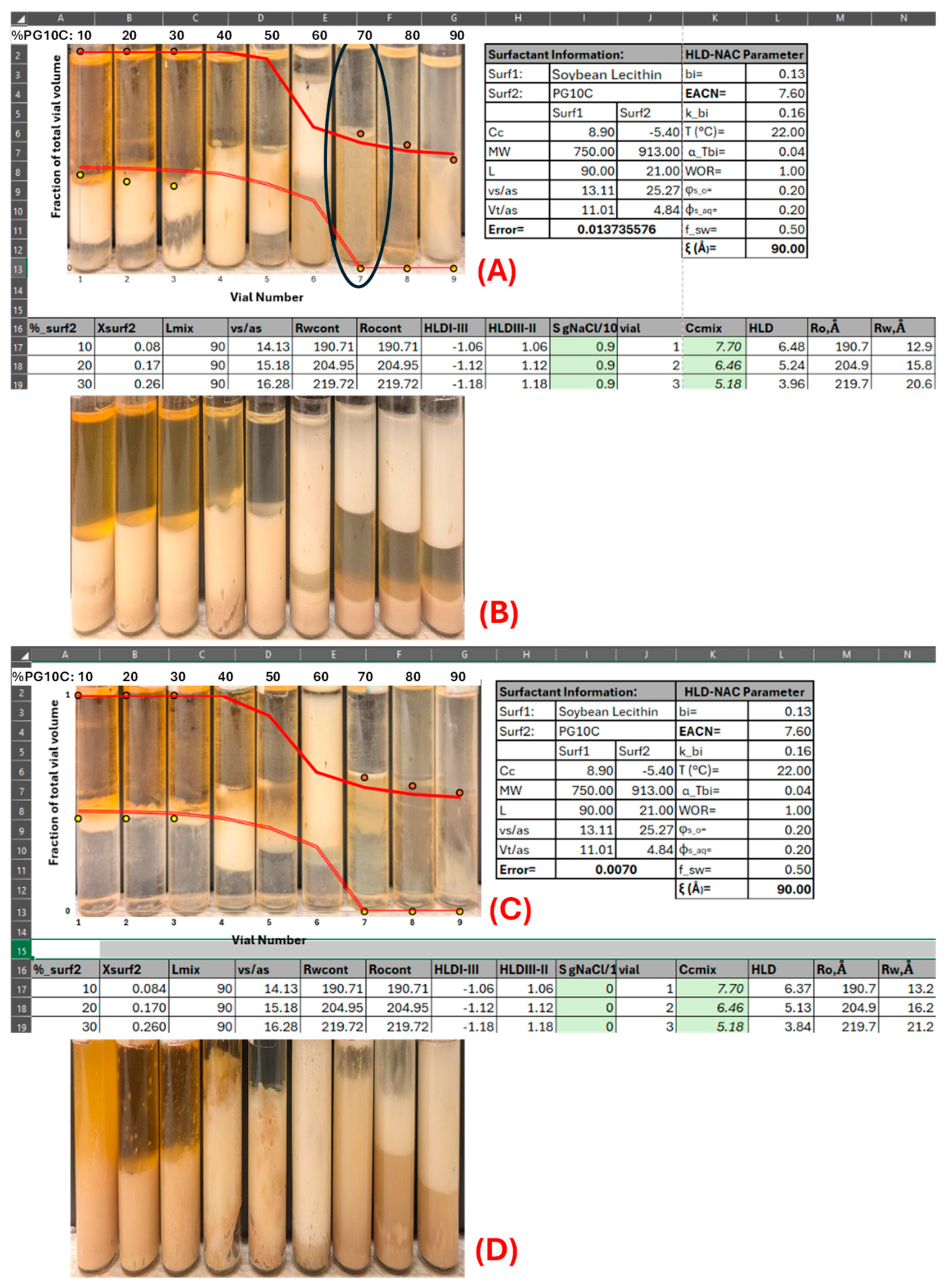
2.1. Determination of HLD Parameters
2.2. Lecithin–Hydrophilic Linker (Le–HL) Phase Scans
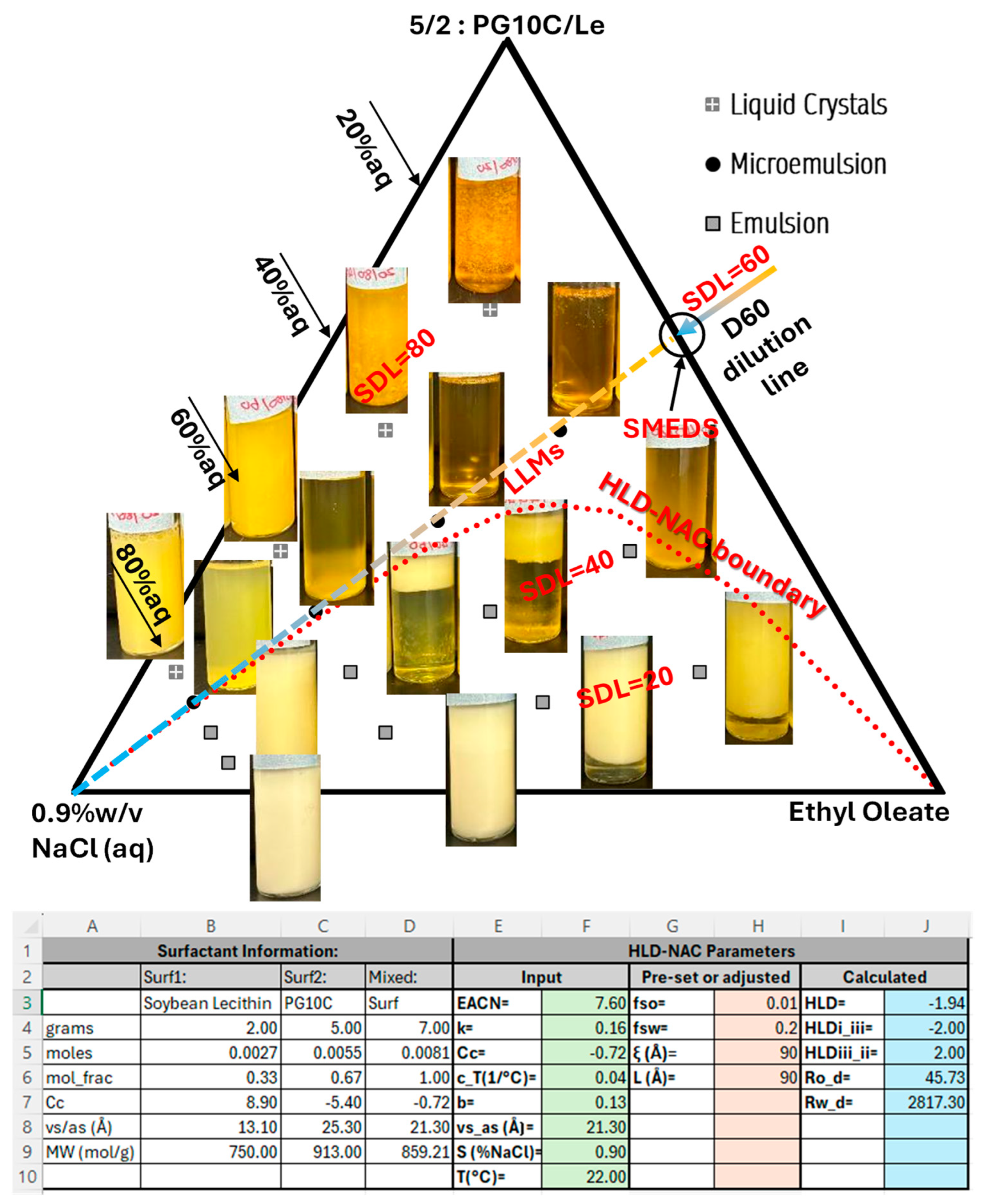
2.3. Ternary Phase Diagram (TPD)
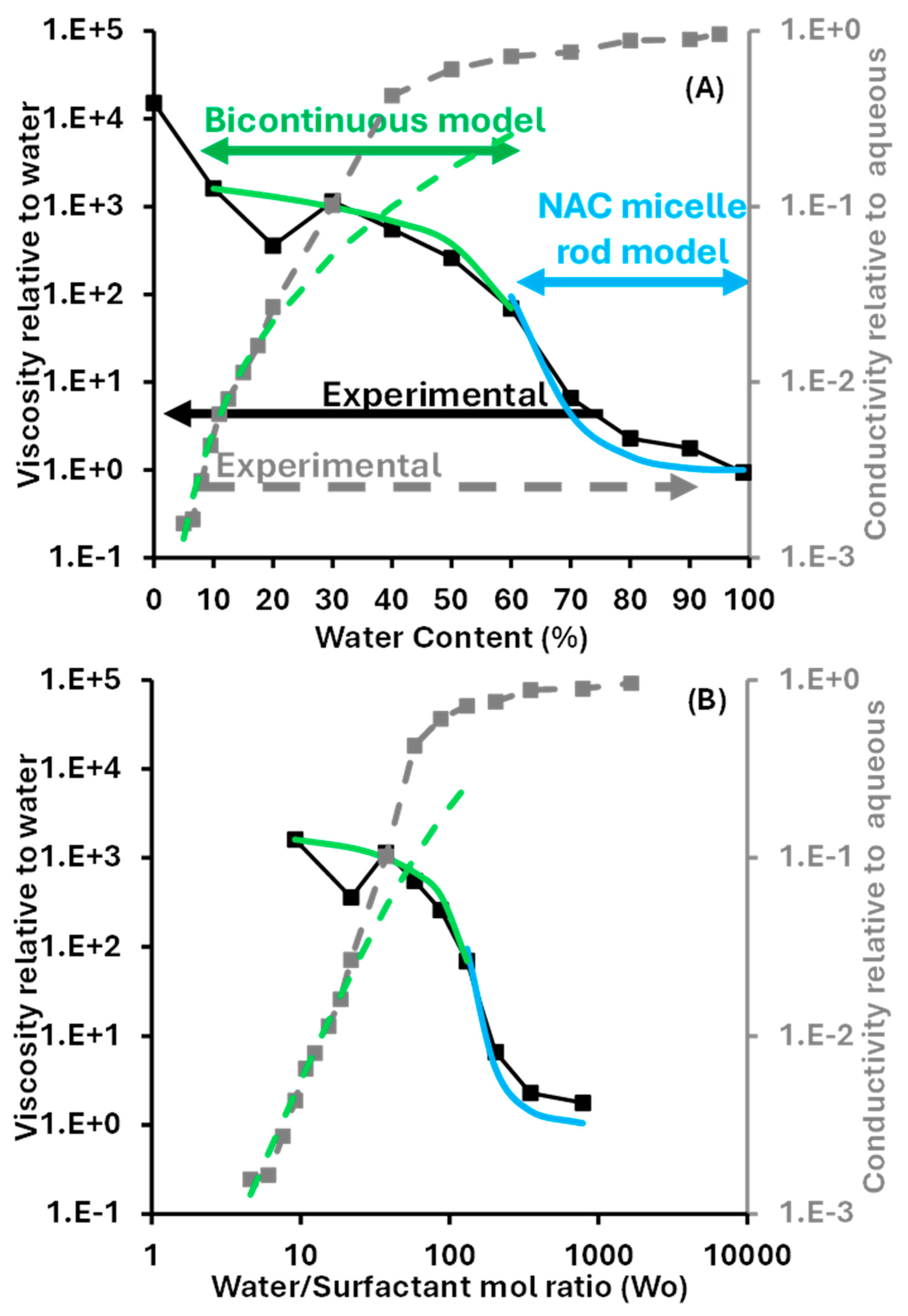
2.4. Conductivity and Viscosity Along SDL = 60 (D60) Dilution Line
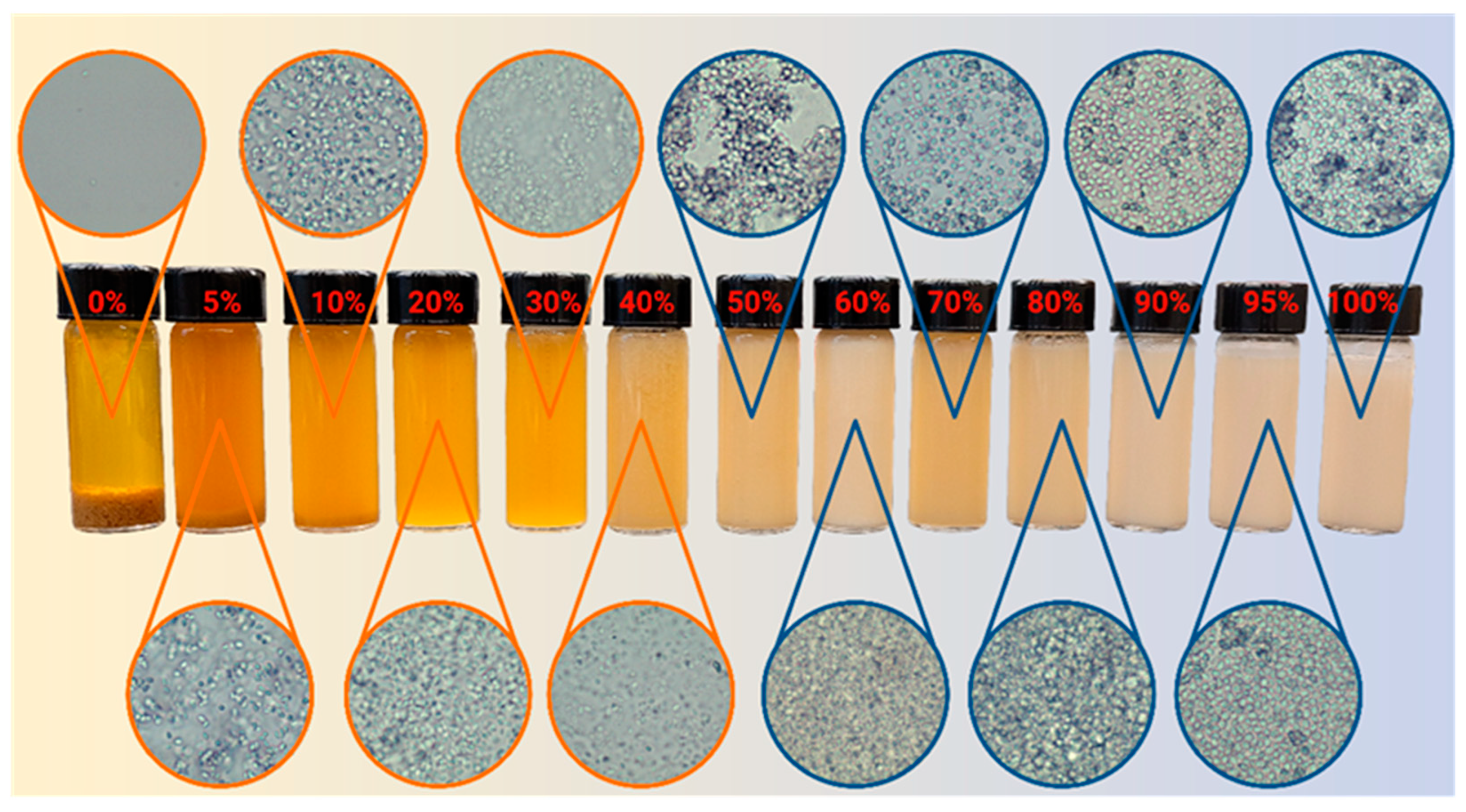
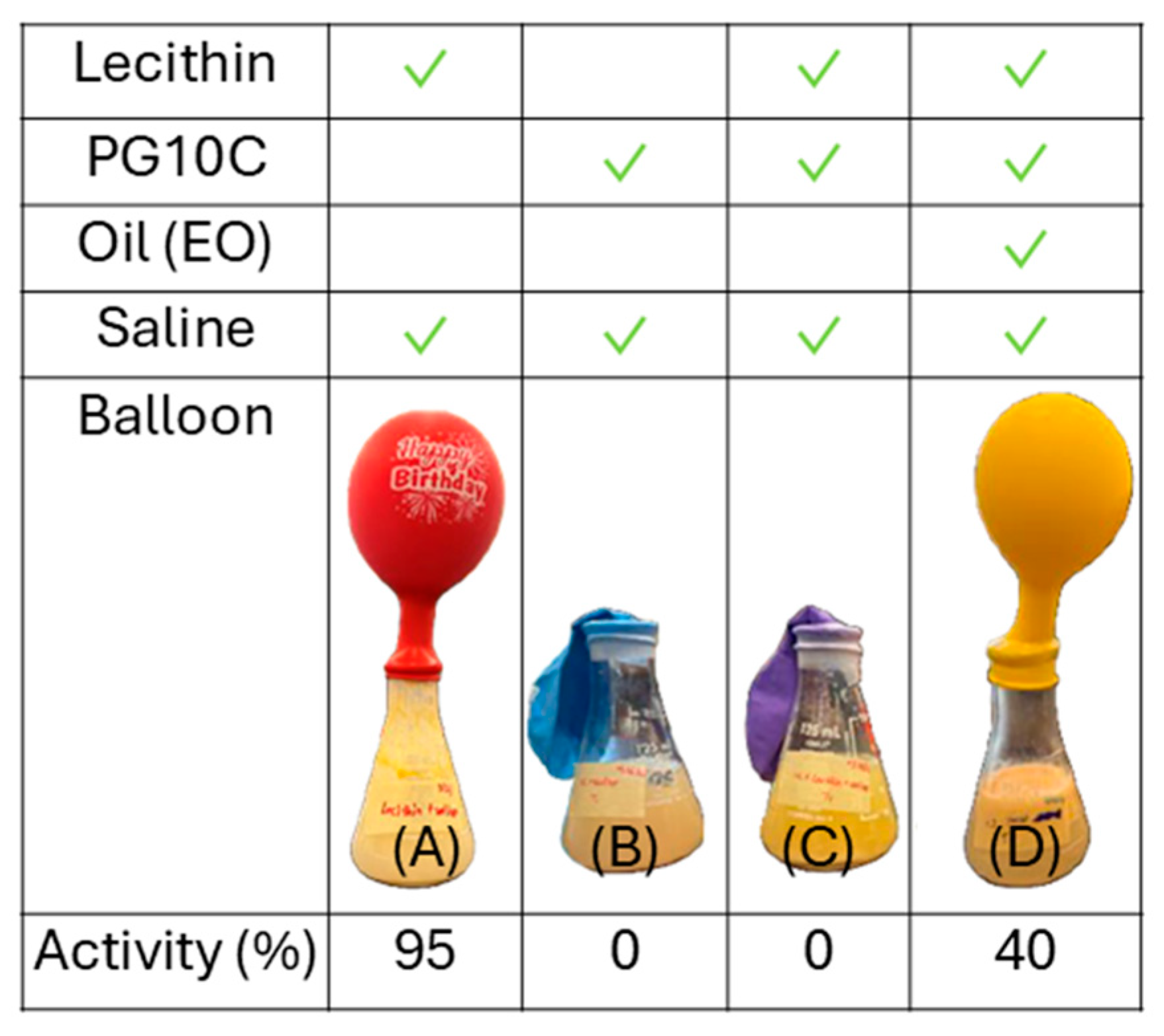
2.5. Yeast Dispersibility
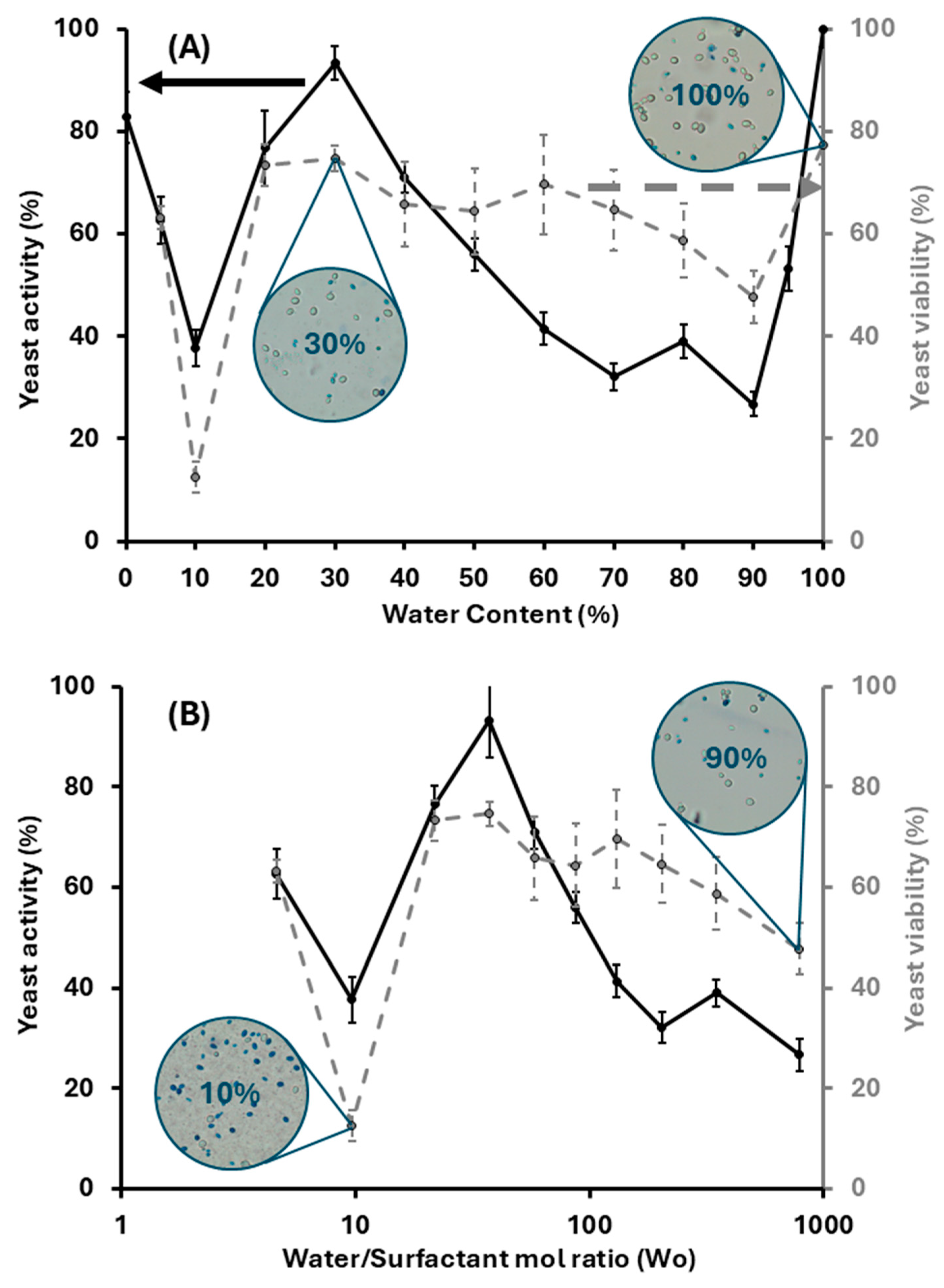
2.6. Yeast Viability and Activity
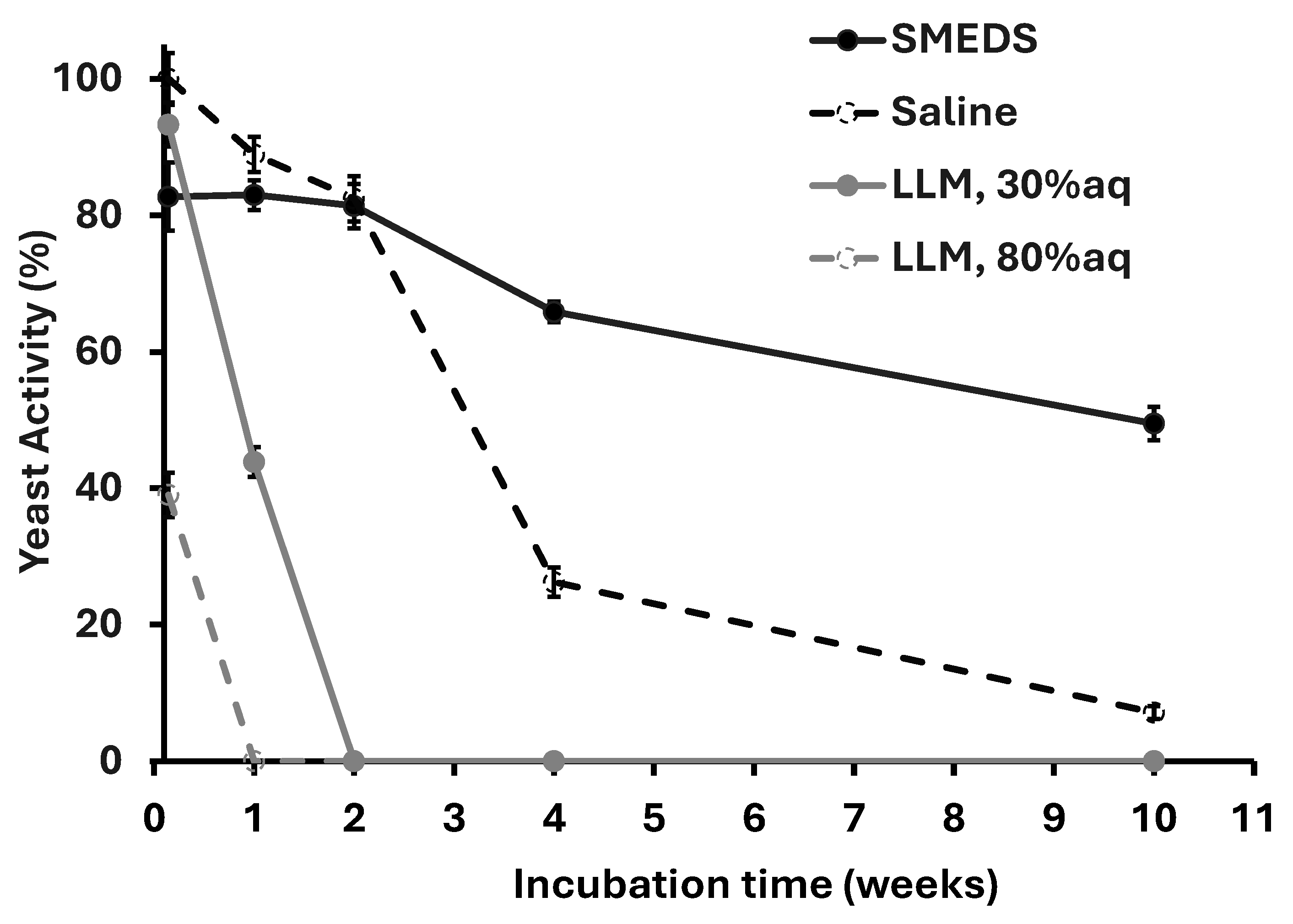
2.7. Long-Term Storage in SMEDS and LLMs
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Estimation of HLD and NAC Parameters for Lecithin-Linker Microemulsions
3.2.2. Lecithin–Hydrophilic Linker Phase Scans
3.2.3. Construction of the Ternary Phase Diagram (TPD)
3.2.4. Preparation of Le–HL µEs (LLMs) and Yeast-Loaded LLMs Along SDL = 60
3.2.5. Electrical Conductivity Studies
3.2.6. Viscosity
3.2.7. Saccharomyces cerevisiae Dispersibility in Le-HL μEs
3.2.8. Saccharomyces cerevisiae Activity
3.2.9. Saccharomyces cerevisiae Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfammatter, N.; Guadalupe, A.A.; Luisi, P.L. Solubilization and Activity of Yeast Cells in Water-in-Oil Microemulsion. Biochem. Biophys. Res. Commun. 1989, 161, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Hoppert, M.; Mlejnek, K.; Seiffert, B.; Mayer, F. Activities of Microorganisms and Enzymes in Water-Restricted Environments: Biological Activities in Aqueous Compartments at Μm-Scale. In Proceedings of the SPIE—The International Society for Optical Engineering; SPIE: Bellingham, WA, USA, 1997; Volume 3111, pp. 501–509. [Google Scholar] [CrossRef]
- Pfammatter, N.; Hochköppler, A.; Luisi, P.L. Solubilization and Growth of Candida Pseudotropicalis in Water-in-oil Microemulsions. Biotechnol. Bioeng. 1992, 40, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Prichanont, S.; Leak, D.J.; Stuckey, D.C. The Solubilisation of Mycobacterium in a Water in Oil Microemulsion for Biotransformations: System Selection and Characterisation. Colloids Surf. A Physicochem. Eng. Asp. 2000, 166, 177–186. [Google Scholar] [CrossRef]
- Prichanont, S.; Leak, D.J.; Stuckey, D.C. Chiral Epoxide Production Using Mycobacterium Solubilized in a Water-in-Oil Microemulsion. Enzym. Microb. Technol. 2000, 27, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Stefan, A.; Palazzo, G.; Ceglie, A.; Panzavolta, E.; Hochkoeppler, A. Water-in-Oil Macroemulsions Sustain Long-Term Viability of Microbial Cells in Organic Solvents. Biotechnol. Bioeng. 2003, 81, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Wu, L.; Zheng, X.; Zhu, S.; Feng, F.; Shen, L. Anti-Yeast Activity of a Food-Grade Dilution-Stable Microemulsion. Appl. Microbiol. Biotechnol. 2010, 87, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Acosta, E.J.; Nguyen, T.; Witthayapanyanon, A.; Harwell, J.H.; Sabatini, D.A. Linker-Based Bio-Compatible Microemulsions. Environ. Sci. Technol. 2005, 39, 1275–1282. [Google Scholar] [CrossRef]
- Nouraei, M.; Collymore, C.; Diosady, L.; Acosta, E. HLD-NAC Design and Evaluation of a Fully Dilutable Lecithin-Linker SMEDDS for Ibuprofen. Int. J. Pharm. 2021, 610, 121237. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, D.A.; Acosta, E.; Harwell, J.H. Linker Molecules in Surfactant Mixtures. Curr. Opin. Colloid Interface Sci. 2003, 8, 316–326. [Google Scholar] [CrossRef]
- Yuan, J.S.; Ansari, M.; Samaan, M.; Acosta, E.J. Linker-Based Lecithin Microemulsions for Transdermal Delivery of Lidocaine. Int. J. Pharm. 2008, 349, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Zarate-Muñoz, S.; Texeira De Vasconcelos, F.; Myint-Myat, K.; Minchom, J.; Acosta, E.J. A Simplified Methodology to Measure the Characteristic Curvature (Cc) of Alkyl Ethoxylate Nonionic Surfactants. J. Surfactants Deterg. 2016, 19, 249–263. [Google Scholar] [CrossRef]
- Nouraei, M.; Acosta, E.J. Predicting Solubilisation Features of Ternary Phase Diagrams of Fully Dilutable Lecithin Linker Microemulsions. J. Colloid Interface Sci. 2017, 495, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Acosta, E.J.; Sundar, S. How to Formulate Biobased Surfactants Through the HLD-NAC Model. In Biobased Surfactants; Hayes, D., Solaiman, D., Ashby, R., Eds.; AOCS Press: London, UK, 2019; pp. 471–510. [Google Scholar] [CrossRef]
- Sundar, S.; Nouraei, M.; Latta, T.; Acosta, E. Hydrophilic-Lipophilic-Difference (HLD) Guided Formulation of Oil Spill Dispersants with Biobased Surfactants. Tenside Surfactants Deterg. 2019, 56, 417–428. [Google Scholar] [CrossRef]
- Acosta, E.J. Formulation Engineering with the Hydrophilic-Lipophilic-Difference (HLD) and Net-Average Curvature (NAC) HLD-NAC: Tutorial; ResearchGate Methods: Toronto, ON, Canada, 2023; p. 12. [Google Scholar] [CrossRef]
- Ontiveros, J.F.; Pierlot, C.; Catté, M.; Molinier, V.; Pizzino, A.; Salager, J.L.; Aubry, J.M. Classification of Ester Oils According to Their Equivalent Alkane Carbon Number (EACN) and Asymmetry of Fish Diagrams of C10E4/Ester Oil/Water Systems. J. Colloid Interface Sci. 2013, 403, 67–76. [Google Scholar] [CrossRef]
- Acosta, E.; Natali, S. Effect of Surfactant Concentration on the Hydrophobicity of Polydisperse Alkyl Ethoxylates. J. Surfactants Deterg. 2022, 25, 79–94. [Google Scholar] [CrossRef]
- Han, J.; Lee, M.J.; Lee, K.; Lee, Y.J.; Kwon, S.H.; Min, J.H.; Lee, E.; Lee, W.; Lee, S.W.; Kim, B.J. Role of Bicontinuous Structure in Elastomeric Electrolytes for High-Energy Solid-State Lithium-Metal Batteries. Adv. Mater. 2023, 35, e2205194. [Google Scholar] [CrossRef]
- Mackie, T.S.; Meares, P. The Diffusion of Electrolytes in a Cation-Exchange Resin Membrane. I. Theoretical. Proc. R. Soc. Lond. Ser. A 1955, 232, 498–509. [Google Scholar] [CrossRef]
- Kiran, S.K.; Acosta, E.J. Predicting the Morphology and Viscosity of Microemulsions Using the HLD-NAC Model. Ind. Eng. Chem. Res. 2010, 49, 3424–3432. [Google Scholar] [CrossRef]
- Krishnan, K.; Chapman, B.; Bates, F.S.; Lodge, T.P.; Almdal, K.; Burghardt, W.R. Effects of Shear Flow on a Polymeric Bicontinuous Microemulsion: Equilibrium and Steady State Behavior. J. Rheol. 2002, 46, 529–554. [Google Scholar] [CrossRef]
- Beuchat, L.R. Influence of Water Activity on Growth, Metabolic Activities and Survival of Yeasts and Molds. J. Food Prot. 1983, 46, 135–141. [Google Scholar] [CrossRef]
- Galvin, K.; McDonald, J.A.; Robinson, B.H.; Knoche, W. Determination of Water Activity in Water-in-Oil Microemulsions. Colloids Surf. 1987, 25, 195–204. [Google Scholar] [CrossRef]
- Cavanagh, R.J.; Smith, P.A.; Stolnik, S. Exposure to a Nonionic Surfactant Induces a Response Akin to Heat-Shock Apoptosis in Intestinal Epithelial Cells: Implications for Excipients Safety. Mol. Pharm. 2019, 16, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Partearroyo, M.A.; Ostolaza, H.; Goñi, F.M.; Barberá-Guillem, E. Surfactant-Induced Cell Toxicity and Cell Lysis. A Study Using B16 Melanoma Cells. Biochem. Pharmacol. 1990, 40, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, L.; Zhao, D.; Zhao, H.; Chen, Y.; Li, Y.; Zeng, Y. Protective Effect of Wheat Gluten Peptides against Ethanol-Stress Damage in Yeast Cell and Identification of Anti-Ethanol Peptides. LWT 2024, 192, 115732. [Google Scholar] [CrossRef]
- Estela-Escalante, W.D.; Moscosa-Santillán, M.; González-Ramírez, J.E.; Rosales-Mendoza, S. Evaluation of the Potential Production of Ethanol by Candida Zemplinina Yeast with Regard to Beer Fermentation. J. Am. Soc. Brew. Chem. 2017, 75, 130–135. [Google Scholar] [CrossRef]
- Munder, M.C.; Midtvedt, D.; Franzmann, T.; Nüske, E.; Otto, O.; Herbig, M.; Ulbricht, E.; Müller, P.; Taubenberger, A.; Maharana, S.; et al. A PH-Driven Transition of the Cytoplasm from a Fluid- to a Solid-like State Promotes Entry into Dormancy. eLife 2016, 5, e09347. [Google Scholar] [CrossRef]
- Aukema, K.G.; Wang, M.; de Souza, B.; O’Keane, S.; Clipsham, M.; Wackett, L.P.; Aksan, A. Core-Shell Encapsulation Formulations to Stabilize Desiccated Bradyrhizobium against High Environmental Temperature and Humidity. Microb. Biotechnol. 2022, 15, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Kuliešienė, N.; Žūkienė, R.; Khroustalyova, G.; Chang, C.-R.; Rapoport, A.; Daugelavičius, R. Changes in Energy Status of Saccharomyces Cerevisiae Cells during Dehydration and Rehydration. Microorganisms 2021, 9, 444. [Google Scholar] [CrossRef]
- Thomas, C.R.; Stenson, J.D.; Zhang, Z. Measuring the Mechanical Properties of Single Microbial Cells. In High Resolution Microbial Single Cell Analytics; Springer: Berlin/Heidelberg, Germany, 2011; Volume 124. [Google Scholar] [CrossRef]
- Han, T.; Zhang, S.; Zhang, C. Unlocking the Secrets behind Liquid Superlubricity: A State-of-the-Art Review on Phenomena and Mechanisms. Friction 2022, 10, 1137–1165. [Google Scholar] [CrossRef]
- Agee, S.; Rowland, T. Single-Celled Science: Yeasty Beasties. Science Buddies. Available online: www.sciencebuddies.org/science-fair-projects/project-ideas/MicroBio_p011/microbiology/yeast-activity-balloons (accessed on 24 November 2024).
- Painting, K.; Kirsop, B. A Quick Method for Estimating the Percentage of Viable Cells in a Yeast Population, Using Methylene Blue Staining. World J. Microbiol. Biotechnol. 1990, 6, 346–347. [Google Scholar] [CrossRef]
- Parker, R.A.; Gabriel, K.T.; Graham, K.; Cornelison, C.T. Validation of Methylene Blue Viability Staining with the Emerging Pathogen Candida Auris. J. Microbiol. Methods 2020, 169, 105829. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doratt Mendoza, J.; Ding, J.; Acosta Alvarez, M.; Acosta, E. Yeast Viability in HLD–NAC-Designed Fully Dilutable Lecithin-Linker Microemulsions. Molecules 2025, 30, 921. https://doi.org/10.3390/molecules30040921
Doratt Mendoza J, Ding J, Acosta Alvarez M, Acosta E. Yeast Viability in HLD–NAC-Designed Fully Dilutable Lecithin-Linker Microemulsions. Molecules. 2025; 30(4):921. https://doi.org/10.3390/molecules30040921
Chicago/Turabian StyleDoratt Mendoza, Juan, Jingwen Ding, Michelle Acosta Alvarez, and Edgar Acosta. 2025. "Yeast Viability in HLD–NAC-Designed Fully Dilutable Lecithin-Linker Microemulsions" Molecules 30, no. 4: 921. https://doi.org/10.3390/molecules30040921
APA StyleDoratt Mendoza, J., Ding, J., Acosta Alvarez, M., & Acosta, E. (2025). Yeast Viability in HLD–NAC-Designed Fully Dilutable Lecithin-Linker Microemulsions. Molecules, 30(4), 921. https://doi.org/10.3390/molecules30040921







