Abstract
Designing supramolecular gelators with targeted properties is very difficult and mainly relies on structural modifications of known gelator molecules. However, very often, even minor modifications can result in the complete loss of gelation capabilities. In the present work, we have studied the influence and role of the silver nanoparticles (AgNPs) and trisodium citrate (TSC) additives on the self-assembly process of alanine derivative gelator (C12Ala) and intermolecular interactions resulting in hydrogel systems of enhanced stability and sustainability. The effect of phase separation and diversity of supramolecular microstructures of gelator internal matrix on the composition of the investigated tricomponent system was studied thoroughly with thermal analysis methods (TGA/DSC), high-resolution nuclear magnetic resonance spectroscopy (HR-MAS NMR), and polarising optical microscopy (POM). The molecular mechanism of gelation and the interactions responsible for enhanced properties of nanosilver hydrogels was determined and described, indicating the synergistic role of TSC and AgNPs in the self-assembly process.
1. Introduction
Soft matter systems like physical gels/hydrogels find broad applications in fuels [1,2], food [3,4] and the pharmaceutical industry [5,6]. The critical parameter for successfully using these materials is their thermal and temporal stability, which depends strongly on the gelator structure and the additives incorporated in the gel. To this day, it remains impossible to predict whether a given molecule has solvent gelling properties or not. Gel research is primarily experimental work. However, sometimes a molecule that does not have solvent-gelling properties on its own acquires this property in the presence of another molecule. An example of such a molecule is N-lauroyl-l-alanine (C12Ala), which on its own gels water very poorly and does not have the gelling properties of a hydrolate enriched with silver nanoparticles. However, the situation changes radically when the gelator–nanoparticle hydrolate system is enriched with a stabiliser molecule. Non-toxic hydrogels enriched with silver nanoparticles (AgNPs) constitute a group of materials with extremely high pharmaceutical potential [7]. In the case of AgNPs containing gels aside from the metal, the stabilators used in nanoparticle synthesis impact the gel properties. One of the most common stabilators used to synthesise negatively charged AgNPs is trisodium citrate (TSC) [8].
The biological activity of silver nanoparticles has been repeatedly confirmed in the fight against microorganisms (including bacteria, viruses, and fungi) or pathological human cancer cells [9,10,11,12]. The bioactive role of nanoparticles, including silver, depends strongly on their size and can have positive as well as negative effects on living organisms. The toxicology of nanoparticles is complicated, and the mechanism of hemotoxicity of silver nanoparticles, for example, is still not fully understood. It is unclear whether it results from the direct impact of nanoparticles on cells or from the release of silver ions. However, we can conclude that the smaller size of nanoparticles results in their more significant biological activity [13,14,15,16]. Another field where silver nanoparticles can be used is photodynamic cancer therapy (FTD). One of the effects of silver ions interactions with cells is the production of reactive oxygen species, especially their singlet form, which causes damage and cell death. The combination of a photosensitiser with silver nanoparticles leads to an increase in the efficiency of the production of free radicals in cells. One possible way to introduce photosensitisers into diseased areas is through the use of hydrogels [17,18]. Due to the alarming statistics of cancer cases, research on improving anti-cancer therapies is currently a civilisational challenge. We already know that antibiotics supported by nanoparticles improve the effectiveness of drugs, but we need personalised therapies with potentially higher therapeutic efficacy [19,20,21,22].
One of the known systems for delivering active substances to the body are gels [23]. A necessary condition for creating such systems for therapeutic purposes is the use of components (solvents and gelators) that have a neutral effect on the organism, can transform pharmaceuticals into an inactive gel form, and release and activate them at the targeted side. However, finding such a gelator to create non-toxic, durable, and stable systems is difficult. The best candidates are hydrogels, which use water to dissolve and immobilise the drug in the gel phase [24,25]. Recent research on different low-molecular-weight gelators showed that the representative of fatty acid amide C12Ala displays the ability to create physical gels with varying types of solvents, including organic solvents, oils, emulsions (oil–water mixture) and water [26,27,28,29,30]. In particular, water is an important solvent that can create gel-based drug carrier systems enriched with gold and silver nanoparticles [9,31].
Although C12Ala (LMWGs; low-molecular-weight gelators) has been shown to form hydrogels, it was found that their stability is time-limited. The pure hydrogel C12Ala-H2O undergoes phase separation within a few hours after gelation. The stable, homogeneous gel (verified by the inverse tube test) disintegrates and begins to resemble the structure of semolina or micellar fluid. While working on the C12Ala gel enriched with silver nanoparticles, we noticed that after a few months of storing the C12Ala-TSC hydrogel at 5 °C, the solid and liquid phases also began to separate, which resulted in the gravitational outflow of water from the sample. This effect does not bode well for the durability of the resulting gel but did not occur in the case of the gel enriched with nanoparticles. The sample enriched with TSC and AgNPs, tightly closed and stored at 5 °C retained its properties for 5 months. In the publication [9] (see Figure 5a,b), we see photos of upside-down vials with stored gels. The C12Ala-TSC-AgNP sample looks homogeneous, but at the bottom of the vial with C12Ala-TSC-H20 gel (top of the image), we can see an area where water has accumulated, and the gel does not look homogeneous but rather resembles a “semolina” structure. So, the question arose about the effect of TSC and AgNPs on the internal structure of the gel and how TSC and silver nanoparticles contribute to its longer stability. In this work, the authors attempt to explain this phenomenon.
2. Materials and Methods
2.1. Hydrogel Preparation
The investigated samples of hydrogels were prepared according to the following procedures. The C12Ala-H2O hydrogels were obtained by dissolving 64 mg and 128 mg of the C12Ala gelator [32] in 1 mL of deionised water to obtain 6 wt.% and 12 wt.% mixture concentrations, respectively. The dissolution was performed using a circulating water bath at 60 °C for 24 h. After dissolving the gelator molecules, the samples were cooled to room temperature for 12 h. The C12Ala-TSC-H2O hydrogels were prepared by dissolving 323 mg of TSC in 1 mL of deionised water, resulting in a molar concentration of Cm = 1.25 M. Next, the prepared solution of TSC was mixed with deionised water in a volume ratio of 0.5%/95.5% and used to prepare hydrogel samples with 6 wt.% and 12 wt.% concentrations of gelator. The procedure for dissolving the gelator in the prepared solution and obtaining the gel phase was the same as for the C12Ala-H2O system. The tube inversion test was performed to confirm no bulk flow. However, only the TSC-enriched sample retained its lack of flow over time. The C12Ala-TSC-AgNP hydrogels were prepared by mixing 995 mL of AgNP water suspension and 5 mL of a 1.25 M water solution of TSC with 64 mg and 128 mg of gelator to obtain 6 wt.% and 12 wt.% hydrogels respectively. The silver nanoparticles with an average size of 30 nm (AgNPs) were synthesised as described in [9]. The chemical structures of the used compounds are depicted in Figure 1. The gelator dissolution process was carried out the same way as for other samples. As a result, we obtained three different gel systems: pure hydrogel C12Ala-H2O (not stable in the concentration of 6 wt.%), hydrogel enriched with TSC (C12Ala-TSC-H2O), and hydrogel enriched with TSC and silver nanoparticles, AgNPs, (C12Ala-TSC-AgNPs). Without the addition of TSC, the C12Ala molecule did not gel the nanoparticle solution containing AgNPs.
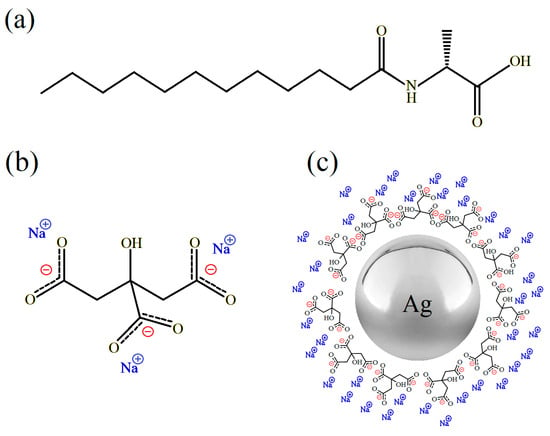
Figure 1.
The chemical structure of (a) C12Ala, (b) TSC, and (c) illustrative view of negatively charged silver nanoparticles stabilised by TSC.
2.2. Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) measurements were performed using a Perkin-Elmer DSC4000 instrument (Waltham, MA, USA). The measurements were conducted in a nitrogen atmosphere at a heating/cooling rate of 15 °C/min in the temperature range of 20 to 100 °C. The heating and cooling rate of 15 K/min was slightly higher than the conventional 10 K/min to estimate the safe limit for storing samples in the gel state. The samples weighing approximately 14.4 mg and 12.4 mg for hydrogels with TSC and TSC-AgNPs, respectively, were loaded in hermetic crucibles. Three consecutive cycles of heating and cooling were recorded for each sample. The measurements allowed us to determine the characteristic endothermic and exothermic peaks for melting and gelation temperatures of the self-assembled matrices of the studied hydrogels.
2.3. Thermogravimetric Analysis (TGA/DTG)
Thermogravimetric analysis (TGA) was performed on a Perkin-Elmer TGA8000 device. The method allows for recording the mass loss of the sample during its thermal degradation. The measurements were performed under a nitrogen atmosphere for TSC and TSC-AgNP hydrogel samples at 10 °C/min heating rates in the temperature range of 20 to 1000 °C. The results were analysed according to the derivative method (DTG).
2.4. NMR Spectroscopy
The 13C NMR spectra of the studied samples were recorded using a Bruker Avance III HD NMR spectrometer (Billerica, MA, USA) coupled to an 11.4 T superconducting magnet operating at 125.76 MHz for the carbon Larmour frequency. All experiments were recorded at room temperature using the solid-state technique for high-resolution NMR spectroscopy. The 13C spectra were recorded using the 1H-13C CP-MAS technique to improve the S/N ratio for recorded signals. In the case of the C12Ala gelator in the solid state, 2.5 mm zirconia rotors and 20 kHz spinning frequency were used for measurements. To record the 13C NMR spectra of TSC solution and hydrogel samples, special 25 μL Kel-F inserts for 4 mm zirconia rotors and 5 kHz of spinning frequency were used. The lower spinning rate and special inserts were used to avoid the risk of mechanically destroying the gel state due to excessive centrifugal force and leakage of the solvent. The contact time during the cross-polarisation sequence was set to 1500 μs, the recycle delay was set to 15 s, and 2048 scans were accumulated, resulting in over 8 h of acquisition time per spectrum. The proton decoupling was achieved with a TPPM sequence at an 80 kHz radiofrequency field.
2.5. Microscopic Observation
The images of the gel microstructures were taken on an Olympus BX53 microscope (Tokyo, Japan). The pure, blank, and AgNP-enriched 12 wt.% gels were cast onto microscope slides and covered with a 130 nm thick coverslip. An image of the nanoparticle AgNP solution with the C12Ala gelator was also taken for comparison. Images were taken immediately after the samples were placed on the slides.
3. Results
3.1. Calorimetric Tests (DSC)
To gain information on thermal events in the blank and silver-enriched sample, DSC scans were performed in the temperature range of 20 to 100 °C. Figure 2a,b show two heating–cooling cycles for blank (C12Ala-TSC-H2O) and silver-enriched (C12Ala-TSC-AgNPs) gels.
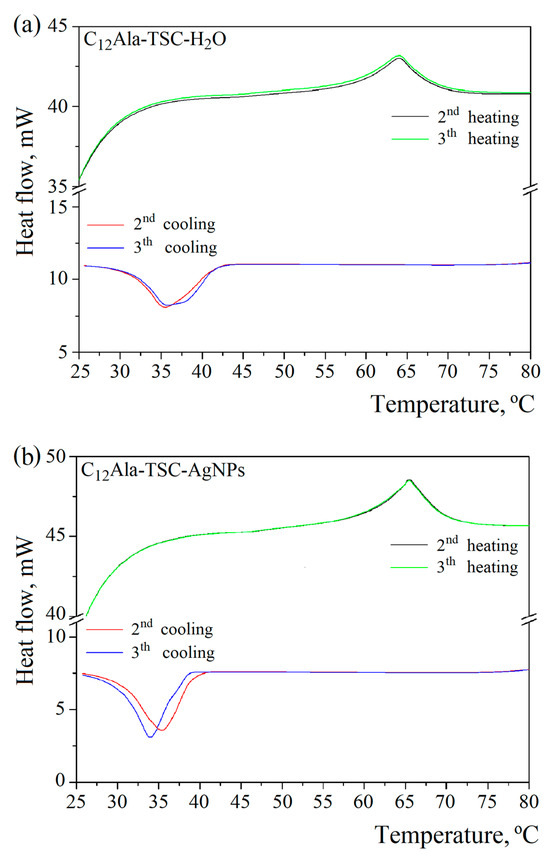
Figure 2.
DSC curves of (a) C12Ala-TSC-H2O and (b) C12Ala-TSC-AgNP gels.
The DSC examination revealed two characteristic peaks, gel–sol and gelation, for both samples. The gel–sol points were 64 °C and 65.5 °C for blank and silver-enriched gels, respectively. The gelation temperature varies from 35.5 °C to 34 °C in the second and third cooling cycles for the enriched sample, and for the blank sample, it remains more stable at 35.5 °C. This indicates silver nanoparticles’ influence on the gelator molecules’ organisation in the gelation process. In each subsequent heating and cooling cycle, this process may take place slightly differently.
3.2. Thermogravimetric Analysis (TGA + DTG)
Two (for liquid and solid phases) and three (for liquid, solid, and nanoparticles phases) characteristic peaks related to decomposition or evaporation (water) of individual components for the blank (C12Ala-TSC-H2O) and silver-enriched samples (C12Ala-TSC-AgNPs), respectively, are visible on the TGA and DTG curves (Figure 3a,b). Figure 3c represents the DTG curves of TSC and C12Ala powder. To compare the degradation temperatures of the individual phases of the systems, Figure 3d shows the normalised mass loss derivatives for both tested samples. The results do not include the pure C12Ala-H2O hydrogel sample because it disintegrated too quickly for its properties to be compared with the stable samples.
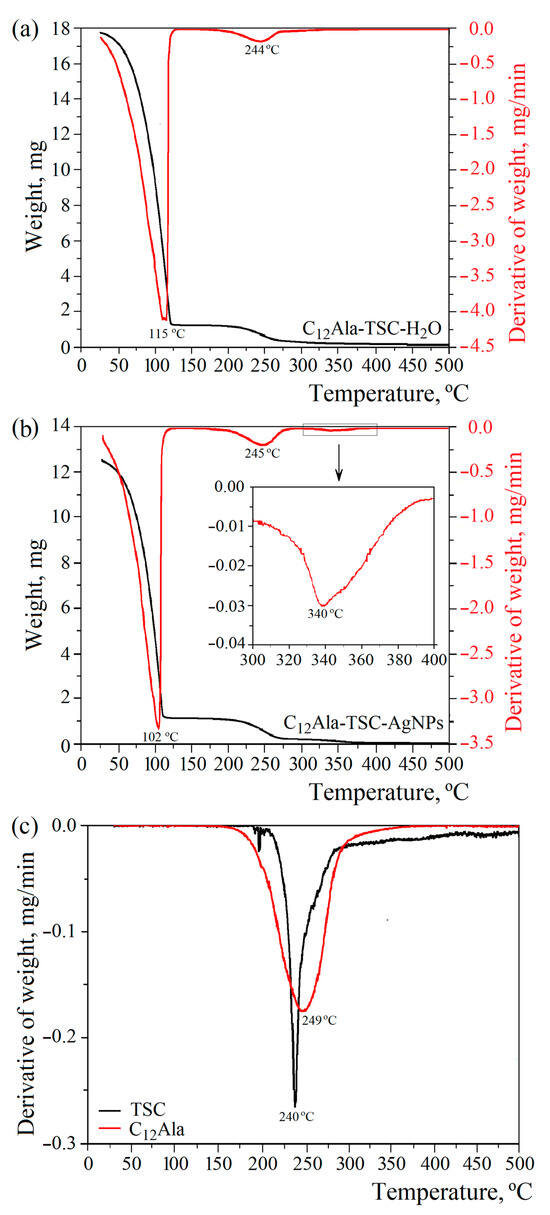
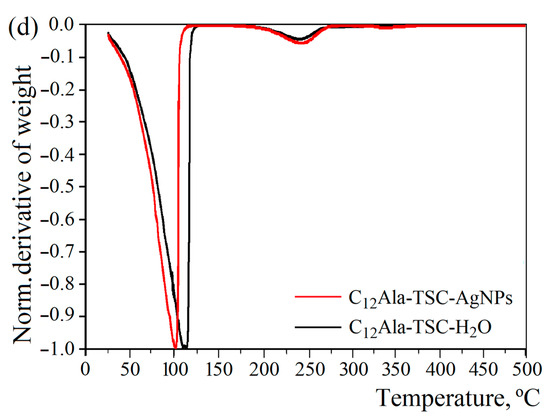
Figure 3.
The 10 °C/min TGA and DTG thermograms of (a) C12Ala-TSC-H2O, (b) C12Ala-TSC-AgNPs, (c) DTG curves of TSC and C12Ala powder, and (d) normalised derivatives of weight loss of gel samples.
The addition of silver nanoparticles, AgNPs, to the hydrogel of C12Ala with TSC influences the temperature water fraction evaporation. The DTG curves (Figure 3b) show a decrease in the inflection point related to the temperature at which the mass loss is the quickest by 13 °C in comparison to the silver-unenriched sample (Figure 3a). The maximum water evaporation rate occurs at 102 °C and 115 °C for the sample with and without silver nanoparticles, respectively. Two possible reasons can cause such behaviour: the intramolecular interactions between the AgNPs and the gelator matrix and the physical nature of the silver nanoparticles themselves. The intramolecular interactions with AgNPs can disturb the mechanism of physical bonding between the gelator molecules, causing a weakening of the gel strength. On the other hand, the physical properties of silver nanoparticles, namely lower heat capacity and higher heat transfer, can cause local heating and additionally weaken the physical bonds between C12Ala molecules. Materials with low heat capacity and high thermal conductivity heat up faster and release accumulated energy more quickly. The heat capacity and thermal conductivity of water and silver are 4200 J/kg·K and 0.6 W/m·K and 237 J/kg·K and 429 W/m·K, respectively. Although adding AgNPs decreases the decomposition temperature, it increases the system’s long-term stability at temperatures below 100 °C. The decomposition process observed at temperatures above 300 °C is related to the melting of AgNPs (Figure 3b, highlighted area). The total weight content of silver nanoparticles in the liquid fraction of the gel is 0.44 % (4.4 mg/mL). Uncrushed silver melts at a temperature of 960 °C. However, silver nanoparticles have a melting point of approximately 360 °C (depending on size), so we can conclude that the peaks visible in this temperature region correspond to the decomposition process of silver nanoparticles. The observed peak in the temperature range 175–275 °C is indistinguishable for TSC and C12Ala due to the similar decomposition temperature of both materials, as shown in Figure 3c. The maximum rate of water evaporation is 3.32 mg/min at 102 °C and 4.10 mg/min at 115 °C for the enriched and blank samples, respectively.
3.3. 13C CPMAS NMR
High-resolution NMR techniques were used to investigate the intra- and intermolecular interactions in all studied systems. The theoretical 13C NMR spectra were calculated for isolated molecules of TSC and C12Ala based on their chemical structures to correctly assign and analyse the obtained experimental results. In Figure 4a,b, the obtained theoretical results are displayed in black, whereas the experimentally recorded spectra in the solid-state are presented in blue. The spectra recorded for C12Ala and TSC in the solid state are more complex, as they reveal intermolecular interactions between molecules, leading to splitting and additional resonance lines. Moreover, the static and random orientation of molecules with respect to the magnetic field causes a chemical shift anisotropy and line broadening due to dipole–dipole interactions. The broadening effects can be partially reduced using the magic angle spinning (MAS) technique, allowing the resonance lines to become distinguishable [33,34]. However, changes in chemical shielding caused by non-equivalent interactions between molecules can still be observed. The discrepancy between theoretical and experimental results originates from the fact that theoretical results do not account for the aforementioned effects. However, to test this assumption, we dissolved TSC and C12Ala in D2O to diminish the intermolecular interactions by dispersing the investigated molecules in a liquid environment, thereby averaging out orientational dipolar interactions through increased rotational and translational molecular dynamics. The experimental 13C NMR spectra obtained for diluted liquid samples of TSC and C12Ala are presented in Figure 4a,b in red. In the case of TSC, experimental and theoretical results show strong agreement, as expected for a small moleucule soluble in aqueous environments. For C12Ala, although the agreement between theoretical and experimental data is much better, especially in the 170–180 ppm range, ir still shows some broadening effects due to residual intermolecular interactions. These observed effects indicate the self-assembly of these molecules, even if gelation does not occur without the addition of TSC, causing slower tumbling of molecules on the NMR timescale due to the formation of structures where diffusion is restricted by interactions. Therefore, the translational and orientational dynamics cannot effectively average out dipole–dipole interactions and the distinct orientation of groups of C12Ala molecules with respect to the external magnetic field. Therefore, it is natural to expect that the spectrum in D2O will not exactly match the theoretical prediction for monomeric C12Ala (in black). On the other hand, to obtain a reasonable S/N ratio, the weight concentration of the gelator molecules in D2O was 6% wt.%, which is relatively high, creating a viscous liquid. Sumita and Joykrishna showed that C12Ala can experience self-assembly already at a critical aggregation concentration (CAC) of 1.04 mM and at pH 12 or lower [35].
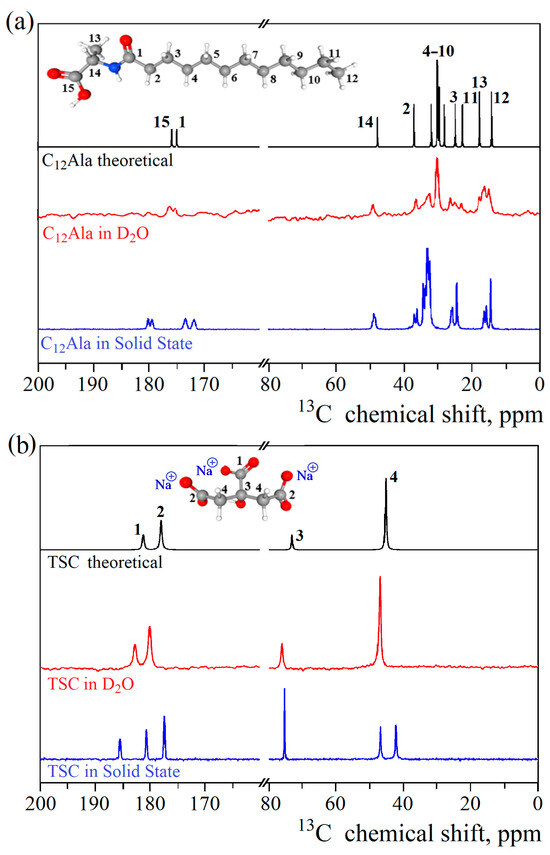
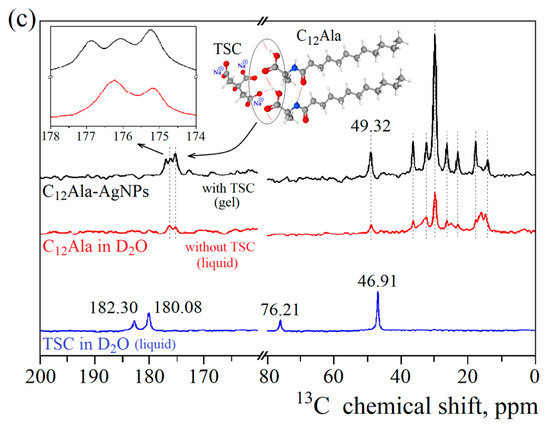
Figure 4.
Theoretical and experimental 13C CPMAS NMR spectra of solid and D2O-dissolved C12Ala (a) and TSC (b), along with experimental spectra of TSC and C12Ala dissolved in D2O and C12Ala-AgNP gel (c).
In Figure 4c, the experimental 13C NMR spectrum for the C12Ala-AgNP gel sample is presented in black, and the 13C spectra for liquid samples of TSC and C12Ala are given in blue and red, respectively. The observed differences in the gel spectrum originate from the intermolecular interactions established in the self-assembly process during gelation in the presence of TSC molecules. Although we cannot detect the TSC signal directly in the gel sample because of the low concentration, we can see its influence indirectly through the interaction with the COO- groups of the C12Ala gelator in the spectral range from 170 to 180 ppm. The observed line at 176 ppm in the liquid sample of C12Ala has been split into two lines in the gel sample due to interaction with TSC, mediated by Na+ cation. The inset in Figure 4c shows the splitting and indicates the relative change in the line intensities relative to the line at 175 ppm, which is expected when a single line splits into more components, but the number of interacting spins remains the same. In the 25–10 ppm spectral range, differences between the spectrum of C12Ala in the gel and liquid state were detected. This region corresponds to the carbons in the hydrophobic tail of the gelator molecule. Compared to the results for the liquid sample, in the gel sample, the spectrum has narrower lines and is less crowded. This indicates that the aliphatic chain’s carbons exhibit a more uniform chemical environment, resulting in better-defined and uniform chemical shifts. Such a situation occurs when the chains become ordered relative to each other and create a uniform structure, e.g., a well-defined lamellar phase, where all the tails reassemble on the inside and polar groups on the outsides. Based on the obtained experimental results and theoretical spectra, we can conclude that TSC plays a crucial role by triggering the gelation process and interacting with the polar head of the gelator molecules through the sodium cations. The mutual influence of the TSC and AgNP electrostatic interactions on C12Ala molecules also causes the ordering of the gelator hydrophobic tails into the lamellar phase.
3.4. Optical Images
To investigate the gelation process in the studied systems, images of the microstructures of the samples were recorded using polarising optical microscopy. Drops of the prepared compositions of the studied systems were cast onto amicroscopic plate, covered with glass coverslips, and subjected immediately to observation. The obtained results are presented in Figure 5. The differences between systems with and without TSC were clearly visible to the naked eye and confirmed by microstructure observation (Figure 5a,b vs. Figure 5c,d). The 12 wt.% mixture was used for the tests because 6 wt.% C12Ala in water (without TSC) did not form a stable gel with a microstructure that could be recorded in optical microscopy.
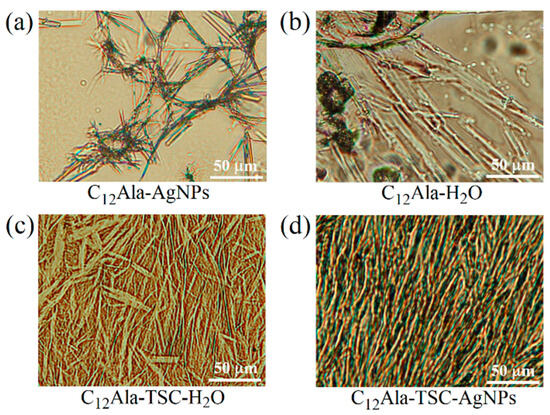
Figure 5.
The polarising optical images of 12 wt% C12Ala gels: C12Ala-AgNPs (a), C12Ala-H2O (b), C12Ala-TSC-H2O (c), and C12Ala-TSC-AgNPs (d).
The polarising optical images were taken at 20× magnification with a field of view of 275 × 200 μm. The systems containing TSC self-assemble into microstructures that significantly differ from those formed without the addition of TSC. Although the added trisodium citrate is below 0.5 wt.% of the total mass, its influence on the intramolecular interactions between gelator molecules is substantial. The gelator solution with hydrolate C12Ala-AgNPs, without TSC, does not exhibit gelling properties. In microscopic images, we can see clusters of gelator fibres surrounded by large pools of water (Figure 5a). In the case of the hydrogels without TSC, such as C12Ala-H2O (Figure 5b), the 12 wt.% of gelator molecules create rod-like aggregates that are loosely packed, resulting in significant areas where no aggregates are present. The small number of junction points between individual aggregates indicates the low thermal stability of the hydrogels created by the C12Ala gelator alone. In the absence of AgNPs, the aggregates of C12Ala stabilised by TSC create a dense mesh of rod-like aggregates of different thicknesses, lengths, and dendrimer-like shapes (Figure 5c). With the addition of silver nanoparticles to the systems, the interactions during the self-assembly process upon gelation change, leading to the creation of clusters of aggregates (Figure 5d). The shape of individual gelator aggregates also changes, assembling into thick, short, and straight rod-like shapes similar to the crystallites that create piles of random orientation. These piles can overlap and create regions of highly dense gelator aggregates and large free spaces in between. To explain such a change in aggregation pattern, we assume that the dispersed AgNPs become the nucleation centres for the aggregation of gelator molecules. The most crucial change in the microstructure of the created hydrogels is observed upon the addition of TSC to systems, both in the presence and absence of AgNPs. Such a matrix contains many more junction points and interactions between aggregates, leading to higher thermal and time stability for the hydrogel. Similar to systems without TSC, adding silver nanoparticles to hydrogels containing TSC influences individual aggregates’ shape and the created matrix’s self-assembly pattern. The microstructure of C12Ala-TSC-AgNP hydrogels consists of thin, long fibres of dendrimer-like shapes showing spatial ordering. The aggregates have a uniform size and form, indicating the role of AgNPs in the systems at the stage of aggregate formation and the supramolecular self-assembly process. A more uniform matrix in C12Ala-TSC-AgNP hydrogels results in higher durability for the systems exposed to temperature and time. Very similar results were obtained in SEM images for 5 wt.% gels with and without AgNPs. In Figure 5a,b, in the publication [9], we can clearly see differences in the structure of the internal gel matrix.
4. Discussion
C12Ala is an amphiphilic compound with a topological Polar Surface Area (PSA) equal to 66.4 Å2 [32] and amino-acid-based surfactants [36] that has a hydrophobic hydrocarbon chain ending with a polar hydrophilic amine and carboxyl groups, so we can expect that, when in contact with polar solutions (e.g., water), it behaves in a characteristic way [37,38,39,40]. Figure 6 shows a simplified diagram of the interaction between neighbouring gelator molecules in different solvent polar conditions. The area of interaction of two neighbouring gelator molecules resembles a cone, with the hydrophobic part of the molecule at the top. C12Ala, when in contact with water, forms micelles inside which the hydrophobic parts of the molecules are enclosed (see Figure 6a). Micelles form a colloidal system similar to lysols or emulsions, depending on the degree of dispersion. The addition of TSC to the C12Ala–H2O solvent results in a lower polarity of gelator molecules; the area of interaction of two neighbouring gelator molecules then resembles a truncated cone, and the mutual interaction of the hydrophobic parts of the molecules decreases. In the tested blank and silver-enriched samples, there is one TSC molecule for approximately 13 C12Ala molecules. This is sufficient to change the geometry of the mutual arrangement of the gelator particles. The micelle changes into a lamella system due to the interaction of TSC with C12Ala (see Figure 6b), as indicated by the change in shape and the shift of the 13C NMR peaks in the range of 170–180 ppm towards higher values, which is visible in the enlarged range in Figure 4c. The polar part of the gelator and silver nanoparticles chelated by TSC has a negative electric charge. Due to the Coulomb interaction, in the silver-enriched sample, C12Ala-TSC-AgNPs, the nanoparticles have the ability to organise lamellar fibres into thicker layers, which are held together by their interaction with positively charged sodium ions. The lamella fibres change into a lamella layer system by interacting with AgNPs with C12Ala (see Figure 6c). As a result, the gel system is expected to have longer, thicker, and more ordered solid fibrer. It should be remembered that the figure shows a schematic interaction of gelator molecules in the absence or presence of stabilisers such as TSC and AgNPs at CMC (critical micelles concentration—Figure 6a) and CAC (critical assembly concentration—Figure 6b,c). In a real system, the ratio of micelles, lamellae, and lamellar layers depends on the concentration of individual components in the sample, such as the gelator, stabiliser, and nanoparticles.
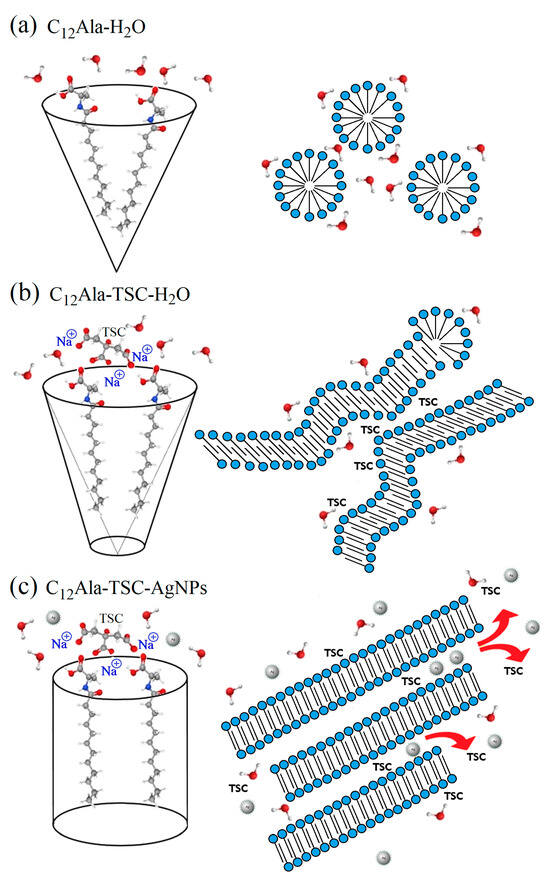
Figure 6.
The simplified diagram of the interaction between neighbouring gelator molecules in different conditions: C12Ala in water (a), C12Ala with TSC in water (b), and C12Ala with TSC in water solution of AgNPs (c).
Now we can understand why, without a small addition of TSC, the C12Ala gelator does not gel in the AgNP solution. Adding negatively charged AgNPs to the C12Ala hydrogel causes structural changes in the gel. The AgNPs separate C12Ala micelles from each other through electrical interaction. A pure hydrogel is formed by short lamellas and micelles, which form gelator clusters or single thin fibres dispersed in water. The disintegration of the pure hydrogel is visible shortly after gelation. In a short time (a few hours), the gelled sample changes into a structure resembling a “micellar fluid”, suggesting an excess of micelles over lamellae in the system. The gelator aggregates precipitate from the gel, and the sample begins to flow. Still, this process is slower than that of a mixture of containing AgNP hydrolate combined only with the gelator C12Ala without the participation of the TSC stabiliser. Adding negatively charged silver particles to the system causes an excess micelles of to form relative to the lamellas due to the Coulomb interaction with AgNPs. The fibres break into smaller, unconnected structures. A larger share of weakly interacting micelles in the sample and the separation of gelator fibres make it impossible for the system to gel. As a result, pure hydrogel without added TSC gels only for a short time, whereas the gel enriched with AgNPs without added TSC does not gel at all. This process is visible in Figure 5a,b. In a pure gel (Figure 5b), large pools of liquid are held together by interconnected gelator chains. Still, the Coulomb interaction with negatively charged AgNPs causes the separation of the gelator in the liquid phase (Figure 5a), and the gelation process becomes impossible. The addition of TSC causes an excess of lamellas over the micelles in this system, and the gel becomes more stable, creating short and twisted or unordered fibres in a rigid gel matrix, as seen in Figure 5c for the 12 wt.% gel and Figure 5a at [9] for the 5 wt.% gel. The appearance of lamellar layers also explains the change in the thermal decay rate of the system enriched with nanoparticles in the TGA measurement compared to the blank system. The temperature ranges and amplitudes of thermal degradation of the individual phases of the systems are always larger for samples enriched with nanoparticles than for the blank samples. Heat transfer through thick and long lamella layers occurs slower than in the case of thin and short fibres found in the blank sample. The fluctuation in the temperature of the silver-enriched sample from 33 °C to 36 °C in the DSC measurement in subsequent cooling cycles also becomes understandable. The mutual arrangement of C12Ala lamellae due to interaction with AgNPs and the formation of lamella layers may differ slightly in each heating and cooling cycle.
In the f C12Ala hydrogel system enriched with silver nanoparticles, AgNPs, the addition of TSC molecules counteracts the electrostatic interaction between the gelator fibres and silver nanoparticles, thereby enabling gelation and maintaining the system’s stability.
5. Conclusions
The conducted research allowed us to answer the question about the mechanism of gelation and the intramolecular interactions between the liquid and solid phases in the pure hydrogel of C12Ala, the hydrogel of C12Ala-TSC, and the silver-enriched hydrogel of C12Ala-TSC-AgNPs. The addition of TSC to water, which interacts with the polar part of the gelator molecule and affects its polarity, changes the forming gel matrix’s internal structure, and increases its stability. The C12Ala hydrogel, with the addition of a 1.25M TSC water solution, exhibits greater thermal and time stability compared to the pure hydrogel due to the increased number of junction points between individual LMWG aggregates composing the internal matrix. A similar effect was observed in the system containing AgNPs. On the other hand, the presence of only silver nanoparticles in the hydrogel composition without TSC molecules causes the disintegration of the internal structure due to electrostatic interactions between the negatively charged nanoparticles and the micelles formed by the gelator molecules, preventing them from combining into a stable structure. In C12Ala-TSC-AgNP hydrogels, the mutual interactions between TSC and AgNPs decrease the negative electrostatic influence of nanoparticles on the LMWG self-assembly process and lead to higher ordering of the created fibrilar aggregates, which increases the homogeneity and strength of the internal matrix. Consequently, we have obtained thermally and long-term stable hydrogels with a homogenous structure and AgNP distribution, improving the potential textural and organoleptic properties of potential pharmaceutical or cosmetic products. The identified role of the TSC molecules in shielding the gelator molecules from electrostatic forces that disturb the self-assembly process introduced by metallic nanoparticles paves the way for the preparation of hydrogel products enriched with other types of nanoparticles.
Author Contributions
Conceptualisation, J.K.; methodology, J.K. and M.B. (Michał Bielejewski); validation, J.K., M.B. (Michał Bielejewski) and O.M.D.; formal analysis, J.K. and M.B. (Michał Bielejewski); investigation, J.K., M.B. (Michał Bielejewski), O.M.D. and M.B. (Mariusz Borkowski); resources, O.M.D. and M.B. (Mariusz Borkowski); data curation, J.K.; writing—original draft preparation, J.K.; writing—review and editing, J.K., M.B. (Michał Bielejewski) and O.M.D.; visualisation, J.K. and O.M.D.; supervision, M.B. (Michał Bielejewski); project administration, J.K.; funding acquisition, O.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Polish National Science Centre, grant number 2019/33/B/NZ7/01608.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Glushkov, D.; Paushkina, K.; Pleshko, A. Gel Fuels: Praparing, Rheology, Atomization, Combustion. Energies 2023, 16, 298. [Google Scholar] [CrossRef]
- Li, M.-G.; Wu, Y.; Cao, Q.-L.; Yuan, X.-Y.; Chen, X.; Han, J.-L.; Wu, W.-T. Rheological Properties of Organic Kerosene Gel Fuel. Gels 2022, 8, 507. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti Telis, V.R. Biopolimer Engineering in Food Procesing; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439844946. [Google Scholar]
- Zhi, L.; Liu, Z.; Wu, C.; Ma, X.; Hu, H.; Liu, H.; Adhikari, B.; Wang, Q.; Shi, A. Advances in preparation and application of food-grade emulsion gels. Food Chem. 2023, 424, 136399. [Google Scholar] [CrossRef]
- Aggarwal, G.; Nagpal, M. Pharmaceutical Polymer Gels in Drug Delivery. In Polymer Gels. Gels Horizons: From Science to Smart Materials; Thakur, V., Thakur, M., Voicu, S., Eds.; Springer: Singapore, 2018; pp. 249–284. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Gündoğdu, E.A.; Cağlar, E.S.; Özgenç, E.; Gonzalez-Alvarez, M.; Gonzalez-Alvarez, I.; Okur, N.U. Polymer based Gels: Recent and Future Applications in Drug Delivery Field. Curr. Drug Deliv. 2023, 20, 1288–1313. [Google Scholar] [CrossRef]
- Sharmin, E.; Batubara, A.S.; Tamboosi, B.A.; Al Khozay, E.B.; Alamoudi, M.K.; Al Aidaroos, O.Z.; Albenayan, J.A.; Lamfom, M.Y.; Sindi, A.A.H.; Al-Madboly, L.A.; et al. PVA nanocomposite hydrogel loaded with silver nanoparticles enriched Nigella sativa oil. Inorg. Nano-Met. Chem. 2021, 52, 1134–1142. [Google Scholar] [CrossRef]
- Rammohan, A.; Kaduk, J.A. Trisodium citrate, Na3(C6H5O7). Acta Cryst. 2016, E72, 793–796. [Google Scholar] [CrossRef]
- Kubiński, K.; Górka, K.; Janeczko, M.; Martyna, A.; Kwaśnik, M.; Masłyk, M.; Zięba, E.; Kowalczuk, J.; Kuśtrowski, P.; Borkowski, M.; et al. Silver Is Not Equal to Silver: Synthesis and Evaluation of Silver Nanoparticles with Low Biological Activity, and Their Incorporation into C12Alanine-Based Hydrogel. Molecules 2023, 28, 1194. [Google Scholar] [CrossRef]
- Anjali Das, C.G.; Ganesh Kumar, V.; Stalin Dhas, T.; Karthick, V.; Govindaraju, K.; Mary Joselin, J.; Baalamurugan, J. Antibacterial activity of silver nanoparticles (biosynthesis): A short review on recent advances. Biocatal. Agric. Biotechnol. 2020, 27, 101593. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Ghiuță, I.; Cristea, D.; Croitoru, C.; Kost, J.; Wenkert, R.; Vyrides, I.; Anayiotos, A.; Munteanu, D. Characterization and antimicrobial activity of silver nanoparticles, biosynthesized using Bacillus species. Appl. Surf. Sci. 2018, 438, 66–73. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of Silver Nanoparticles to Red Blood Cells: Size Dependent Adsorption, Uptake, and Hemolytic Activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Reipa, V.; Hitchins, V.M.; Goering, P.L.; Malinauskas, R.A. Physicochemical characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol. Sci. 2011, 123, 133–143. [Google Scholar] [CrossRef]
- Riaz Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. Vitr. 2017, 38, 179–192. [Google Scholar] [CrossRef]
- Lucky, S.S.; Chee Soo, K.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Biogenic Silver Nanoparticles for Targeted Cancer Therapy and Enhancing Photodynamic Therapy. Cells 2023, 12, 2012. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 10 September 2024).
- Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 10 September 2024).
- Liu, G.; Yang, L.; Chen, G.; Xu, F.; Yang, F.; Yu, H.; Li, L.; Dong, X.; Han, J.; Cao, C.; et al. A Review on Drug Delivery System for Tumor Therapy. Front. Pharmacol. 2021, 12, 735446. [Google Scholar] [CrossRef]
- Fornaguera, C.; García-Celma, M.J. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef]
- Sastri, T.K.; Gupta, V.N.; Chakraborty, S.; Madhusudhan, S.; Kumar, H.; Chand, P.; Jain, V.; Veeranna, B.; Gowda, D.V. Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends. Gels 2022, 8, 316. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Motulsky, A.; Lafleur, M.; Couffin-Hoarau, A.C.; Hoarau, D.; Boury, F.; Benoit, J.P.; Leroux, J.C. Characterization and biocompatibility of organogels based on L-alanine for parenteral drug delivery implants. Biomaterials 2005, 26, 6242–6253. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Pal, A. Physical Gelation of Binary Mixtures of Hydrocarbons Mediated by n-Lauroyl-L-Alanine and Characterization of Their Thermal and Mechanical Properties. J. Phys. Chem. B 2008, 112, 4918–4927. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Chhikara, B.S.; Govindaraj, A.; Bhattacharya, S.; Rao, C.N.R. Synthesis and properties of novel nanocomposites made of ingle-walled carbon nanotubes and low molecular mass organogels and their thermo-responsive behavior triggered by near IR radiation. J. Mater. Chem. 2008, 18, 2593–2600. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [PubMed]
- Miroslaw, B.; Demchuk, O.M.; Luboradzki, R.; Tyszczuk-Rotko, K. Low-Molecular-Weight Organogelators Based on N-dodecanoyl-L-amino Acids—Energy Frameworks and Supramolecular Synthons. Materials 2023, 16, 702. [Google Scholar] [CrossRef]
- Kowalczuk, J.; Łapiński, A.; Stolarczyk, E.; Demchuk, O.M.; Kubiński, K.; Janeczko, M.; Martyna, A.; Masłyk, M.; Turczyniak-Surdacka, S. New Supramolecular Drug Carriers: The Study of Organogel Conjugated Gold Nanoparticles. Molecules 2021, 26, 7462. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 13854541, N-Lauroyl-L-Alanine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/N-Lauroyl-L-alanine (accessed on 22 October 2024).
- Antzutkin, O.N. Sideband manipulation in magic-angle-spinning nuclear magnetic resonance. Prog. Nucl. Mag. Reson. Spectrosc. 1999, 35, 203–266. [Google Scholar] [CrossRef]
- Brown, S.P. Probing proton-proton proximities in the solid state. Prog. Nucl. Mag. Reson. Spectrosc. 2007, 50, 199–251. [Google Scholar] [CrossRef]
- Ghosh, A.; Dey, J. Effect of Hydrogen Bonding on the Physicochemical Properties andBilayer Self-Assembly Formation of N-(2-Hydroxydodecyl)-L-alanine inAqueous Solution. Langmuir 2008, 24, 6018–6026. [Google Scholar] [CrossRef]
- Borkowski, M.; Orvalho, S.; Warszyński, P.; Demchuk, O.M.; Jarek, E.; Zawala, J. Experimental and theoretical study of adsorption of synthesized amino acid core derived surfactants at an air/water interface. Phys. Chem. Chem. Phys. 2022, 24, 3854–3864. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, A.; Illa, O.; Ortuño, R.M. Amphiphiles in aqueous solution: Well beyond a soap bubble. Chem. Soc. Rev. 2013, 42, 8200–8219. [Google Scholar] [CrossRef] [PubMed]
- Choucair, A.; Eisenberg, A. Control of amphiphilic block copolymer morphologies using solution conditions. Eur. Phys. J. E 2003, 10, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications; Elsevier Science: Amsterdam, The Netherlands, 2000; ISBN 0-444-82441-3. [Google Scholar]
- Luo, C.; Yang, B.; Zhou, Y.; Yang, J.; Han, F.; Xu, B. Gelation properties and application based on amino acids gelators with four kinds of edible oils. Colloids Surf. A 2020, 585, 124184. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).