Biological Evaluation, Phytochemical Screening, and Fabrication of Indigofera linifolia Leaves Extract-Loaded Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Phytochemical Analysis
2.2. TFC and TPC in the Crude Extract and Prepared NPs
2.3. Characterization of the Crude Extract
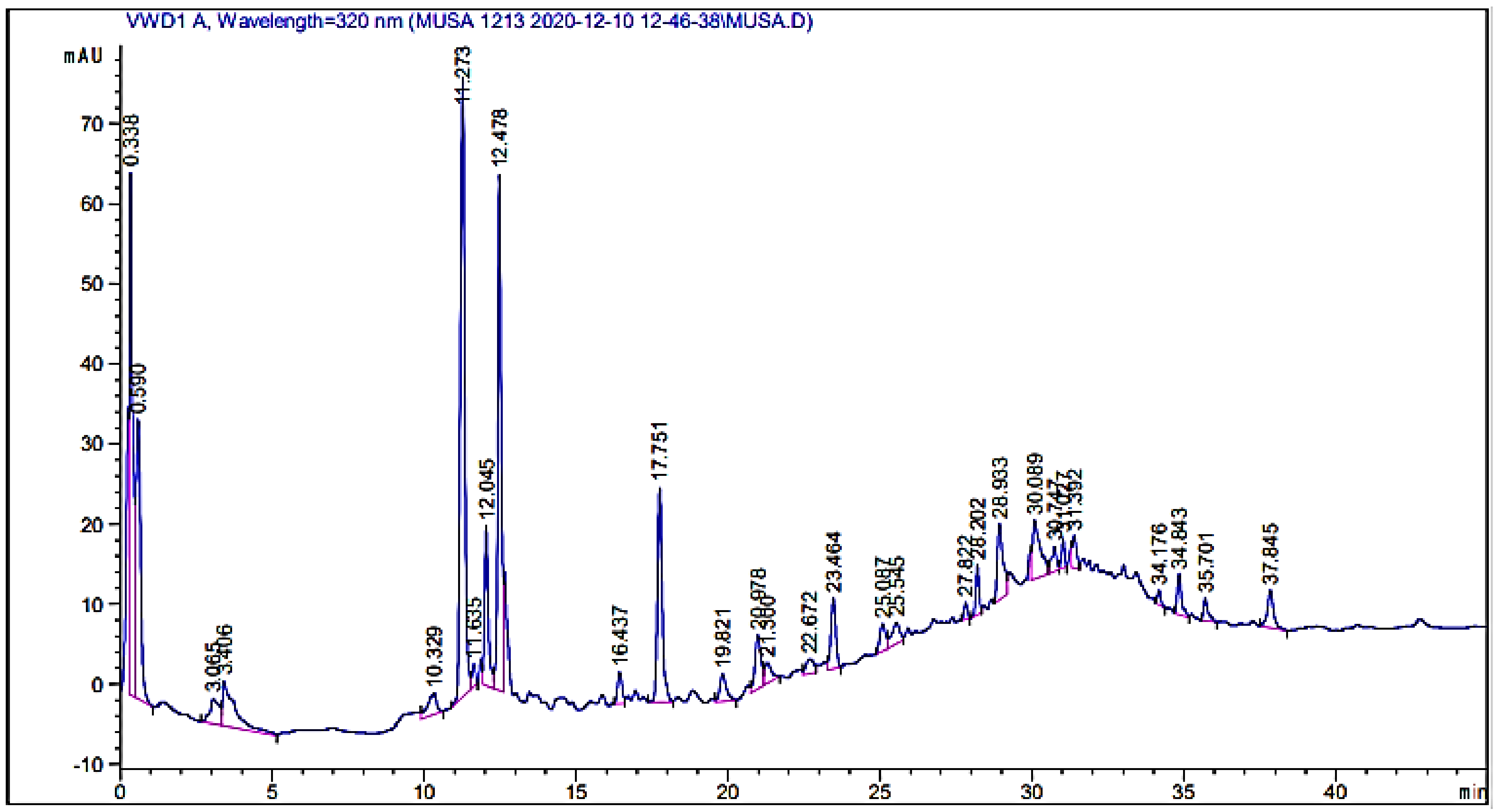
2.3.1. HPLC-UV Analysis of the Crude Extract
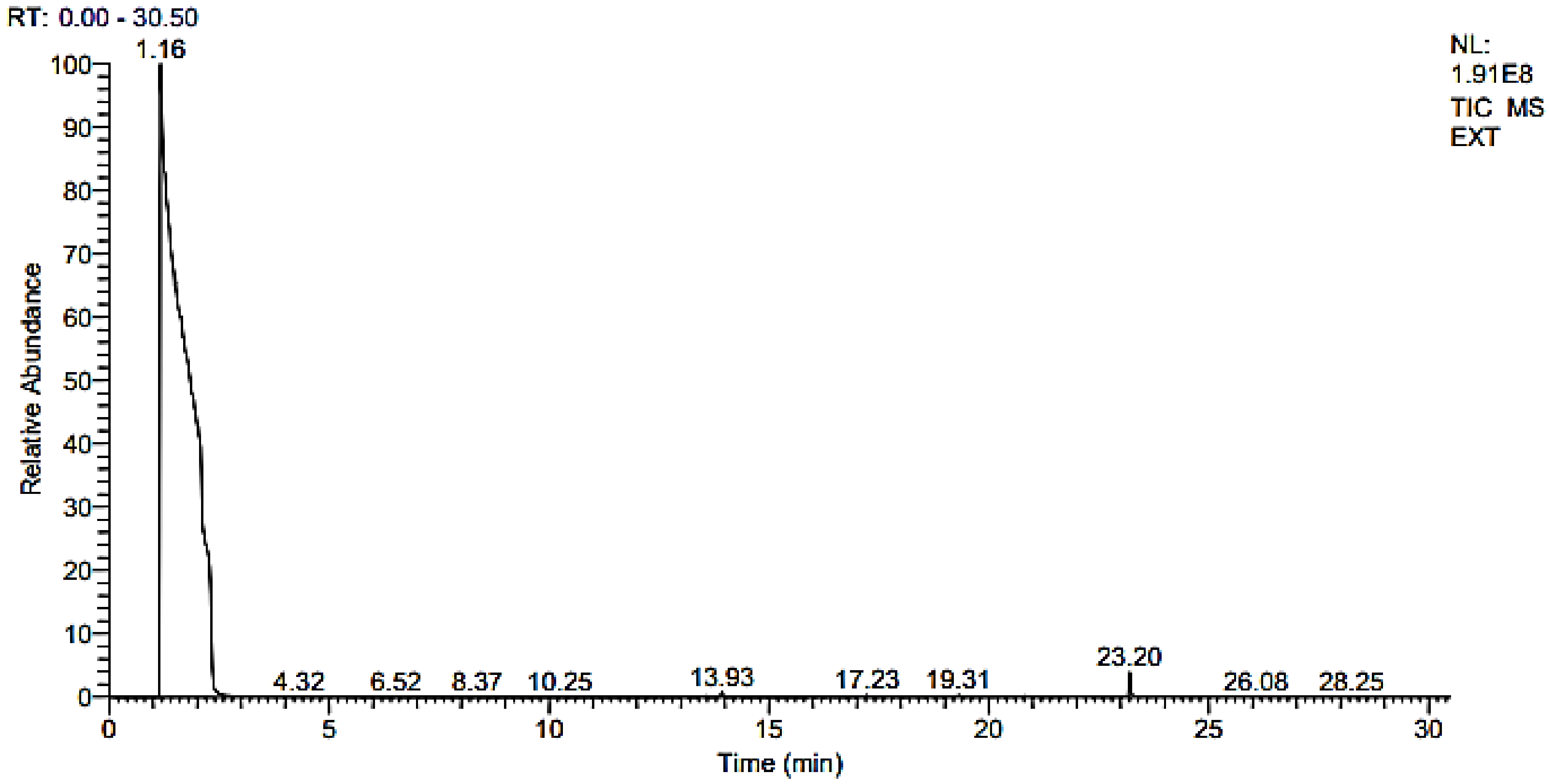
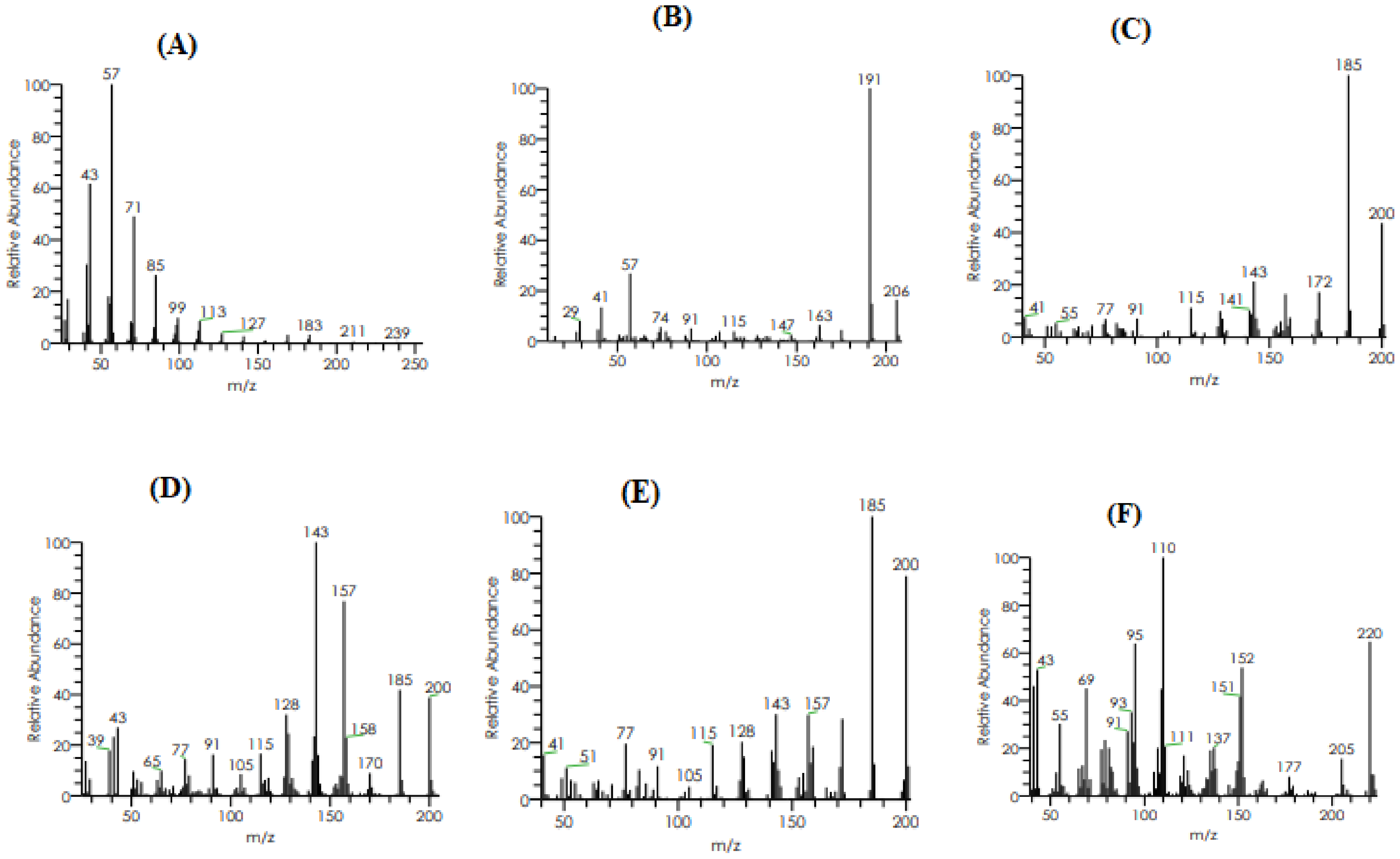
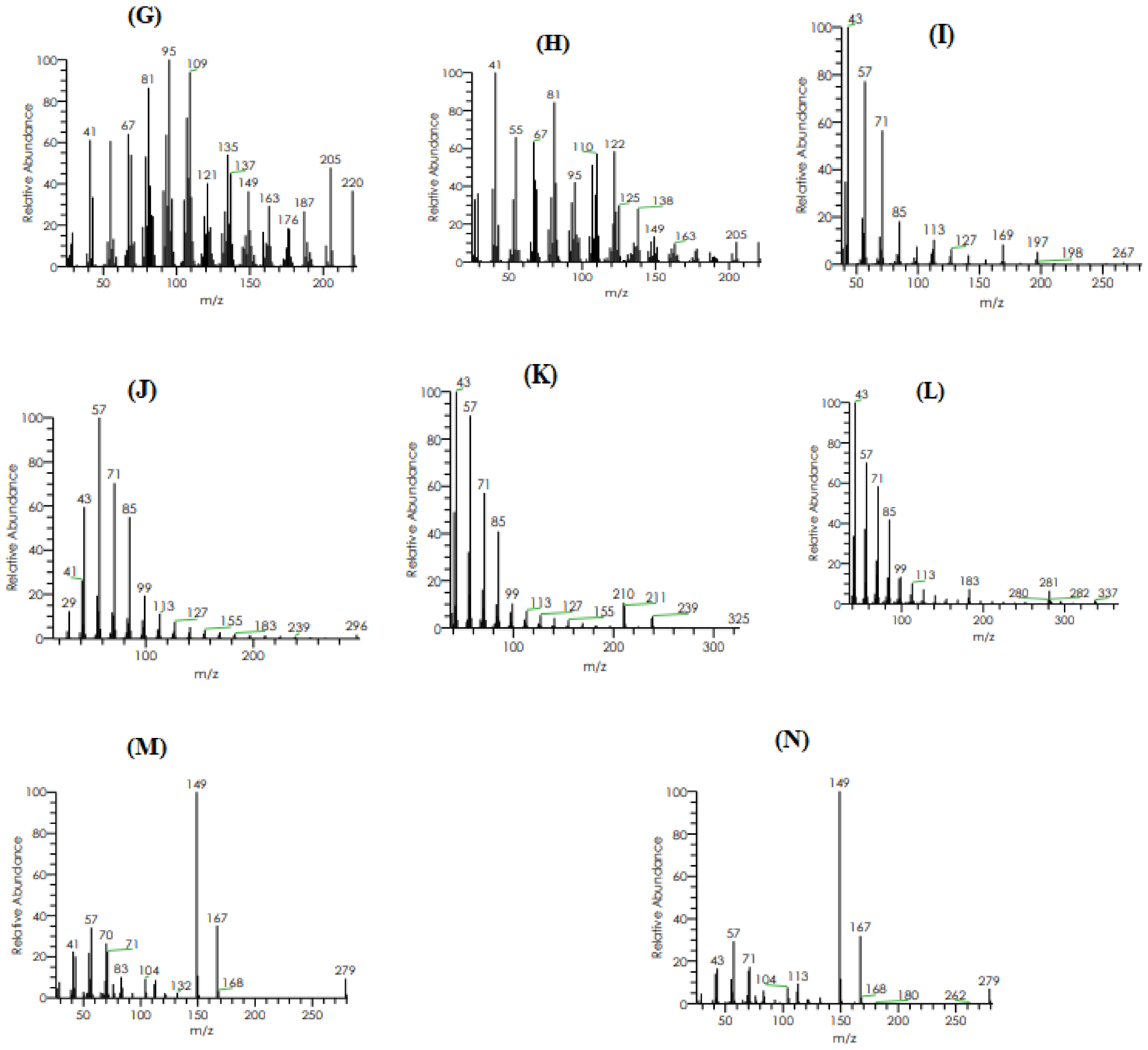
2.3.2. GC–MS Analysis
2.4. Characterization of the Prepared NPs
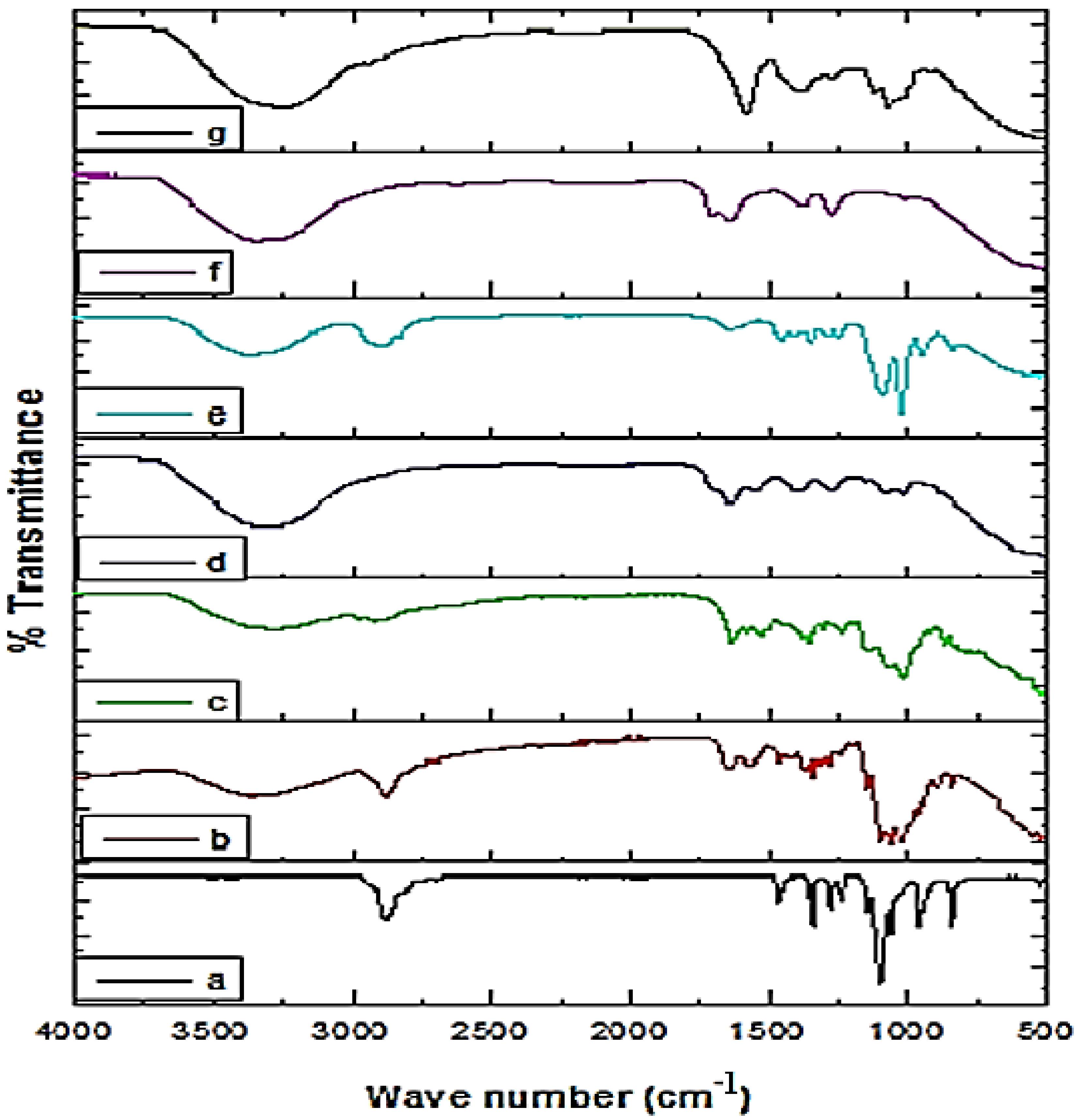
2.4.1. FTIR Analysis of the Crude Extract and Fabricated NPs
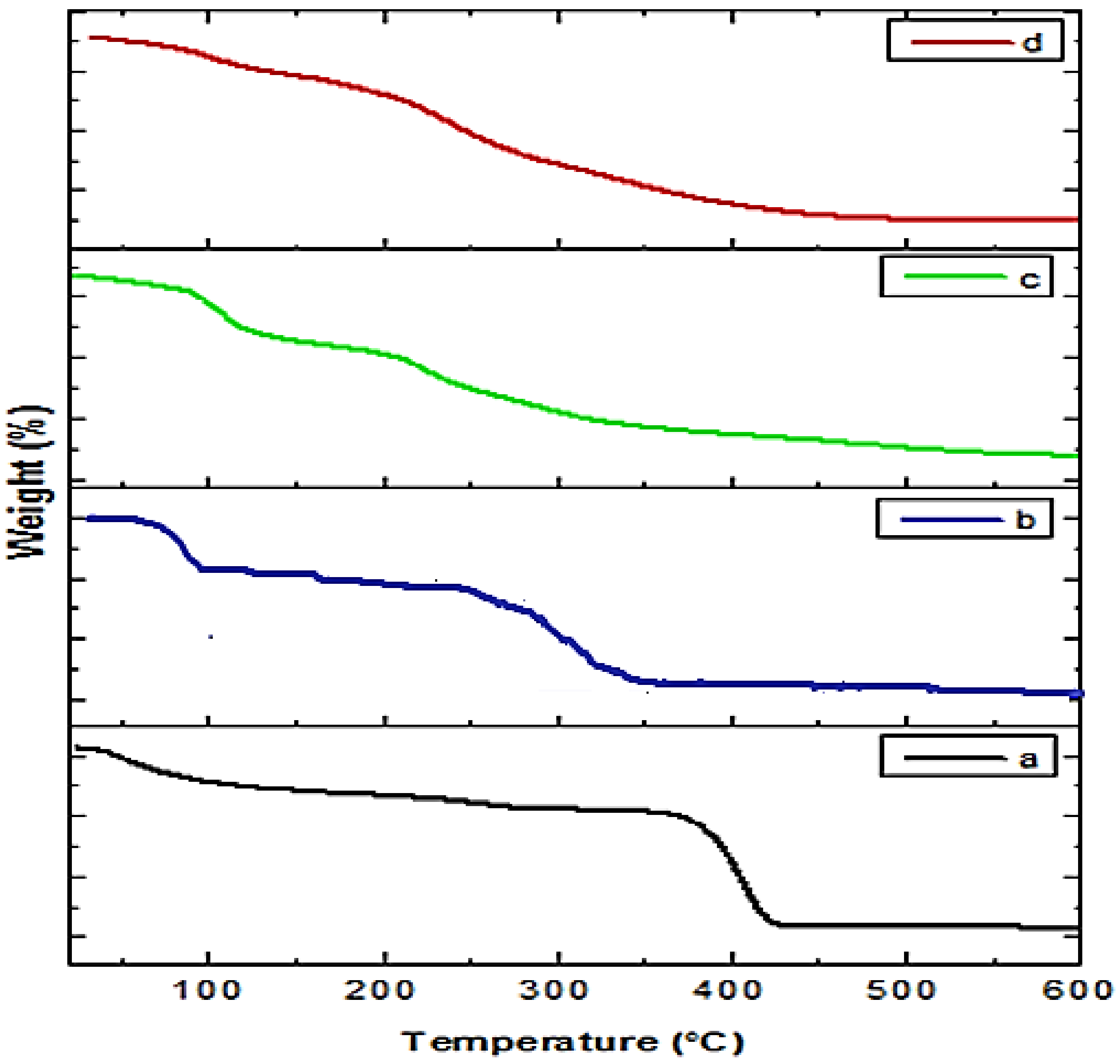
2.4.2. TGA of Crude Extract and Prepared NPs
2.4.3. SEM Analysis of Plant Extract-Loaded NPs
2.5. Antibacterial Activity of Crude Extract and Prepared NPs
2.6. Determination of Free Radical Scavenging Activity of Crude Extract and Prepared NPs by DPPH Assay
2.7. In Vitro Antidiabetic Activities of Crude Extract and Prepared NPs
2.7.1. In Vitro α-Glucosidase Enzyme Inhibition
2.7.2. In Vitro α-Amylase Enzyme Inhibition
3. Materials and Methods
3.1. Chemicals Used
3.2. Plant Sample Collection
3.3. Identification
3.4. Crude Extract Preparation
3.5. Preliminary Phytochemical Analysis of a Crude Extract of Indigofera Linifolia
3.6. Determination of Total Phenolic Content (TPC) and Total Flavonoid Contents (TFC)
3.7. HPLC-UV Analysis of the Crude Extract
3.8. GC–MS Analysis
3.9. Locust Bean Gum (LBG) Extraction
3.10. Synthesis of NPs
Characterization of NPs
3.11. Antimicrobial Activity of Extract and Prepared NPs
3.12. Determination of Free Radical Scavenging Activity by DPPH Assay
3.13. Antidiabetic Potential of Extract and NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability Statement
References
- Memariani, Z.; Abbas, S.Q.; Ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef] [PubMed]
- Cordell, G.A. Biodiversity and Drug Discovery—A Symbiotic Relationship. Phytochemistry 2000, 55, 463–480. [Google Scholar] [CrossRef]
- Ul Hassan, S.S.; Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Tasneem, U. Emerging biopharmaceuticals from marine actinobacteria. Environ. Toxicol. Pharmacol. 2017, 49, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Farhan, A.; Asad, M.I.; Khan, M.R.; Hassan, S.S.U.; Haq, I.-U.; Bungau, S. An Extensive Pharmacological Evaluation of New Anti-Cancer Triterpenoid (Nummularic Acid) from Ipomoea batatas through In Vitro, In Silico, and In Vivo Studies. Molecules 2022, 27, 2474. [Google Scholar] [CrossRef]
- Adang, A.; Hermkens, P. The Contribution of Combinatorial Chemistry to Lead Generation an Interim Analysis. Curr. Med. Chem. 2001, 8, 985–998. [Google Scholar] [CrossRef]
- Mendelsohn, R.; Balick, M.J. The Value of Undiscovered Pharmaceuticals in Tropical Forests. Econ. Bot. 1995, 49, 223–228. [Google Scholar] [CrossRef]
- Malmiri, H.J.; Jahanian, M.A.G.; Berenjian, A. Potential Applications of Chitosan Nanoparticles as Novel Support in Enzyme Immobilization. Am. J. Biochem. Biotechnol. 2012, 8, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Schmid, G. Large Clusters and Colloids. Metals in the Embryonic State. Chem. Rev. 1992, 92, 1709–1727. [Google Scholar] [CrossRef]
- Ul Hassan, S.S.; Jin, H.-Z.; Abu-Izneid, T.; Rauf, A.; Ishaq, M.; Suleria, H.A.R. Stress-driven discovery in the natural products: A gateway towards new drugs. Biomed. Pharmacother. 2019, 109, 459–467. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, A.; Uthaman, S.; Park, I.-K. Polyethylene Glycol Nanoparticles as Promising Tools for Anticancer Therapeutics. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 10; pp. 205–231. [Google Scholar] [CrossRef]
- Soumya, R.S.; Raghu, K.G.; Abraham, A. Locust bean gum-based micro- and nanomaterials for biomedical applications. In Micro- and Nanoengineered Gum-Based Biomaterials for Drug Delivery and Biomedical Applications; Elsevier: Berlin, Germany, 2022; Chapter 9; pp. 241–253. [Google Scholar]
- Siddhuraju, P.; Vijayakumari, K.; Janardhanan, K. Studies on the Underexploited Legumes, Indigofera linifolia and Sesbania bispinosa: Nutrient Composition and Antinutritional Factors. Int. J. Food Sci. Nutr. 1995, 46, 195–203. [Google Scholar] [CrossRef]
- Muhammad, I.; Luo, W.; Shoaib, R.M.; Li, G.-L.; Ul Hassan, S.S.; Yang, Z.-H.; Xiao, X.; Tu, G.-L.; Yan, S.-K.; Ma, X.-P.; et al. Guaiane-type sesquiterpenoids from Cinnamomum migao H. W. Li: And their anti-inflammatory activities. Phytochemistry 2021, 190, 112850. [Google Scholar] [CrossRef]
- Xie, Y.G.; Zhao, X.C.; Ul Hassan, S.S.; Zhen, X.-Y.; Muhammad, I.; Yan, S.-K.; Yuan, X.; Li, H.-L.; Jin, H.-Z. One new sesquiterpene and one new iridoid derivative from Valeriana amurensis. Phytochem. Lett. 2019, 32, 6–9. [Google Scholar] [CrossRef]
- Ul Hassan, S.S.; Muhammad, I.; Abbas, S.Q.; Hassan, M.; Majid, M.; Jin, H.Z.; Bungau, S. Stress driven discovery of natural products from actinobacteria with antioxidant and cytotoxic activities including docking and admet properties. Int. J. Mol. Sci. 2021, 22, 11432. [Google Scholar] [CrossRef]
- Muhammad, I.; Xiao, Y.Z.; Ul Hassan, S.S.; Xiao, X.; Yan, S.K.; Guo, Y.Q.; Ma, X.-P.; Jin, H.Z. Three new guaiane-type sesquiterpenoids and a monoterpenoid from Litsea lancilimba Merr. Nat. Prod. Res. 2020, 36, 3271–3279. [Google Scholar] [CrossRef]
- Tripathi, I.P.; Mishra, M.K.; Pardhi, Y.; Dwivedi, A.; Dwivedi, N.; Kamal, A.; Gupta, P. HPLC Analysis of Methanolic Extract of Some Medicinal Plant Leaves of Myrtaceae Family. Int. Pharm. Sci. 2012, 2, 49–53. [Google Scholar]
- Suryanti, V.; Marliyana, S.D.; Putri, H.E. Effect of Germination on Antioxidant Activity, Total Phenolics, β-Carotene, Ascorbic Acid and α-Tocopherol Contents of Lead Tree Sprouts (Leucaena leucocephala (Lmk.) de Wit). Int. Food Res. J. 2016, 23, 167–172. [Google Scholar]
- Hamid, A.A.; Aiyelaagbe, O.O.; Usman, L.A.; Ameen, O.M.; Lawal, A. Antioxidants: Its Medicinal and Pharmacological Applications. Afr. J. Pure Appl. Chem. 2010, 4, 142–151. [Google Scholar]
- Ngueyem, T.A.; Brusotti, G.; Caccialanza, G.; Finzi, P.V. The Genus Bridelia: A Phytochemical and Ethnopharmacological Review. J. Ethnopharmacol. 2009, 124, 339–349. [Google Scholar] [CrossRef]
- Kabra, A.; Sharma, R.; Hano, C.; Kabra, R.; Martins, N.; Singh Baghel, U. Phytochemical Composition, Antioxidant, and Antimicrobial Attributes of Different Solvent Extracts from Myrica esculenta Buch.-Ham. Ex. D. Don Leaves. Biomolecules 2019, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; MacÊdo, R.O.; Medeiros, A.C.D. Thermal Characterization of Dried Extract of Medicinal Plant by DSC and Analytical Techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Tiţa, B.; Fuliaş, A.; Bandur, G.; Marian, E.; Tiţa, D. Compatibility Study between Ketoprofen and Pharmaceutical Excipients Used in Solid Dosage Forms. J. Pharm. Biomed. Anal. 2011, 56, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Ahmad, K.; Khalil, A.T.; Amin, F.; Lutfullah, G.; Khan, K.; Shah, G.; Ahmad, A. Monotheca buxifolia Mediated Synthesis of Gold Nanoparticles and Its Diverse Biomedical Applications. Mater. Res. Express 2019, 6, 125416. [Google Scholar] [CrossRef]
- Correia, L.P.; Santana, C.P.; Medeiros, A.C.D.; MacÊdo, R.O. Sideroxylon obtusifolium Herbal Medicine Characterization Using Pyrolysis GC/MS, SEM and Different Thermoanalytical Techniques. J. Therm. Anal. Calorim. 2016, 123, 993–1001. [Google Scholar] [CrossRef]
- Renukadevi, K.P.; Suhani Sultana, S. Determination of Antibacterial, Antioxidant and Cytotoxicity Effect of Indigofera tinctoria on Lung Cancer Cell Line NCI-H69. Int. J. Pharmacol. 2011, 7, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, M.; Jacob, K.; Govindaraj, Y. Antibacterial Activity and Mutagenicity of Leaves of Indigofera tinctoria. Linn. J. Exp. Integr. Med. 2012, 2, 263–269. [Google Scholar] [CrossRef]
- Tan, J.B.L.; Yap, W.J.; Tan, S.Y.; Lim, Y.Y.; Lee, S.M. Antioxidant Content, Antioxidant Activity, and Antibacterial Activity of Five Plants from the Commelinaceae Family. Antioxidants 2014, 3, 758. [Google Scholar] [CrossRef]
- Ferreira, L. Nanoparticles as Tools to Study and Control Stem Cells. J. Cell. Biochem. 2009, 108, 746–752. [Google Scholar] [CrossRef]
- Azevedo, C.; Macedo, M.H.; Sarmento, B. Strategies for the Enhanced Intracellular Delivery of Nanomaterials. Drug Discov. Today 2018, 23, 944–959. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ul Hassan, S.S.; Shaikh, A.L. Marine actinobacteria as a drug treasure house. Biomed. Pharmacother. 2017, 87, 46–57. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Srinivasan, S.; Wankhar, W.; Rathinasamy, S.; Rajan, R. Free Radical Scavenging Potential and HPTLC Analysis of Indigofera tinctoria Linn (Fabaceae). J. Pharm. Anal. 2016, 6, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, I.; Ul Hassan, S.S.; Cheung, S.; Li, X.; Wang, R.; Zhang, W.D.; Yan, S.K.; Zhang, Y.; Jin, H.Z. Phytochemical study of Ligularia subspicata and valuation of its anti-inflammatory activity. Fitoterapia 2021, 148, 104800. [Google Scholar] [CrossRef]
- Khan, I.; Abbas, T.; Anjum, K.; Abbas, S.Q.; Shagufta, B.I.; Shah, S.A.A.; Akhter, N.; Ul Hassan, S.S. Antimicrobial potential of aqueous extract of Camellia sinensis against representative microbes. Pak. J. Pharm. Sci. 2019, 32, 631–636. [Google Scholar]
- Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Alqarni, A.O.; Jan, M.S.; Hussain, F.; Zafar, R.; Rashid, U.; Abbas, M.; Tariq, M.; et al. Antioxidant Molecules Isolated from Edible Prostrate Knotweed: Rational Derivatization to Produce More Potent Molecules. Oxid. Med. Cell. Longev. 2022, 2022, 3127480. [Google Scholar] [CrossRef]
- Mahmood, F.; Khan, J.A.; Mahnashi, M.H.; Jan, M.S.; Javed, M.A.; Rashid, U.; Sadiq, A.; Hassan, S.S.U.; Bungau, S. Anti-Inflammatory, Analgesic and Antioxidant Potential of New (2S,3S)-2-(4-isopropylbenzyl)-2-methyl-4-nitro-3-phenylbutanals and Their Corresponding Carboxylic Acids through In Vitro, In Silico and In Vivo Studies. Molecules 2022, 27, 4068. [Google Scholar] [CrossRef]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and Antioxidant Activity of Curcumin after Encapsulated by Nano and Pickering Emulsion Based on Chitosan-Tripolyphosphate Nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef]
- Barceló, A.; Rajpathak, S. Incidence and Prevalence of Diabetes Mellitus in the Americas. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2001, 10, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Atlas, D. International diabetes federation. In IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015; p. 33. [Google Scholar]
- Tafesse, T.B.; Hymete, A.; Mekonnen, Y.; Tadesse, M. Antidiabetic Activity and Phytochemical Screening of Extracts of the Leaves of Ajuga remota Benth on Alloxan-Induced Diabetic Mice. BMC Complement. Altern. Med. 2017, 17, 243. [Google Scholar] [CrossRef] [Green Version]
- Muhtadi; Primarianti, A.U.; Sujono, T.A. Antidiabetic Activity of Durian (Durio zibethinus Murr.) and Rambutan (Nephelium lappaceum L.) Fruit Peels in Alloxan Diabetic Rats. Procedia Food Sci. 2015, 3, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Balomajumder, C.; Roy, P. Hypoglycemic and Hypolipidemic Effects of Flavonoid Rich Extract from Eugenia Jambolana Seeds on Streptozotocin Induced Diabetic Rats. Food Chem. Toxicol. 2008, 46, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical Constituents of Some Nigerian Medicinal Plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Girão Da Cruz, M.T.; Cordeiro, M.N.D.S.; Milhazes, N.; Borges, F.; Marques, M.P.M. Phenolic Acid Derivatives with Potential Anticancer Properties—A Structure-Activity Relationship Study. Part 1: Methyl, Propyl and Octyl Esters of Caffeic and Gallic Acids. Bioorg. Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [Green Version]
- Kielhorn, S.; Thorngate, J.H. Oral Sensations Associated with the Flavan-3-Ols (+)-Catechin and (−)-Epicatechin. Food Qual. Prefer. 1999, 10, 109–116. [Google Scholar] [CrossRef]
- Zeb, A. A Reversed Phase HPLC-DAD Method for the Determination of Phenolic Compounds in Plant Leaves. Anal. Methods 2015, 7, 7753–7757. [Google Scholar] [CrossRef]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Di Marco, G.; Canini, A.; Atik Bekkara, F. GC/MS Analysis, and Antioxidant and Antimicrobial Activities of Alkaloids Extracted by Polar and Apolar Solvents from the Stems of Anabasis articulata. Med. Chem. Res. 2019, 28, 754–767. [Google Scholar] [CrossRef]
- Dakia, P.A.; Blecker, C.; Robert, C.; Wathelet, B.; Paquot, M. Composition and Physicochemical Properties of Locust Bean Gum Extracted from Whole Seeds by Acid or Water Dehulling Pre-Treatment. Food Hydrocoll. 2008, 22, 807–818. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In Vitro Antioxidant and Free Radical Scavenging Activity of Different Parts of Tabebuia pallida Growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [Green Version]
- Telagari, M.; Hullatti, K. In-Vitro α-Amylase and α-Glucosidase Inhibitory Activity of Adiantum caudatum Linn. and Celosia argentea Linn. Extracts and Fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [CrossRef] [Green Version]



| Phytochemical | Reagent | Observation | Results |
|---|---|---|---|
| Flavonoids | Ferric chloride | Appearance of yellow color and after the addition of HCl become colorless. | + |
| Glycosides | Keller Killiani | Formation of a red to brown layer. | + |
| Tannins | Gelatin | Brownish-green precipitates. | + |
| Triterpenoids | Liebermann Burchard | Reddish-brown boundary. | + |
| Retention Time (min) | Phytochemical Compounds | Sample Peak Area | Identification Reference |
|---|---|---|---|
| 10.329 | Mandelic acid | 55.548 | Ref. Stand |
| 11.273 | Bis-HHDP-hex(pedunculagin) | 797.264 | [19] |
| 12.478 | Caffeic acid | 510.522 | Ref. Stand |
| 16.437 | Hydroxy bezoic acid | 38.4805 | Ref. Stand |
| 20.978 | Chlorogenic acid | 91.0721 | Ref. Stand |
| 22.672 | Morin | 32.6104 | Ref. Stand |
| 23.464 | Syringic acid | 100.907 | [20] |
| 25.087 | 5-0-dicaffeoylquinic acid | 42.0698 | [19] |
| 25.545 | Kaempferol-3-(caffeoyl-diglucoside)-7-rhamnosyl | 50.79067 | [19] |
| 27.822 | Kaempferol-3-(p-coumaroyl-diglucoside)-7-glucoside | 15.8819 | [19] |
| 30.089 | Quercetin | 131.189 | Ref. Stand |
| 31.027 | Qurcetin-3-(caffeoyldiglucoside)-7-glucoside | 24.0199 | [19] |
| 34.843 | Quercitin-3-O-rutinoside | 47.3937 | [19] |
| 37.845 | Glucose | 4.6713 | [21] |
| Retention Time (min) | Molecular Mass (g/mol) | Molecular Formula | %Area | Area | Peak Height | Compound Name |
|---|---|---|---|---|---|---|
| 13.08 | 254 | C18H38 | 0.00 | 258,385.77 | 93,693.15 | 2,6,10-Trimethylpentadecane |
| 13.93 | 206 | C14H22O | 0.08 | 5,736,551.10 | 1,410,675.04 | Phenol, 2,4-bis(1,1-dimethylethyl)- |
| 14.30 | 200 | C15H20 | 0.01 | 466,139.33 | 131,832.12 | Isolongifolene, 4,5,9,10-dehydro- |
| 14.30 | 200 | C15H20 | 0.01 | 466,139.33 | 131,832.12 | 9-Methyl-S-octahydroanthracene |
| 14.30 | 200 | C15H20 | 0.01 | 466,139.33 | 131,832.12 | 9-Methyl-S-octahydrophenanthrene |
| 15.95 | 220 | C15H24O | 0.01 | 363,808.41 | 123,197.77 | á-Himachalenoxide |
| 15.95 | 220 | C15H24O | 0.01 | 363,808.41 | 123,197.77 | Longifolenaldehyde |
| 15.95 | 220 | C15H24O | 0.01 | 363,808.41 | 123197.77 | ç-Gurjunenepoxide-(1) |
| 17.23 | 282 | C20H42 | 0.02 | 1,260,254.99 | 450,404.86 | Crocetane |
| 17.23 | 296 | C20H42 | 0.02 | 1,260,254.99 | 450,404.86 | Heneicosane |
| 18.68 | 324 | C23H48 | 0.01 | 371,092.40 | 137,687.26 | 9-n-Hexylheptadecane |
| 20.31 | 366 | C26H54 | 0.00 | 145,679.34 | 86,758.74 | Octadecane, 3-ethyl-5-(2-ethylbutyl)- |
| 23.20 | 390 | C24H38O4 | 0.26 | 18,393,028.17 | 7,282,283.86 | Diisooctyl phthalate |
| 23.20 | 278 | C16H22O4 | 0.26 | 18,393,028.17 | 7,282,283.86 | Mono(2-ethylhexyl) phthalate |
| Microbial Strain | Zone of Inhibition (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Crude Extract | PEG | Chitosan | LGB | Ext+Ch NPs | Ext+PEG NPs | Ext+LGB NPs | |
| Escherichia coli | 13.2 ± 0.9 | 8.0 ± 0.5 | 8.3 ± 0.6 | 8.0 ± 0.5 | 12.6 ± 0.4 | 17 ± 0.3 | 10 ± 0.5 |
| Klebsiella pneumoniae | 10.8 ± 0.4 | 9.1 ± 0.7 | 8.2 ± 0.5 | 7.8 ± 0.7 | 12.6 ± 0.6 | 16.6 ± 0.6 | 11 ± 0.3 |
| Salmonella typhi | 8.4 ± 0.2 | 7.9 ± 0.4 | 9.6 ± 0.7 | 7.4 ± 0.4 | 11 ± 0.7 | 14 ± 0.4 | 9 ± 0.5 |
| S. No | Sample | Concentration (µg/mL) | DPPH% Scavenging | IC50 |
|---|---|---|---|---|
| 1 | Crude extract | 1000 | 87.83 ± 0.66 | 53 |
| 500 | 83.27 ± 0.84 | |||
| 250 | 74.19 ± 0.24 | |||
| 125 | 60.49 ± 0.58 | |||
| 62.5 | 57.16 ± 0.49 | |||
| 2 | Ext+Ch NPs | 1000 | 76.32 ± 0.24 | 45 |
| 500 | 68.64 ± 0.41 | |||
| 250 | 64.27 ± 0.58 | |||
| 125 | 57.47 ± 0.89 | |||
| 62.5 | 53.52 ± 0.82 | |||
| 3 | Ext+PEG NPs | 1000 | 88.81 ± 0.39 | 41 |
| 500 | 81.36 ± 0.45 | |||
| 250 | 75.72 ± 0.34 | |||
| 125 | 71.47 ± 0.31 | |||
| 62.5 | 63.71 ± 0.23 | |||
| 4 | Ext+LGB NPs | 1000 | 84.59 ± 0.36 | 39 |
| 500 | 81.81 ± 0.39 | |||
| 250 | 73.84 ± 0.58 | |||
| 125 | 68.73 ± 0.91 | |||
| 62.5 | 62.38 ± 0.89 | |||
| 5 | Ascorbic acid | 1000 | 94.88 ± 0.56 | 30 |
| 500 | 86.59 ± 0.45 | |||
| 250 | 78.64 ± 0.76 | |||
| 125 | 69.14 ± 0.25 | |||
| 62.5 | 62.87 ± 0.53 |
| Sample | Concentration (µg/mL) | % α-Glucosidase Inhibition Mean ± SEM | IC50 (µg/mL) | % α-Amylase Inhibition | IC50 (µg/mL) |
|---|---|---|---|---|---|
| Mean ± SEM | |||||
| Crude extract | 1000 | 66.15 ± 0.92 | 120 | 71.16 ± 0.13 | 104 |
| 500 | 60.74 ± 0.45 | 64.41 ± 0.71 | |||
| 250 | 58.99 ± 0.18 | 60.06 ± 0.23 | |||
| 125 | 51.31 ± 0.15 | 54.72 ± 0.91 | |||
| 62.5 | 43.37 ± 1.02 | 49.67 ± 0.37 | |||
| Ext+Ch NPs | 1000 | 72.58 ± 0.84 | 100 | 79.72 ± 0.89 | 86 |
| 500 | 69.07 ± 0.98 | 73.22 ± 0.16 | |||
| 250 | 67.98 ± 0.64 | 67.02 ± 0.23 | |||
| 125 | 51.75 ± 0.56 | 61.97 ± 0.56 | |||
| 62.5 | 49.78 ± 1.78 | 54.58 ± 0.78 | |||
| Ext+PEG | 1000 | 63.15 ± 0.45 | 126 | 67.97 ± 0.09 | 112 |
| 500 | 57.45 ± 0.73 | 61.27 ± 0.30 | |||
| 250 | 55.04 ± 0.03 | 56.22 ± 0.13 | |||
| 125 | 39.69 ± 1.03 | 51.82 ± 0.07 | |||
| 62.5 | 37.06 ± 1.08 | 47.18 ± 0.15 | |||
| Ext+LGB | 1000 | 77.59 ± 0.20 | 83 | 83.37 ± 0.21 | 78 |
| 500 | 71.62 ± 0.09 | 76.29 ± 0.08 | |||
| 250 | 64.60 ± 0.65 | 71.57 ± 0.34 | |||
| 125 | 58.46 ± 0.34 | 68.47 ± 0.36 | |||
| 62.5 | 54.92 ± 1.34 | 61.88 ± 0.67 | |||
| Acarbose | 1000 | 92.11 ± 0.84 | 69 | 86.72 ± 1.14 | 74 |
| 500 | 86.13 ± 1.34 | 80.54 ± 0.54 | |||
| 250 | 80.47± 1.82 | 73.76 ± 1.02 | |||
| 125 | 76.32 ± 1.22 | 69.22 ± 2.02 | |||
| 62.5 | 71.18 ± 2.02 | 64.28 ± 1.75 |
| Name of Polymer | Formula | Molecular Weight | Supplier |
|---|---|---|---|
| PEG 6000 | H(OCH2CH2)nOH | 5000–7500 | Sigma-Aldrich, Germany |
| Chitosan | C56H103N9O39 | 1526 | Sigma-Aldrich, Germany |
| LBG (extract from seed) | -- | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talha, M.; Islam, N.U.; Zahoor, M.; Sadiq, A.; Nawaz, A.; Khan, F.A.; Gulfam, N.; Alshamrani, S.A.; Nahari, M.H.; Alshahrani, M.A.; et al. Biological Evaluation, Phytochemical Screening, and Fabrication of Indigofera linifolia Leaves Extract-Loaded Nanoparticles. Molecules 2022, 27, 4707. https://doi.org/10.3390/molecules27154707
Talha M, Islam NU, Zahoor M, Sadiq A, Nawaz A, Khan FA, Gulfam N, Alshamrani SA, Nahari MH, Alshahrani MA, et al. Biological Evaluation, Phytochemical Screening, and Fabrication of Indigofera linifolia Leaves Extract-Loaded Nanoparticles. Molecules. 2022; 27(15):4707. https://doi.org/10.3390/molecules27154707
Chicago/Turabian StyleTalha, Muhammad, Noor Ul Islam, Muhammad Zahoor, Abdul Sadiq, Asif Nawaz, Farhat Ali Khan, Naila Gulfam, Saleh A. Alshamrani, Mohammed H. Nahari, Mohammed Abdulrahman Alshahrani, and et al. 2022. "Biological Evaluation, Phytochemical Screening, and Fabrication of Indigofera linifolia Leaves Extract-Loaded Nanoparticles" Molecules 27, no. 15: 4707. https://doi.org/10.3390/molecules27154707
APA StyleTalha, M., Islam, N. U., Zahoor, M., Sadiq, A., Nawaz, A., Khan, F. A., Gulfam, N., Alshamrani, S. A., Nahari, M. H., Alshahrani, M. A., Mahnashi, M. H., & Hassan, S. S. u. (2022). Biological Evaluation, Phytochemical Screening, and Fabrication of Indigofera linifolia Leaves Extract-Loaded Nanoparticles. Molecules, 27(15), 4707. https://doi.org/10.3390/molecules27154707







