Site-Specific Fluorescent Labeling of RNA Interior Positions
Abstract
1. Introduction
2. Exploitation of Natural Posttranslational Modifications
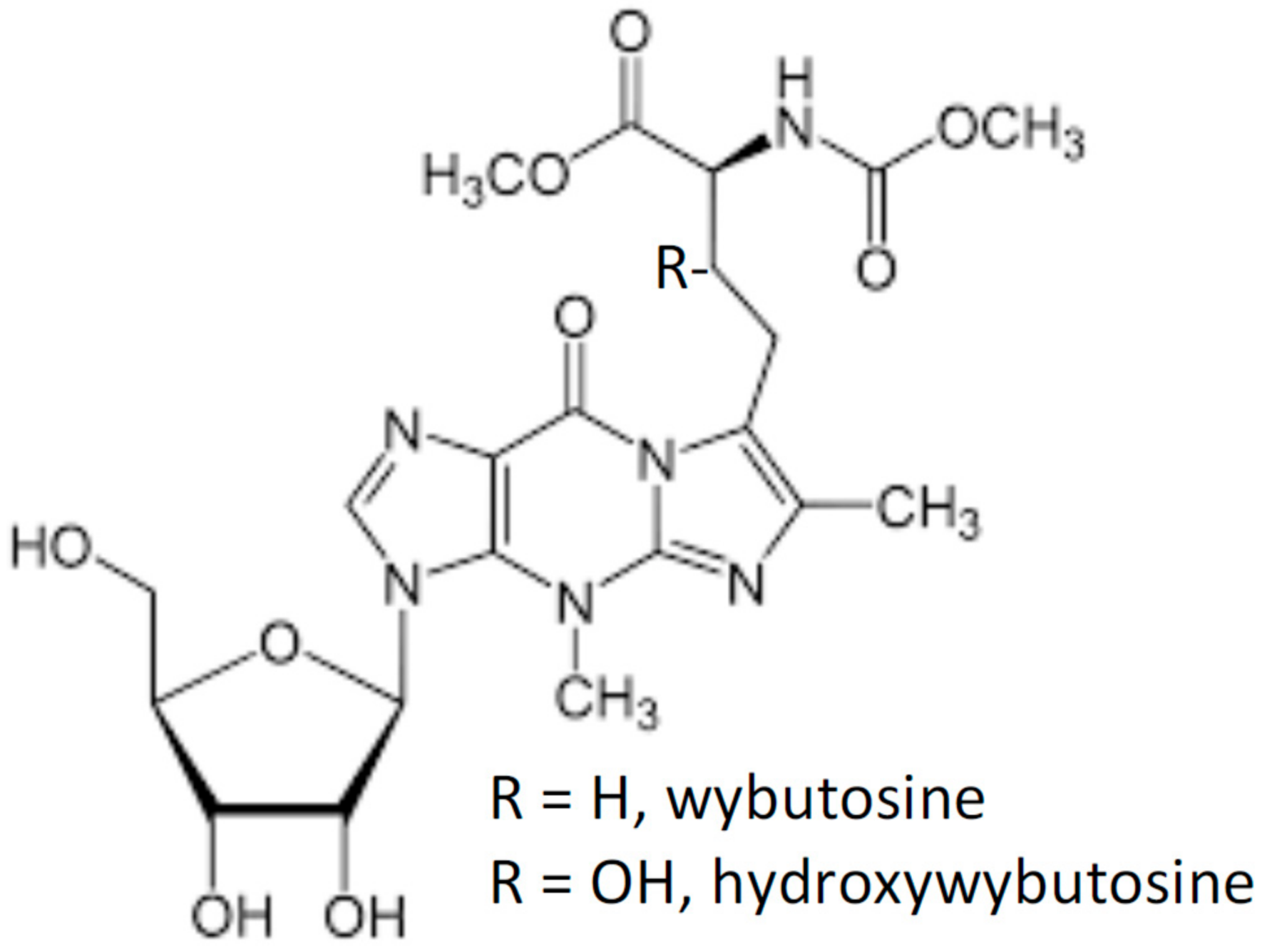
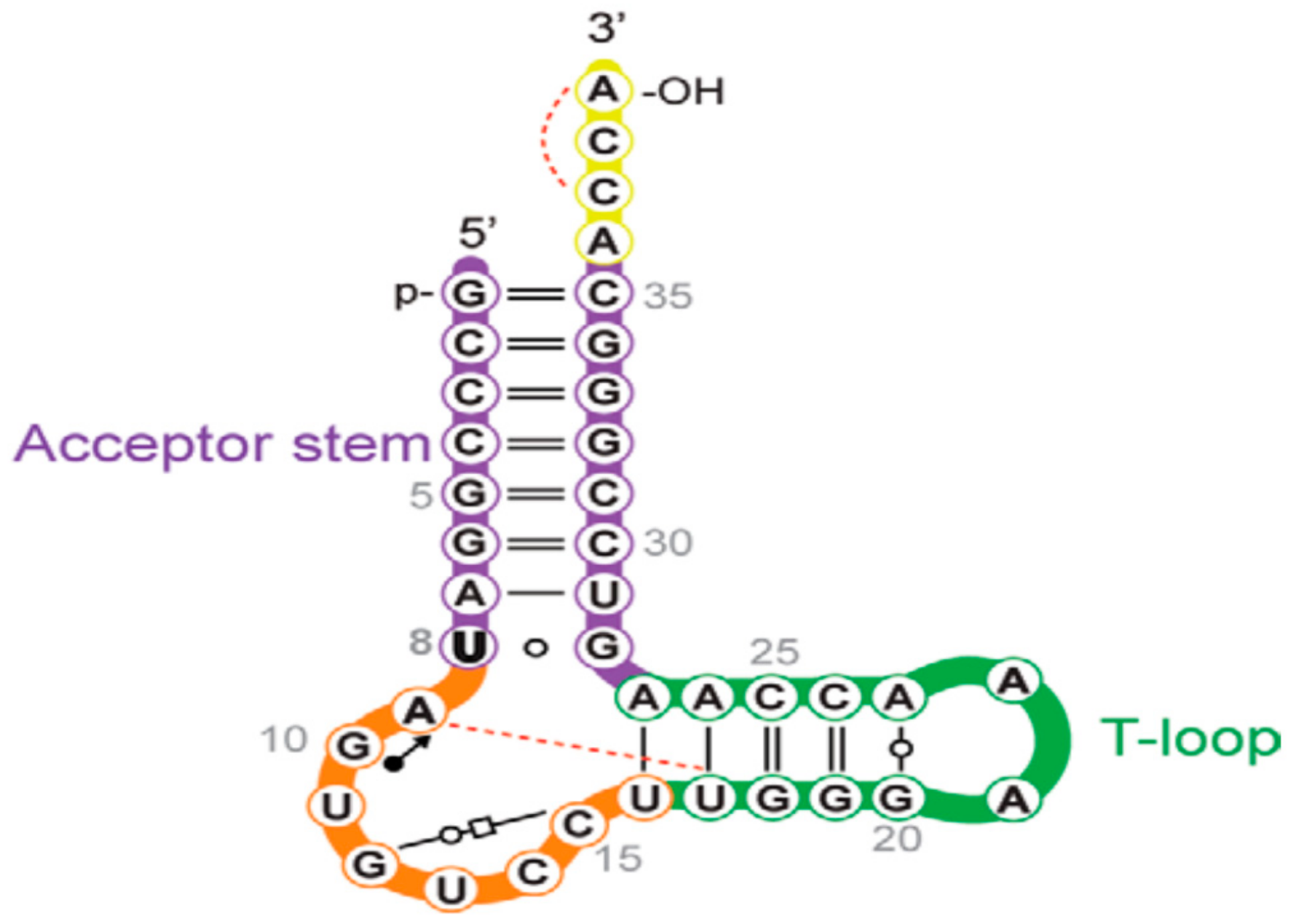
2.1. Wybutosine
2.2. DHU
2.3. acp3U
2.4. s4U
2.5. Extensions beyond tRNA Labeling
3. Repurposing Enzymatic Transferase Reactions
3.1. SAM-Dependent Methyl Transferases (SAM-Mtases)
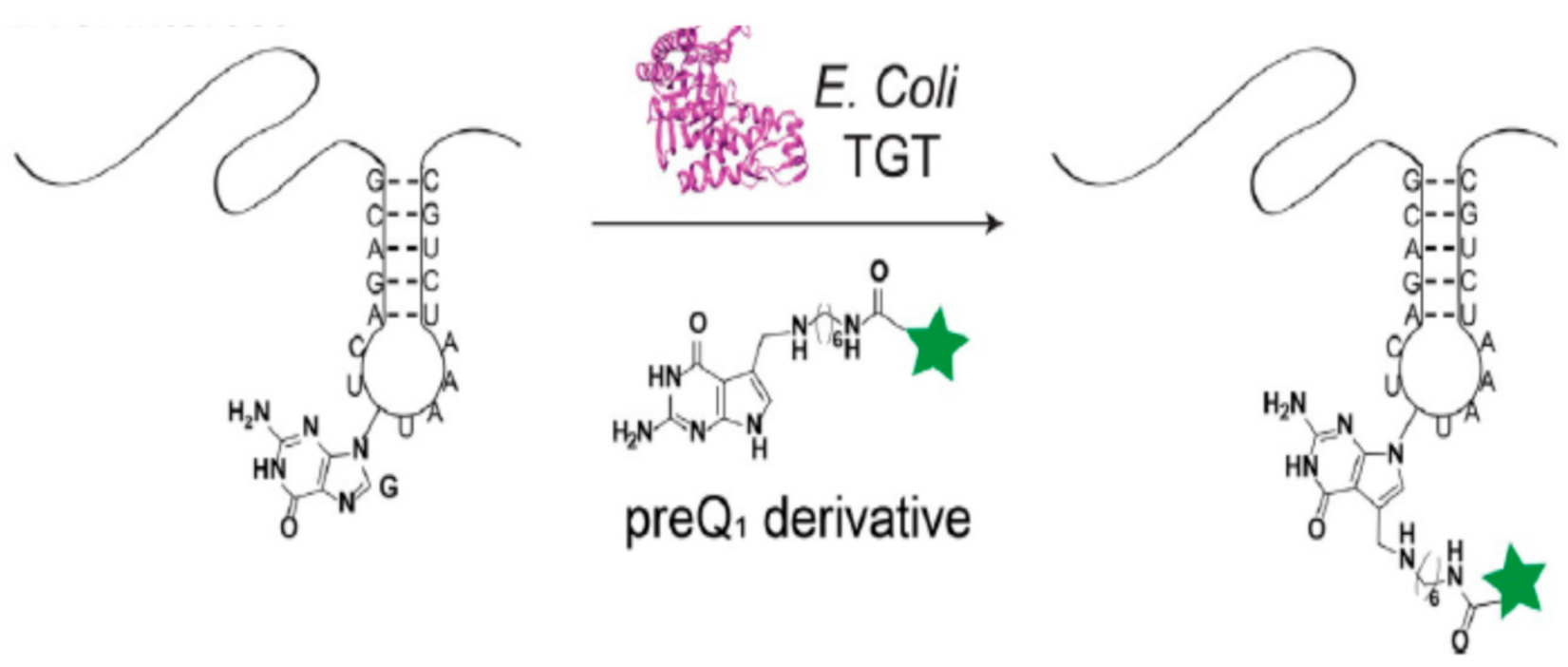
3.2. tRNA Guanine Transglycosylase (TGT)
4. Nucleic Acid-Assisted Labeling of Intact RNAs
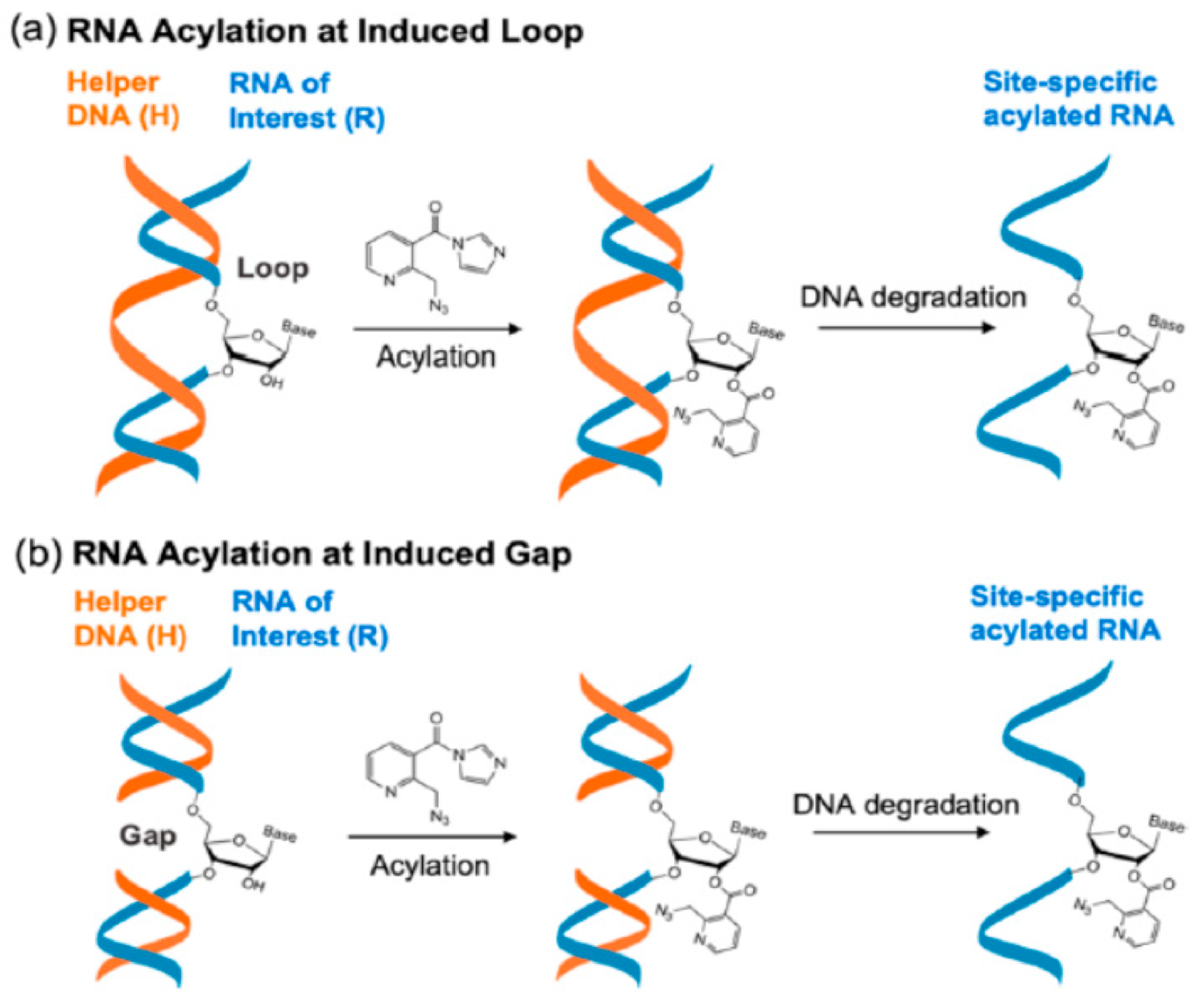
4.1. RNA Acylation at DNA Induced Loops or Gaps
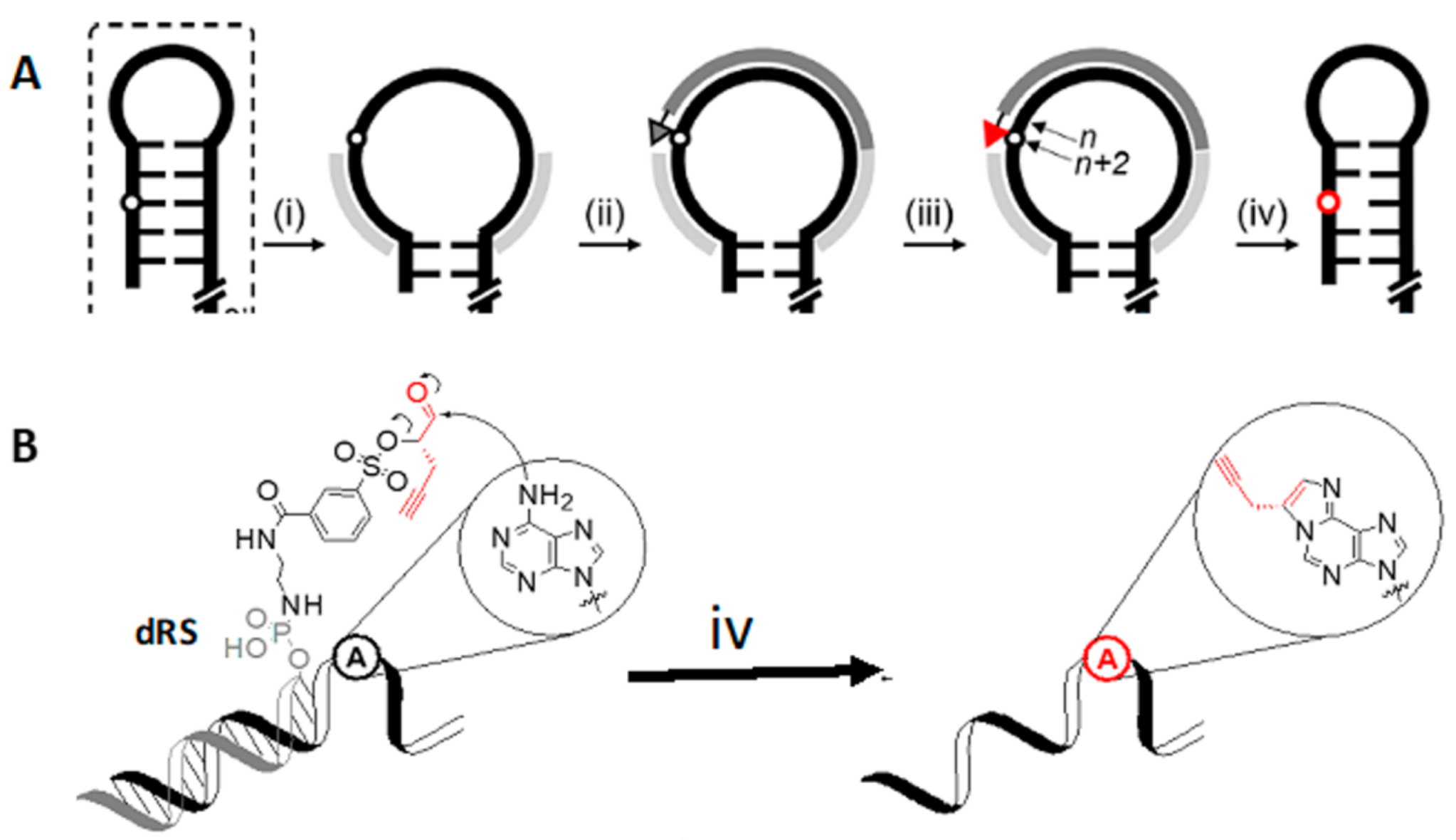
4.2. DNA Reactive Sequence Targeting of an Interior Adenosine
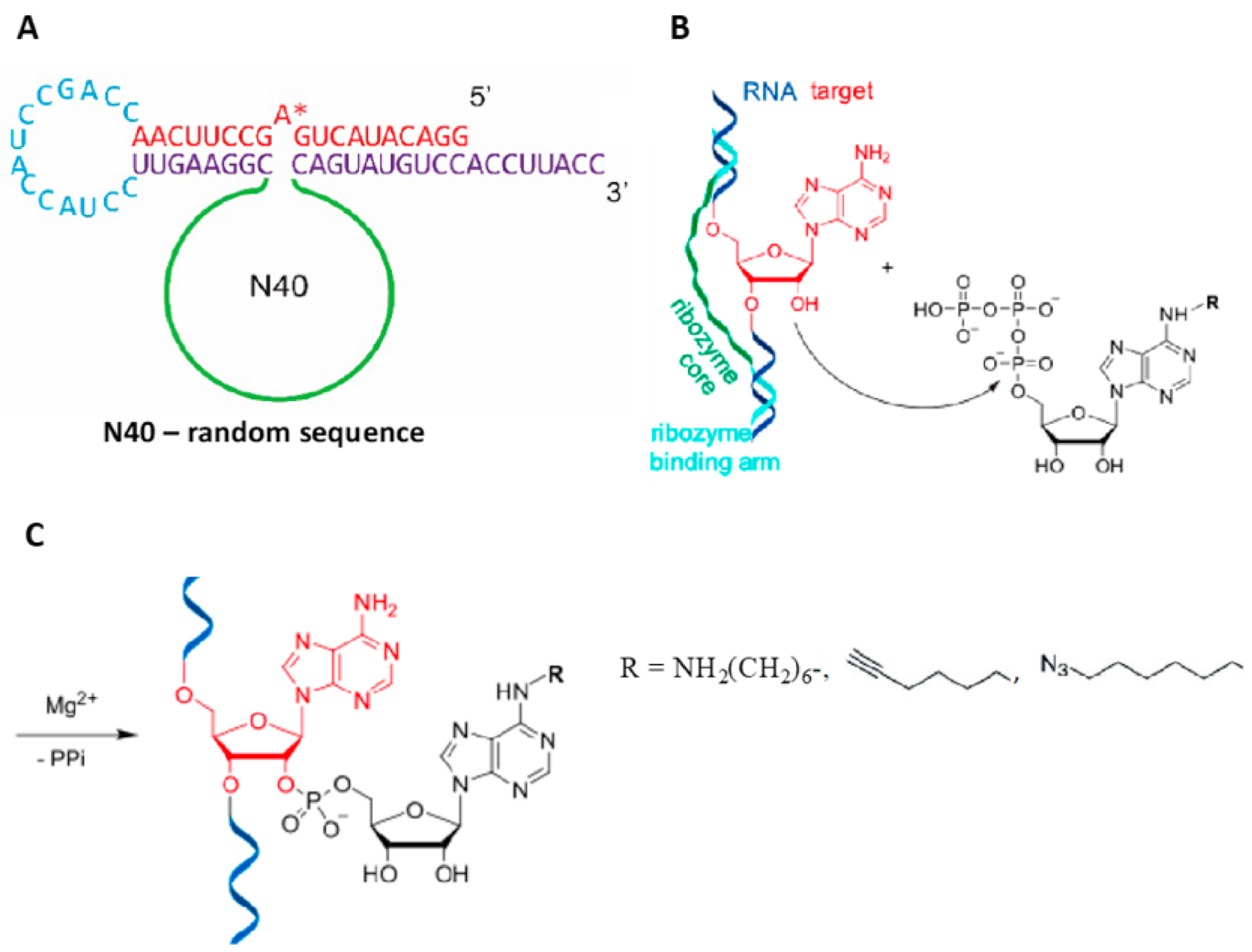
4.3. Evolving Ribozymes
5. Site-Specific Labeling Requiring De Novo RNA Preparation
5.1. Position-Selective Labeling of RNA (PLOR)
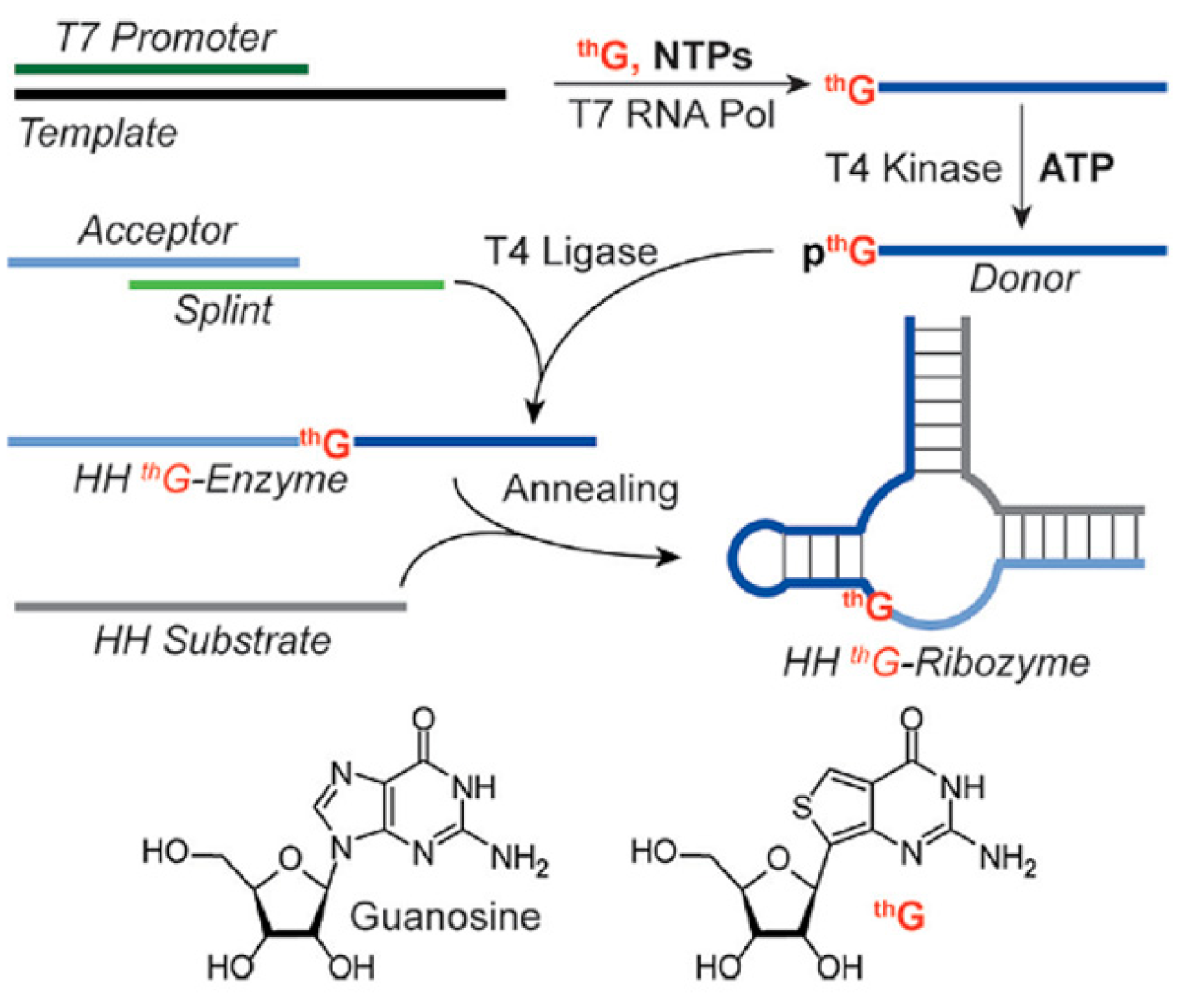
5.2. thG-Containing RNAs
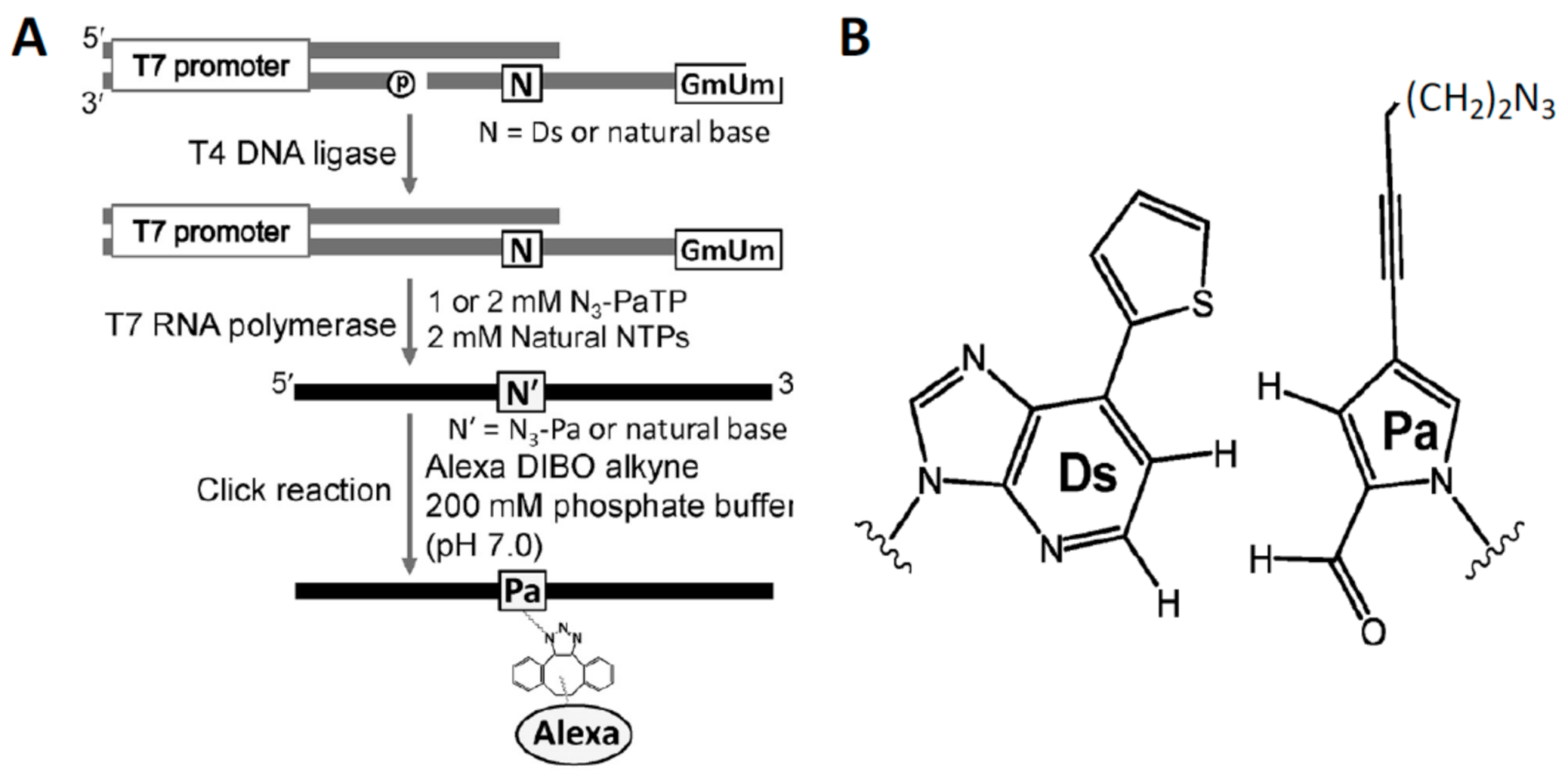
5.3. Unnatural Base Pairs
6. Conclusions
Funding
Conflicts of Interest
References
- Sinkeldam, R.W.; Greco, N.J.; Tor, Y. Fluorescent analogs of biomolecular building blocks: Design, properties, and applications. Chem. Rev. 2010, 110, 2579–2619. [Google Scholar] [CrossRef]
- Xu, W.; Chan, K.M.; Kool, E.T. Fluorescent nucleobases as tools for studying DNA and RNA. Nat. Chem. 2017, 9, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hudson, R.H. Base-modified fluorescent purine nucleosides and nucleotides for use in oligonucleotide probes. J. Photochem. Photobiol. C. Photochem. Rev. 2018, 36, 48–73. [Google Scholar] [CrossRef]
- Bood, M.; Sarangamath, S.; Wranne, M.S.; Grøtli, M.; Wilhelmsson, L.M. Fluorescent nucleobase analogues for base–base FRET in nucleic acids: Synthesis, photophysics and applications. Beilstein J. Org. Chem. 2018, 14, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.Y.; Dziuba, D.; Benhida, R.; Demchenko, A.P.; Burger, A. Probing of Nucleic Acid Structures, Dynamics, and Interactions With Environment-Sensitive Fluorescent Labels. Front. Chem. 2020, 8, 112. [Google Scholar] [CrossRef]
- Klöcker, N.; Weissenboeck, F.P.; Rentmeister, A. Covalent labeling of nucleic acids. Chem. Soc. Rev. 2020, 49, 8749–8773. [Google Scholar] [CrossRef] [PubMed]
- Hanspach, G.; Trucks, S.; Hengesbach, M. Strategic labelling approaches for RNA single-molecule spectroscopy. RNA Biol. 2019, 16, 1119–1132. [Google Scholar] [CrossRef]
- Tomkuvienė, M.; Mickutė, M.; Vilkaitis, G.; Klimašauskas, S. Repurposing enzymatic transferase reactions for targeted labeling and analysis of DNA and RNA. Curr. Opin. Biotechnol. 2019, 55, 114–123. [Google Scholar] [CrossRef]
- George, J.T.; Srivatsan, S.G. Bioorthogonal chemistry-based RNA labeling technologies: Evolution and current state. Chem. Commun. 2020, 56, 12307–12318. [Google Scholar] [CrossRef]
- McCown, P.J.; Ruszkowska, A.; Kunkler, C.N.; Breger, K.; Hulewicz, J.P.; Wang, M.C.; Springer, N.A.; Brown, J.A. Naturally occurring modified ribonucleosides. Wiley Interdiscip Rev. RNA 2020, 11, e1595. [Google Scholar] [CrossRef]
- Itaya, T.; Kanai, T. Synthesis and structure of the hypermodified nucleoside of rat liver phenylalanine transfer ribonucleic Acid. Chem. Pharm. Bull. 2002, 50, 1318–1326. [Google Scholar] [CrossRef][Green Version]
- de Crécy-Lagard, V.; Brochier-Armanet, C.; Urbonavicius, J.; Fernandez, B.; Phillips, G.; Lyons, B.; Noma, A.; Alvarez, S.; Droogmans, L.; Armengaud, J.; et al. Biosynthesis of wyosine derivatives in tRNA: An ancient and highly diverse pathway in Archaea. Mol. Biol. Evol. 2010, 27, 2062–2077. [Google Scholar]
- Eisinger, J.; Feuer, B.; Yamane, T. Luminescence and binding studies on tRNA-Phe. Proc. Natl. Acad. Sci. USA 1970, 65, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, K.; Cantor, C.R. Studies of transfer RNA tertiary structure by singlet-singlet energy transfer. Proc. Natl. Acad. Sci. USA 1970, 65, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.R.; Tor, Y. tRNA(Phe) binds aminoglycoside antibiotics. Bioorg. Med. Chem. 1999, 9, 1979–1991. [Google Scholar] [CrossRef]
- Dwyer, B.G.; Johnson, E.; Cazares, E.; Holman, K.L.M.; Kirk, S.R. Ruthenium anticancer agent KP1019 binds more tightly than NAMI-A to tRNAPhe. J. Inorg. Biochem. 2018, 182, 177–183. [Google Scholar] [CrossRef]
- Paulsen, H.; Robertson, J.M.; Wintermeyer, W. Effect of ribosome binding and translocation on the anticodon of tRNAPhe as studied by wybutine fluorescence. Nucleic Acids Res. 1982, 10, 2651–2663. [Google Scholar] [CrossRef]
- Robertson, J.M.; Paulsen, H.; Wintermeyer, W. Pre-steady-state kinetics of ribosomal translocation. J. Mol. Biol. 1986, 192, 351–360. [Google Scholar] [CrossRef]
- RajBhandary, U.L.; Faulkner, R.D.; Stuart, A. Studies on Polynucleotides LXXIX. Yeast phenylalanine transfer ribonucleic acid: Products obtained by degradation with pancreatic ribonucleasE. J. Biol. Chem. 1968, 243, 575–583. [Google Scholar] [CrossRef]
- Thiebe, R.; Zachau, H.G. A specific modification next to the anticodon of phenylalanine transfer ribonucleic acid. Eur. J. Biochem. 1968, 5, 546–555. [Google Scholar] [CrossRef]
- MODOMICS a Database of RNA Modifications. Available online: https://iimcb.genesilico.pl/modomics/ (accessed on 12 February 2021).
- Finet, O.; Yague-Sanz, C.; Kruger, L.K.; Migeot, V.; Ernst, F.G.; Lafontaine, D.L.J.; Tran, P.; Wéry, M.; Morillon, A.; Hermand, D. Transcription-Wide Mapping of Dihydrouridine (d) Reveals That Mrna ihydrouridylation is Essential for Meiotic Chromosome Segregation. Available online: http://dx.doi.org/10.2139/ssrn.3569550 (accessed on 15 February 2021).
- Betteridge, T.; Liu, H.; Gamper, H.; Kirillov, S.; Cooperman, B.S.; Hou, Y.M. Fluorescent labeling of tRNAs for dynamics experiments. RNA 2007, 13, 1594–1601. [Google Scholar] [CrossRef]
- Liu, C.; Betteridge, T.; Hou, Y.M. Fluorophore labeling to monitor tRNA dynamics. Methods Enzymol. 2009, 469, 69–93. [Google Scholar]
- Wintermeyer, W.; Zachau, H.G. Fluorescent derivatives of yeast tRNAPhe. Eur. J. Biochem. 1979, 98, 465–475. [Google Scholar] [CrossRef]
- Pan, D.; Qin, H.; Cooperman, B.S. Synthesis and functional activity of tRNAs labeled with fluorescent hydrazides in the D-loop. RNA 2009, 15, 346–354. [Google Scholar] [CrossRef]
- Stevens, B.; Chen, C.; Farrell, I.; Zhang, H.; Kaur, J.; Broitman, S.L.; Smilansky, Z.; Cooperman, B.S.; Goldman, Y.E. FRET-based identification of mRNAs undergoing translation. PLoS ONE 2012, 7, 38344. [Google Scholar]
- Tu, C.; Santo, L.; Mishima, Y.; Raje, N.; Smilansky, Z.; Zoldan, J. Monitoring protein synthesis in single live cancer cells. Integr. Biol. 2016, 8, 645–653. [Google Scholar] [CrossRef]
- Kaur, J.; Raj, M.; Cooperman, B.S. Fluorescent labeling of tRNA dihydrouridine residues: Mechanism and distribution. RNA 2011, 17, 1393–1400. [Google Scholar] [CrossRef]
- Pan, D.; Kirillov, S.V.; Cooperman, B.S. Kinetically competent intermediates in the translocation step of protein synthesis. Mol. Cell 2007, 25, 519–525. [Google Scholar] [CrossRef]
- Grigoriadou, C.; Marzi, S.; Kirillov, S.; Gualerzi, C.O.; Cooperman, B.S. A quantitative kinetic scheme for 70 S translation initiation complex formation. J. Mol. Biol. 2007, 373, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.; Li, H.; Ghelfi, M.D.; Goldman, Y.E.; Cooperman, B.S. Ataluren and aminoglycosides stimulate read-through of nonsense codons by orthogonal mechanisms. Proc. Natl. Acad. Sci. USA 2021, 118, 2020599118. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Stevens, B.; Kaur, J.; Cabral, D.; Liu, H.; Wang, Y.; Zhang, H.; Rosenblum, G.; Smilansky, Z.; Goldman, Y.E.; et al. Single-molecule fluorescence measurements of ribosomal translocation dynamics. Mol. Cell 2011, 42, 367–377. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Broitman, S.L.; Reiche, M.; Farrell, I.; Cooperman, B.S.; Goldman, Y.E. Dynamics of translation by single ribosomes through mRNA secondary structures. Nat. Struct. Mol. Biol. 2013, 20, 582–588. [Google Scholar] [CrossRef]
- Rosenblum, G.; Chen, C.; Kaur, J.; Cui, X.; Zhang, H.; Asahara, H.; Chong, S.; Smilansky, Z.; Goldman, Y.E.; Cooperman, B.S. Quantifying elongation rhythm during full-length protein synthesis. J. Am. Chem. Soc. 2013, 135, 11322–11329. [Google Scholar] [CrossRef]
- Jamiolkowski, R.M.; Chen, C.; Cooperman, B.S.; Goldman, Y.E. tRNA Fluctuations Observed on Stalled Ribosomes Are Suppressed during Ongoing Protein Synthesis. Biophys. J. 2017, 113, 2326–2335. [Google Scholar] [CrossRef]
- Barhoom, S.; Kaur, J.; Cooperman, B.S.; Smorodinsky, N.I.; Smilansky, Z.; Ehrlich, M.; Elroy-Stein, O. Quantitative single cell monitoring of protein synthesis at subcellular resolution using fluorescently labeled tRNA. Nucleic Acids Res. 2011, 39, 129. [Google Scholar] [CrossRef] [PubMed]
- Plochowietz, A.; Farrell, I.; Smilansky, Z.; Cooperman, B.S.; Kapanidis, A.N. In vivo single-RNA tracking shows that most tRNA diffuses freely in live bacteria. Nucleic Acids Res. 2017, 45, 926–937. [Google Scholar] [CrossRef]
- Dhakal, R.; Tong, C.; Anderson, S.; Kashina, A.S.; Cooperman, B.; Bau, H.H. Dynamics of intracellular stress-induced tRNA trafficking. Nucleic Acids Res. 2019, 47, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Rai, A.; Hur, E.M.; Smilansky, Z.; Chang, K.T.; Min, K.T. DSCR1 is required for both axonal growth cone extension and steering. J. Cell Biol. 2016, 213, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, J.; Chan, S.W.; Farnum, J.B.; Thomas, W.M.; Smilansky, Z.; Vanderklish, P.W. A heterogeneous tRNA granule structure exhibiting rapid, bi-directional neuritic transport. Eur. J. Cell Biol. 2018, 97, 168–179. [Google Scholar] [CrossRef]
- Koltun, B.; Ironi, S.; Gershoni-Emek, N.; Barrera, I.; Hleihil, M.; Nanguneri, S.; Sasmal, R.; Agasti, S.S.; Nair, D.; Rosenblum, K. Measuring mRNA translation in neuronal processes and somata by tRNA-FRET. Nucleic Acids Res. 2020, 48, 32. [Google Scholar] [CrossRef]
- Alroy, I.; Mansour, W.; Klepfish, M.; Sheinberger, Y. Expanding small-molecule target space to mRNA translation regulation. Drug Discov. Today 2020. [Google Scholar] [CrossRef] [PubMed]
- Barhoom, S.; Farrell, I.; Shai, B.; Dahary, D.; Cooperman, B.S.; Smilansky, Z.; Elroy-Stein, O.; Ehrlich, M. Dicodon monitoring of protein synthesis (DiCoMPS) reveals levels of synthesis of a viral protein in single cells. Nucleic Acids Res. 2013, 41, 177. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pampillo, M.; Guo, F.; Liu, S.; Cooperman, B.S.; Farrell, I.; Dahary, D.; Gan, B.S.; O’Gorman, D.B.; Smilansky, Z.; et al. Monitoring collagen synthesis in fibroblasts using fluorescently labeled tRNA pairs. J. Cell Physiol. 2014, 229, 1121–1129. [Google Scholar] [CrossRef]
- Volkov, I.L.; Lindén, M.; Aguirre Rivera, J.; Ieong, K.W.; Metelev, M.; Elf, J.; Johansson, M. tRNA tracking for direct measurements of protein synthesis kinetics in live cells. Nat. Chem. Biol. 2018, 14, 618–626. [Google Scholar] [CrossRef]
- Meyer, B.; Wurm, J.P.; Sharma, S.; Immer, C.; Pogoryelov, D.; Kötter, P.; Lafontaine, D.L.J.; Wöhnert, J.; Entian, K.-D. Ribosome biogenesis factor Tsr3 is the aminocarboxypropyl transferase responsible for 18S rRNA hypermodification in yeast and humans. Nucleic Acids Res. 2016, 44, 4304–4316. [Google Scholar] [CrossRef]
- Umitsu, M.; Nishimasu, H.; Noma, A.; Suzuki, T.; Ishitani, R.; Nureki, O. Structural basis of AdoMet-dependent aminocarboxypropyl transfer reaction catalyzed by tRNA-wybutosine synthesizing enzyme, TYW2. Proc. Natl. Acad. Sci. USA 2009, 106, 15616–15621. [Google Scholar] [CrossRef]
- Meyer, B.; Wurm, J.P.; Kötter, P.; Leisegang, M.S.; Schilling, V.; Buchhaupt, M.; Held, M.; Bahr, U.; Karas, M.; Heckel, et.al. The Bowen-Conradi syndrome protein Nep1 (Emg1) has a dual role in eukaryotic ribosome biogenesis, as an essential assembly factor and in the methylation of Ψ1191 in yeast 18S rRNA. Nucleic Acids Res. 2011, 39, 1526–1537. [Google Scholar] [CrossRef]
- Fei, J.; Wang, J.; Sternberg, S.H.; MacDougall, D.D.; Elvekrog, M.M.; Pulukkunat, D.K.; Englander, M.T.; Gonzalez, R.L., Jr. A highly purified, fluorescently labeled in vitro translation system for single-molecule studies of protein synthesis. Methods Enzymol. 2010, 472, 221–259. [Google Scholar]
- Blanchard, S.C.; Kim, H.D.; Gonzalez, R.L., Jr.; Puglisi, J.D.; Chu, S. tRNA dynamics on the ribosome during translation. Proc. Natl. Acad. Sci. USA 2004, 101, 12893–12898. [Google Scholar] [CrossRef]
- Blanchard, S.C.; Gonzalez, R.L.; Kim, H.D.; Chu, S.; Puglisi, J.D. tRNA selection and kinetic proofreading in translation. Nat. Struct. Mol. Biol. 2004, 11, 1008–1014. [Google Scholar] [CrossRef]
- Wasserman, M.R.; Alejo, J.L.; Altman, R.B.; Blanchard, S.C. Multiperspective smFRET reveals rate-determining late intermediates of ribosomal translocation. Nat. Struct. Mol. Biol. 2016, 23, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Fei, J.; Gonzalez, R.L., Jr. The ribosome uses cooperative conformational changes to maximize and regulate the efficiency of translation. Proc. Natl. Acad. Sci. USA 2014, 111, 12073–12078. [Google Scholar] [CrossRef]
- Tsai, A.; Puglisi, J.D.; Uemura, S. Probing the Translation Dynamics of Ribosomes Using Zero-Mode Waveguides. Prog. Mol. Biol. Transl. Sci. 2016, 139, 1–43. [Google Scholar] [PubMed]
- Bimai, O.; Arragain, S.; Golinelli-Pimpaneau, B. Structure-based mechanistic insights into catalysis by tRNA thiolation enzymes. Curr. Opin. Struct. Biol. 2020, 65, 69–78. [Google Scholar] [CrossRef]
- Lauhon, C.T.; Erwin, W.M.; Ton, G.N. Substrate specificity for 4-thiouridine modification in Escherichia coli. J. Biol. Chem. 2004, 279, 23022–23029. [Google Scholar] [CrossRef]
- Neumann, P.; Lakomek, K.; Naumann, P.T.; Erwin, W.M.; Lauhon, C.T.; Ficner, R. Crystal structure of a 4-thiouridine synthetase-RNA complex reveals specificity of tRNA U8 modification. Nucleic Acids Res. 2014, 42, 6673–6685. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shang, J.; Qin, Z.; Tong, A.; Xiang, Y. Selective and sensitive fluorescence “turn-on” detection of 4-thiouridine in nucleic acids via oxidative amination. Chem. Commun. 2019, 55, 13096–13099. [Google Scholar] [CrossRef] [PubMed]
- Bou-Nader, C.; Pecqueur, L.; Barraud, P.; Fontecave, M.; Tisné, C.; Sacquin-Mora, S.; Hamdane, D. Conformational Stability Adaptation of a Double-Stranded RNA-Binding Domain to Transfer RNA Ligand. Biochemistry 2019, 58, 2463–2473. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.H. Innovation by Evolution: Bringing New Chemistry to Life (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2019, 58, 14420–14426. [Google Scholar] [CrossRef] [PubMed]
- Motorin, Y.; Burhenne, J.; Teimer, R.; Koynov, K.; Willnow, S.; Weinhold, E.; Helm, M. Expanding the chemical scope of RNA:methyltransferases to site-specific alkynylation of RNA for click labeling. Nucleic Acids Res. 2011, 39, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, A.; Weissenboeck, F.P.; Rentmeister, A. Tag-Free Internal RNA Labeling and Photocaging Based on mRNA Methyltransferases. Angew. Chem. Int. Ed. Engl. 2020. [Google Scholar] [CrossRef]
- Gu, X.; Santi, D.V. The T-arm of tRNA is a substrate for tRNA (m5U54)-methyltransferase. Biochemistry 1991, 30, 2999–3002. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.S.; Zoltek, M.A.; Simon, M.D. Reengineering a tRNA Methyltransferase To Covalently Capture New RNA Substrates. J. Am. Chem. Soc. 2019, 141, 17460–17465. [Google Scholar] [CrossRef]
- Busby, K.N.; Devaraj, N.K. Enzymatic covalent labeling of RNA with RNA transglycosylation at guanosine (RNA-TAG). Methods Enzym. 2020, 641, 373–399. [Google Scholar]
- Xiao, L.; Habibian, M.; Kool, E.T. Site-Selective RNA Functionalization via DNA-Induced Structure. J. Am. Chem. Soc. 2020, 142, 16357–16363. [Google Scholar] [CrossRef]
- Zhao, M.; Steffen, F.D.; Börner, R.; Schaffer, M.F.; Sigel, R.K.O.; Freisinger, E. Site-specific dual-color labeling of long RNAs for single-molecule spectroscopy. Nucleic Acids Res. 2018, 46, e13. [Google Scholar] [CrossRef]
- Zhao, M.; Börner, R.; Sigel, R.K.O.; Freisinger, E. Site-Specific Dual-Color Labeling of Long RNAs. Methods Mol Biol. 2020, 2106, 253–270. [Google Scholar]
- Li, Y.; Fin, A.; McCoy, L.; Tor, Y. Polymerase-Mediated Site-Specific Incorporation of a Synthetic Fluorescent Isomorphic G Surrogate into RNA. Angew. Chem. Int. Ed. 2017, 56, 1303–1307. [Google Scholar] [CrossRef]
- Egloff, D.; Oleinich, I.A.; Zhao, M.; König, S.L.; Sigel, R.K.; Freisinger, E. Sequence-Specific Post-Synthetic Oligonucleotide Labeling for Single-Molecule Fluorescence Applications. ACS Chem. Biol. 2016, 11, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Ghaem Maghami, M.; Scheitl, C.P.M.; Höbartner, C. Direct in Vitro Selection of Trans.-Acting Ribozymes for Posttranscriptional, Site-Specific, and Covalent Fluorescent Labeling of RNA. J. Am. Chem. Soc. 2019, 141, 19546–19549. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ghaem Maghami, M.; Dey, S.; Lenz, A.K.; Höbartner, C. Repurposing Antiviral Drugs for Orthogonal RNA-Catalyzed Labeling of RNA. Angew Chem. Int. Ed. Engl. 2020, 59, 9335–9339. [Google Scholar] [CrossRef]
- Liu, Y.; Holmstrom, E.; Yu, P.; Tan, K.; Zuo, X.; Nesbitt, D.J.; Sousa, R.; Stagno, J.R.; Wang, Y.X. Incorporation of isotopic, fluorescent, and heavy-atom-modified nucleotides into RNAs by position-selective labeling of RNA. Nat. Protoc. 2018, 13, 987–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Liu, Y. Optimization and characterization of position-selective labelling of RNA (PLOR) for diverse RNA and DNA sequences. RNA Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Optimization of N-hydroxysuccinimide ester coupling with aminoallyl-modified RNA for fluorescent labeling. Bioengineered 2020, 11, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.W.; Romesberg, F.E. Expansion of the Genetic Alphabet: A Chemist’s Approach to Synthetic Biology. Acc. Chem. Res. 2018, 51, 394–403. [Google Scholar] [CrossRef]
- Kimoto, M.; Hirao, I. Genetic alphabet expansion technology by creating unnatural base pairs. Chem. Soc. Rev. 2020, 49, 7602–7626. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Kimoto, M.; Kawai, R.; Yokoyama, S.; Hirao, I. Characterization of fluorescent, unnatural base pairs. Tetrahedron 2007, 63, 3528–3537. [Google Scholar] [CrossRef]
- Kimoto, M.; Mitsui, T.; Yamashige, R.; Sato, A.; Yokoyama, S.; Hirao, I. A new unnatural base pair system between fluorophore and quencher base analogues for nucleic acid-based imaging technology. J. Am. Chem. Soc. 2010, 132, 15418–15426. [Google Scholar] [CrossRef]
- Someya, T.; Ando, A.; Kimoto, M.; Hirao, I. Site-specific labeling of RNA by combining genetic alphabet expansion transcription and copper-free click chemistry. Nucleic Acids Res. 2015, 43, 6665–6676. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooperman, B.S. Site-Specific Fluorescent Labeling of RNA Interior Positions. Molecules 2021, 26, 1341. https://doi.org/10.3390/molecules26051341
Cooperman BS. Site-Specific Fluorescent Labeling of RNA Interior Positions. Molecules. 2021; 26(5):1341. https://doi.org/10.3390/molecules26051341
Chicago/Turabian StyleCooperman, Barry S. 2021. "Site-Specific Fluorescent Labeling of RNA Interior Positions" Molecules 26, no. 5: 1341. https://doi.org/10.3390/molecules26051341
APA StyleCooperman, B. S. (2021). Site-Specific Fluorescent Labeling of RNA Interior Positions. Molecules, 26(5), 1341. https://doi.org/10.3390/molecules26051341





