Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains
Abstract
1. Introduction
2. Results
2.1. Carbohydrates Profile
2.2. Concentration of Total Polyphenols and Antioxidative Activity
2.3. Browning Index and Concentrations of 5-Hydroxymethylfurfural (HMF)
3. Discussion
3.1. Carbohydrates Profile
3.2. Concentration of Total Polyphenols and Antioxidative Activity
3.3. Browning Index and Concentrations of 5-Hydroxymethylfurfural (HMF)
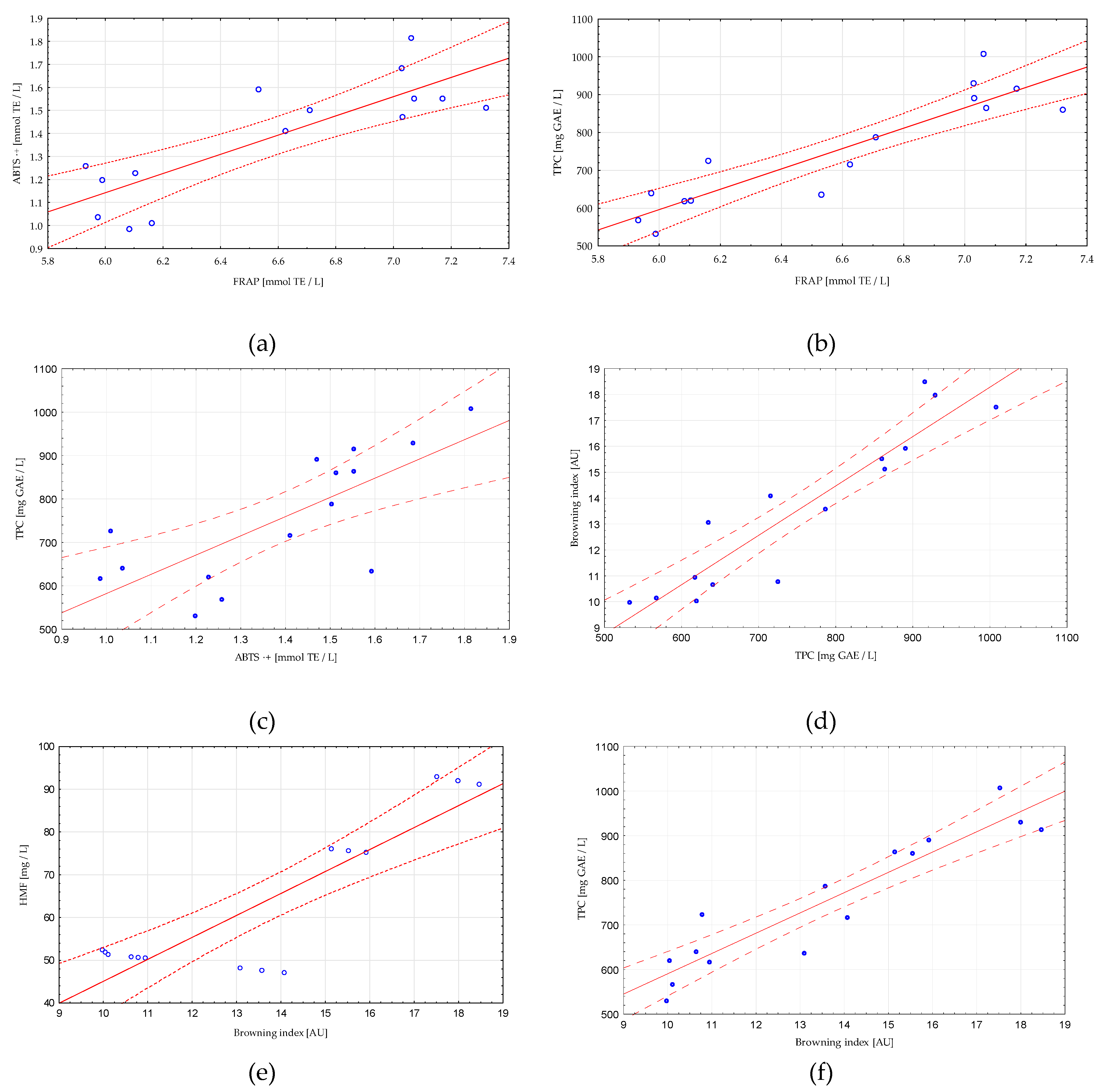
3.4. Correlation and Linear Regression Analysis
4. Materials and Methods
4.1. Raw Material
4.2. Prepartation of the Congress Worts
4.3. Analytic Methods
4.3.1. High-Performance Liquid Chromatography (HPLC) Analysis of Carbohydrate Profile
4.3.2. High-Performance Liquid Chromatography (HPLC) Analysis of 5-Hydroxymethylfurfural (HMF) Content
4.3.3. Total Polyphenols Content
4.3.4. Antioxidative Activity
Ability to Iron Ions Reduction (FRAP)
Ability to Cation Radical ABTS•+ Reduction
4.3.5. Browning Index
4.3.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar]
- Kondo, K. Beer and health: Preventive effects of beer components on lifestyle-related diseases. Biofactors 2004, 22, 303–310. [Google Scholar] [CrossRef]
- Kołota, A.; Oczkowski, M.; Gromadzka-Ostrowska, J. Wpływ występujących w piwie związków polifenolowych na organizm–przegląd literatury. Alcohol. Drug Addict. 2014, 27, 273–281. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Bartoszek-Pączkowska, A. Bioaktywne Fitozwiązki W Chemoprewencji Przewlekłych Chorób Niezakaźnych–Owoce I Warzywa Czy Suplementy Diety? Żywność. Nauka. Technol. Jakość 2019, 1, 5–14. [Google Scholar]
- Blazewicz, J.; Kawa-Rygielska, J. Zalety technologiczne nietypowo suszonych słodów specjalnych. Przemysł Ferment. Owocowo Warzywny 2018, 1, 16–17. [Google Scholar] [CrossRef]
- Coghe, S.; Vanderhaegen, B.; Pelgrims, B.; Basteyns, A.V.; Delvaux, F.R. Characterization of dark specialty malts: New insights in color evaluation and pro-and antioxidative activity. J. Am. Soc. Brew. Chem. 2003, 61, 125–132. [Google Scholar] [CrossRef]
- Błażewicz, J.; Kawa-Rygielska, J.; Gasinski, A. Słody żytnie w ocenie technologicznej. Przemysł Ferment. Owocowo Warzywny 2019, 63. [Google Scholar] [CrossRef]
- Błażewicz, J.; Kawa-Rygielska, J.; Gasior, J. Słody specjalne z nasion roślin strączkowych. Przemysł Ferment. i Owocowo Warzywny 2019, 63. [Google Scholar] [CrossRef]
- Malt, V. Available online: https://vikingmalt.pl/oferta/ (accessed on 21 August 2020).
- Carvalho, D.O.; Gonçalves, L.M.; Guido, L.F. Overall antioxidant properties of malt and how they are influenced by the individual constituents of barley and the malting process. Compr. Rev. Food Sci. Food Saf. 2016, 15, 927–943. [Google Scholar] [CrossRef]
- Coghe, S.; Martens, E.; D’Hollander, H.; Dirinck, P.J.; Delvaux, F.R. Sensory and instrumental flavour analysis of wort brewed with dark specialty malts. J. Inst. Brew. 2004, 110, 94–103. [Google Scholar] [CrossRef]
- Baranwal, D. Malting: An indigenous technology used for improving the nutritional quality of grains: A review. Asian J. Dairy Food Res. 2017, 36, 179–183. [Google Scholar] [CrossRef][Green Version]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of phenolic content in different barley varieties and corresponding malts by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, H.M.; Ames, J.M.; Chandra, S. Critical stages of the brewing process for changes in antioxidant activity and levels of phenolic compounds in ale. J. Am. Soc. Brew. Chem. 2003, 61, 203–209. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Morales, F.J. Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews. J. Agric. Food Chem. 2005, 53, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Echavarrı’a, A.P.; Paga’n, J.; Ibarz, A. Melanoidins formed by maillard reaction in food and their biological activity. Food. Eng. Rev. 2012, 4, 203–223. [Google Scholar] [CrossRef]
- Michalska, A.; Zielinski, H. Produkty reakcji Maillarda w zywnosci. Żywność Nauka Technol. Jakość 2007, 14, 5–16. [Google Scholar]
- Aljahdali, N.; Gadonna-Widehem, P.; Anton, P.M.; Carbonero, F. Gut Microbiota Modulation by Dietary Barley Malt Melanoidins. Nutrients 2020, 12, 241. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bin-Jumah, M.; Othman, S.I.; Khafaga, A.F.; Shaheen, H.M.; Mohamed, E. The Toxicological Aspects of the Heat-Borne Toxicant 5-Hydroxymethylfurfural in Animals: A Review. Molecules 2020, 25, 1941. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.; Gonçalves, J.; Figueira, J.A.; Ornelas, L.P.; Branco, R.N.; Câmara, J.S.; Pereira, J.A. Beer volatile fingerprinting at different brewing steps. Food Chem. 2020, 326, 126856. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of phenolics content and antioxidant activity of different beer types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef] [PubMed]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piórecki, N. Physicochemical and antioxidative properties of Cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gąsior, J.; Pietrzak, W. Volatile Compounds Content, Physicochemical Parameters, and Antioxidant Activity of Beers with Addition of Mango Fruit (Mangifera Indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Gąsior, J.; Głowacki, A. Assessment of Volatiles and Polyphenol Content, Physicochemical Parameters and Antioxidant Activity in Beers with Dotted Hawthorn (Crataegus punctata). Foods 2020, 9, 775. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Pérez-Jiménez, J. What Contribution Is Beer to the Intake of Antioxidants in the Diet? In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 441–448. [Google Scholar]
- Tubaro, F. Antioxidant Activity of Beer’s Maillard Reaction Products: Features and Health Aspects. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 449–457. [Google Scholar]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef]
- Coghe, S.; D’Hollander, H.; Verachtert, H.; Delvaux, F.R. Impact of dark specialty malts on extract composition and wort fermentation. J. Inst. Brew. 2005, 111, 51–60. [Google Scholar] [CrossRef]
- Briggs, D.E.; Brookes, P.A.; Stevens, R.B.C.A.; Boulton, C.A. Brewing: Science and Practice; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Oracz, J.; Nebesny, E. Effect of roasting parameters on the physicochemical characteristics of high-molecular-weight Maillard reaction products isolated from cocoa beans of different Theobroma cacao L. groups. Eur. Food. Res. Technol. 2019, 245, 111–128. [Google Scholar] [CrossRef]
- Nanamori, M.; Watanabe, T.; Shinano, T.; Kihara, M.; Kawahara, K.; Yamada, S.; Osaki, M. Changes in saccharide, amino acid and S-methylmethionine content during malting of barley grown with different nitrogen and sulfur status. J. Sci. Food Agric. 2011, 91, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Dodig, D.; Basić, Z.; Vančetović, J.; Titan, P.; Đurić, N.; Tolimir, N. Free asparagine and sugars profile of cereal species: The potential of cereals for acrylamide formation in foods. Food Addit. Contam. Part A 2017, 34, 705–713. [Google Scholar]
- Vinje, M.A.; Duke, S.H.; Henson, C.A. Comparison of factors involved in starch degradation in barley germination under laboratory and malting conditions. J. Am. Soc. Brew. Chem. 2015, 73, 195–205. [Google Scholar] [CrossRef]
- Ferreira, I.M. Beer Carbohydrates. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 291–298. [Google Scholar]
- Mohsin, G.F.; Schmitt, F.J.; Kanzler, C.; Epping, J.D.; Buhrke, D.; Hornemann, A. Melanoidin formed from fructosylalanine contains more alanine than melanoidin formed from d-glucose with L-alanine. Food Chem. 2020, 305, 125459. [Google Scholar] [CrossRef]
- Zhao, H. Endogenous Antioxidants and Antioxidant Activities of Beers. In Processing and Impact on Antioxidants in Beverages; Academic Press: Cambridge, MA, USA, 2014; pp. 15–24. [Google Scholar]
- Dybkowska, E.; Sadowska, A.; Rakowska, R.; Debowska, M.; Swiderski, F.; Swiader, K. Assessing polyphenols content and antioxidant activity in coffee beans according to origin and the degree of roasting. Rocz. Panstw. Zakl. Hig. 2017, 68, 347–353. [Google Scholar]
- Murakami, M.; Yamaguchi, T.; Takamura, H.; Atoba, T.M. Effects of thermal treatment on radical-scavenging activity of single and mixed polyphenolic compounds. J. Food Sci. 2004, 69, FCT7–FCT10. [Google Scholar] [CrossRef]
- Jayabalan, R.; Subathradevi, P.; Marimuthu, S.; Sathishkumar, M.; Swaminathan, K. Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chem. 2008, 109, 227–234. [Google Scholar] [CrossRef]
- Woffenden, H.M.; Ames, J.M.; Chandra, S.; Anese, M.; Nicoli, M.C. Effect of kilning on the antioxidant and pro-oxidant activities of pale malts. J. Agric. Food Chem. 2002, 50, 4925–4933. [Google Scholar] [CrossRef]
- Fogarasi, A.L.; Kun, S.; Tankó, G.; Stefanovits-Bányai, É.; Hegyesné-Vecseri, B. A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 2015, 167, 1–6. [Google Scholar] [CrossRef]
- Inns, E.L.; Buggey, L.A.; Booer, C.; Nursten, H.E.; Ames, J.M. Effect of modification of the kilning regimen on levels of free ferulic acid and antioxidant activity in malt. J. Agric. Food Chem. 2011, 59, 9335–9343. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, L.; Cao, L.; Lin, W.; Ye, X. Sonication inhibited browning but decreased polyphenols contents and antioxidant activity of fresh apple (malus pumila mill, cv. Red Fuji) juice. Int. J. Food. Sci. Tech. 2015, 52, 8336–8342. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chen, K.T.; Lin, J.A.; Chen, Y.T.; Chen, Y.A.; Wu, J.T.; Hsieh, C.W. Recent advances in processing technology to reduce 5-hydroxymethylfurfural in foods. Trends Food Sci. Technol. 2019, 93, 271–280. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Lachenmeier, D.W. The margin of exposure of 5-hydroxymethylfurfural (HMF) in alcoholic beverages. Environ. Health Toxicol. 2012, 27, e2012016. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Hydroxymethylfurfural determination in cereal and insect bars by high-performance liquid chromatography-mass spectrometry employing a functionalized mesostructured silica as sorbent in solid-phase extraction. J. Chromatogr. A 2020, 1622, 461124. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Aggarwal, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) formation, occurrence and potential health concerns: Recent developments. Toxin Rev. 2020, 1–17. [Google Scholar] [CrossRef]
- Akıllıoglu, H.G.; Mogol, B.A.; Gökmen, V. Degradation of 5-hydroxymethylfurfural during yeast fermentation. Food Addit. Contam. 2011, 28, 1629–1635. [Google Scholar] [CrossRef]
- Viegas, O.; Prucha, M.; Gökmen, V.; Ferreira, I.M. Parameters affecting 5-hydroxymethylfurfural exposure from beer. Food Addit. Contam. Part A 2018, 35, 1464–1471. [Google Scholar] [CrossRef]
- Rufian-Henares, J.A.; De la Cueva, S.P. Assessment of hydroxymethylfurfural intake in the Spanish diet. Food Addit. Contam. 2008, 25, 1306–1312. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Nilsson, J.; Pillai, D.; Önning, G.; Persson, C.; Nilsson, Å.; Åkesson, B. Comparison of the 2, 2′-azinobis-3-ethylbenzotiazo-line-6-sulfonic acid (ABTS) and ferric reducing anti-oxidant power (FRAP) methods to assess the total antioxidant capacity in extracts of fruit and vegetables. Mol. Nutr. Food Res. 2005, 49, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.S.; Kim, H.Y.; Hwang, I.G.; Lee, S.H.; Jeong, H.S. Characteristics of the thermal degradation of glucose and maltose solutions. Prev. Nutr. Food Sci. 2015, 20, 102. [Google Scholar] [CrossRef] [PubMed]
- Bastola, K.P.; Guragain, Y.N.; Bhadriraju, V.; Vadlani, P.V. Evaluation of standards and interfering compounds in the determination of phenolics by Folin-Ciocalteu assay method for effective bioprocessing of biomass. Am. J. Anal. Chem. 2017, 8, 416–431. [Google Scholar] [CrossRef]
- Kroh, L.W.; Schulz, A. News on the Maillard reaction of oligomeric carbohydrates: A survey. Food Nahr. 2001, 45, 160–163. [Google Scholar] [CrossRef]
- Pietrzak, W.; Kawa-Rygielska, J. Simultaneous saccharification and ethanol fermentation of waste wheat–rye bread at very high solids loading: Effect of enzymatic liquefaction conditions. Fuel 2015, 147, 236–242. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z. Characteristics of Cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chem. 2020, 312, 125968. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chen, S.L.; Jin, S.Y.; Chen, C.S. Relative reactivities of glucose and galactose in browning and pyruvaldehyde formation in sugar/glycine model systems. Food Chem. 2005, 92, 597–605. [Google Scholar] [CrossRef]
Sample Availability: Samples of the worts are available from the authors. |




| Series | Variant | Maltose | Dextrin | Maltotriose | Glucose |
|---|---|---|---|---|---|
| g/L | |||||
| P | 51.59 ± 0.01 a,A | 23.49 ± 0.04 d,E | 13.57 ± 0.02 b,A | 5.65 ±0.19 b,c,A | |
| I | CJ | 45.86 ± 0.40 c | 21.86 ± 0.40 e | 12.21 ± 0.10 e | 3.96 ± 0.15 d |
| CC | 50.50 ± 0.04 b | 26.09 ± 0.22 b | 13.46 ± 0.01 c | 5.48 ± 0.14 c | |
| PC | 34.74 ± 0.02 c | 18.03 ± 0.30 f | 9.13 ± 0.01 f | 0.47 ± 0.06 e | |
| JP | 51.69 ± 0.33 a | 28.43 ± 0.08 a | 13.87 ± 0.08 a | 5.80 ± 0.02 b | |
| JB | 50.14 ± 0.06 b | 25.31 ± 0.23 c | 13.34 ± 0.00 d | 6.48 ± 0.04 a | |
| II | CC20 | 46.12 ± 0.18 B | 35.06 ± 0.17 C | 12.68 ± 0.10 B | nd |
| CC25 | 45.50 ± 0.23 C | 33.73 ± 0.32 D | 11.83 ± 0.12 D | 2.80 ± 0.40 C | |
| CC30 | 45.71 ± 0.41 B,C | 37.22 ± 0.22 B | 12.05 ± 0.11 C | 4.18 ± 0.34 B | |
| CC35 | 41.17 ± 0.22 D | 37.43 ± 0.22 B | 10.90 ± 0.06 E | 5.60 ± 0.08 A | |
| CC40 | 39.50 ± 0.06 E | 39.85 ± 0.16 A | 10.74 ± 0.00 F | nd | |
| Series | Variant | Total Polyphenol Content (F–C) | FRAP | ABTS•+ | |
|---|---|---|---|---|---|
| mg GAE/L | mmol TE/L | mmol TE/L | % Inhibition | ||
| P | 192.58 ± 8.66 d,F | 1.23 ± 0.01 f,D | 1.44 ± 0.09 c,B | 22.17 ± 0.87 | |
| I | CJ | 252.84 ±12.81 c | 1.66 ± 0.03 c | 1.76 ± 0.17 c | 26.82 ± 1.70 |
| CC | 404.38 ± 5.98 a | 2.07 ± 0.03 a | 2.17 ± 0.17 b | 32.78 ± 1.80 | |
| PC | 303.90 ± 7.83 b | 1.44 ± 0.01 d | 1.46 ± 0.11 c | 22.53 ± 1.13 | |
| JP | 397.03 ± 7.63 a | 1.90 ± 0.02 b | 2.70 ± 0.40 a | 40.33 ± 4.06 | |
| JB | 176.02 ± 0.59 d | 1.32 ± 0.02 e | 1.46 ± 0.05 c | 22.53 ± 0.51 | |
| II | CC20 | 593.82 ± 6.23 E | 6.01 ± 0.09 C | 1.23 ± 0.03 C | 19.19 ± 0.31 |
| CC25 | 628.79 ± 10.73 D | 6.10 ± 0.09 C | 1.01 ± 0.03 D | 16.06 ± 0.26 | |
| CC30 | 776.21 ± 10.78 C | 6.62 ± 0.09 B | 1.50 ± 0.09 B | 23.11 ± 0.92 | |
| CC35 | 871.77 ± 16.55 B | 7.14 ± 0.15 A | 1.51 ± 0.04 B | 23.26 ± 0.41 | |
| CC40 | 922.32 ± 6.96 A | 7.09 ± 0.07 A | 1.68 ± 0.13 A | 25.73 ± 1.34 | |
| Series | Variant | Browning Index | HMF |
|---|---|---|---|
| AU | mg/L | ||
| P | 0.62 ± 0.04 f,F | 0.66 ± 0.00 f,F | |
| I | CJ | 1.80 ± 0.01 d | 19.65 ± 0.00 c |
| CC | 4.43 ± 0.04 b | 20.90 ± 0.01 b | |
| PC | 3.16 ± 0.01 c | 5.54 ± 0.03 e | |
| JP | 5.57 ± 0.03 a | 24.31 ± 0.17 a | |
| JB | 1.05 ± 0.00 e | 12.32 ± 0.04 d | |
| II | CC20 | 10.05 ± 0.07 E | 51.81 ± 0.54 C |
| CC25 | 10.79 ± 0.16 D | 50.62 ± 0.14 D | |
| CC30 | 13.58 ± 0.50 C | 47.62 ± 0.54 E | |
| CC35 | 15.53 ± 0.39 B | 75.61 ± 0.43 B | |
| CC40 | 17.99 ± 0.48 A | 91.94 ± 0.85 A |
| Variable | FRAP | ABTS•+ | TPC | BI | HMF | Dextrin | Maltotriose | Maltose | Glucose |
|---|---|---|---|---|---|---|---|---|---|
| FRAP | 1.00 | 0.83 | 0.91 | 0.94 | 0.79 | 0.87 | −0.87 | −0.86 | 0.32 |
| ABTS•+ | 1.00 | 0.75 | 0.84 | 0.70 | 0.95 | −0.62 | −0.72 | −0.01 | |
| TPC | 1.00 | 0.93 | 0.87 | 0.82 | −0.91 | −0.91 | 0.17 | ||
| BI | 1.00 | 0.88 | 0.94 | −0.88 | −0.91 | 0.11 | |||
| HMF | 1.00 | 0.80 | −0.88 | −0.98 | −0.17 | ||||
| Dextrin | 1.00 | −0.70 | −0.81 | −0.06 | |||||
| Maltotriose | 1.00 | 0.94 | −0.19 | ||||||
| Maltose | 1.00 | 0.04 | |||||||
| Glucose | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gąsior, J.; Kawa-Rygielska, J.; Kucharska, A.Z. Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains. Molecules 2020, 25, 3882. https://doi.org/10.3390/molecules25173882
Gąsior J, Kawa-Rygielska J, Kucharska AZ. Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains. Molecules. 2020; 25(17):3882. https://doi.org/10.3390/molecules25173882
Chicago/Turabian StyleGąsior, Justyna, Joanna Kawa-Rygielska, and Alicja Z. Kucharska. 2020. "Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains" Molecules 25, no. 17: 3882. https://doi.org/10.3390/molecules25173882
APA StyleGąsior, J., Kawa-Rygielska, J., & Kucharska, A. Z. (2020). Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains. Molecules, 25(17), 3882. https://doi.org/10.3390/molecules25173882





