Effects of Orange Extracts on Longevity, Healthspan, and Stress Resistance in Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
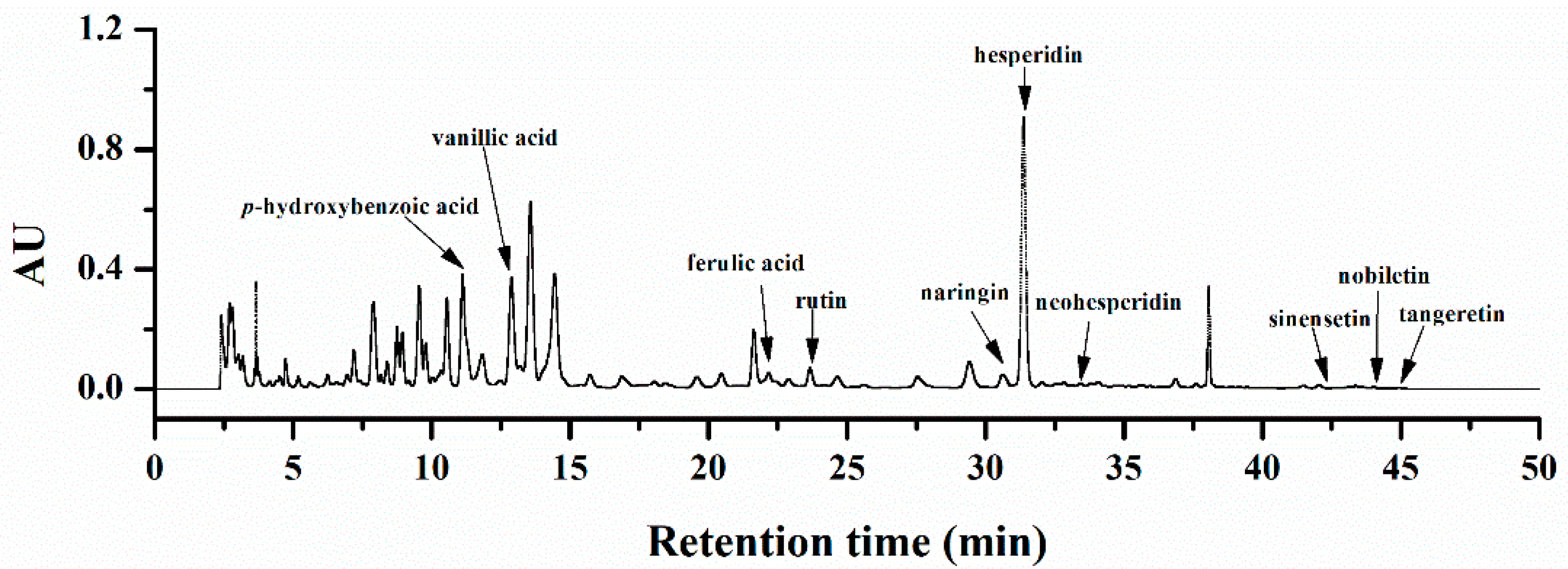
2.1. Chemical Characterization of Orange Extracts
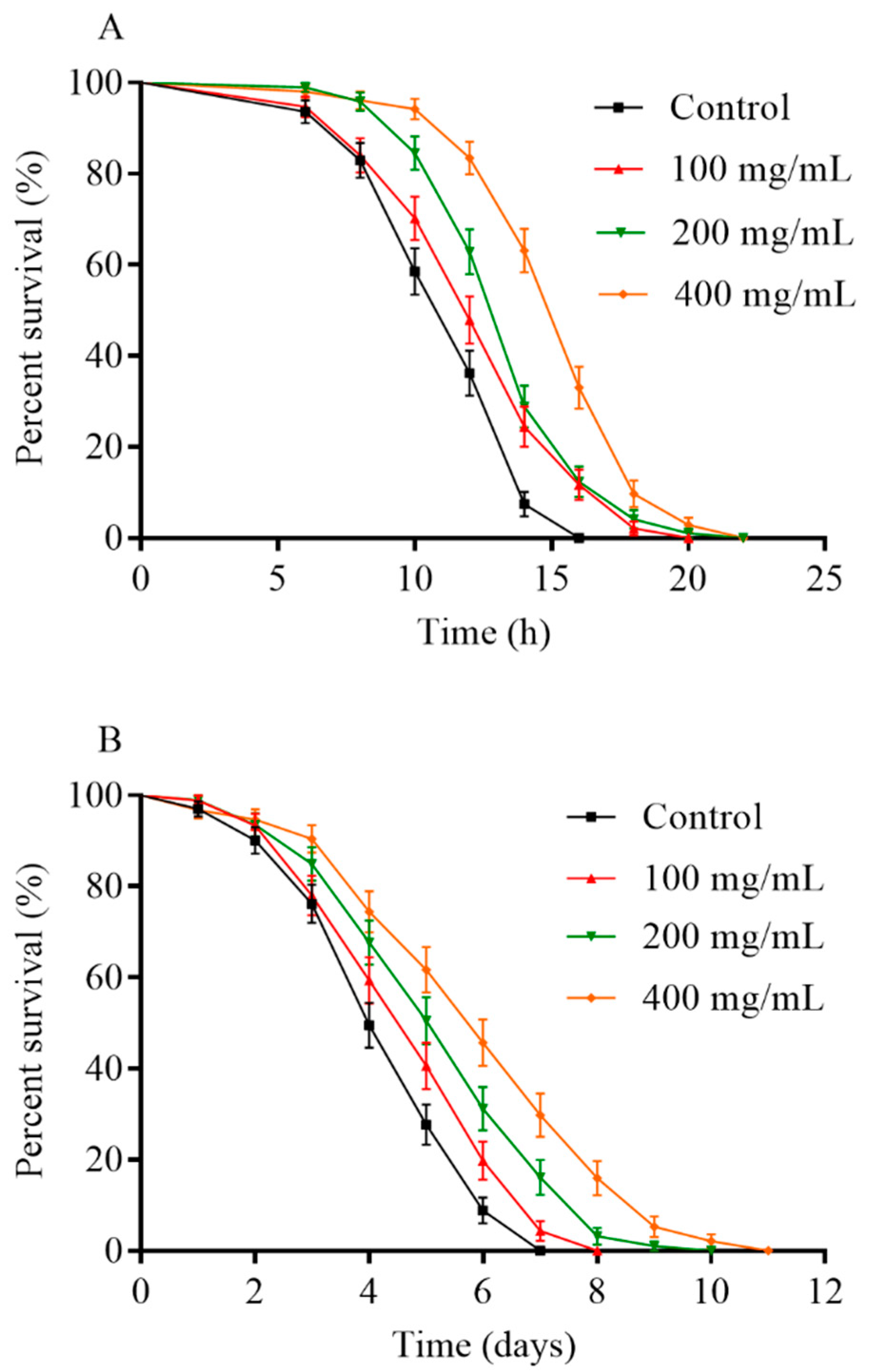
2.2. Orange Extracts Prolonged the Lifespan of Wild-Type C. elegans
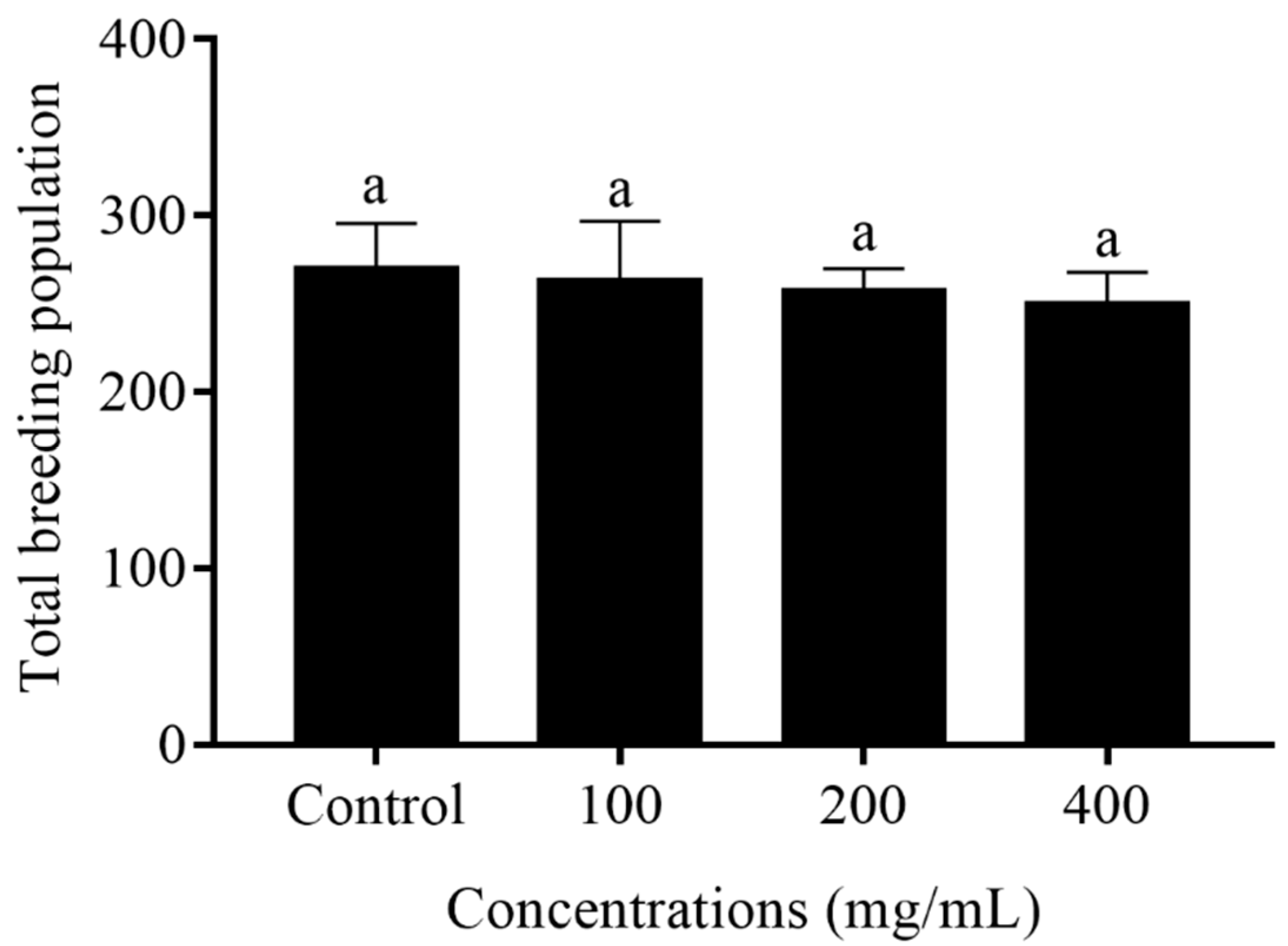
2.3. Reproduction Capacity of C. elegans Treated with Orange Extracts
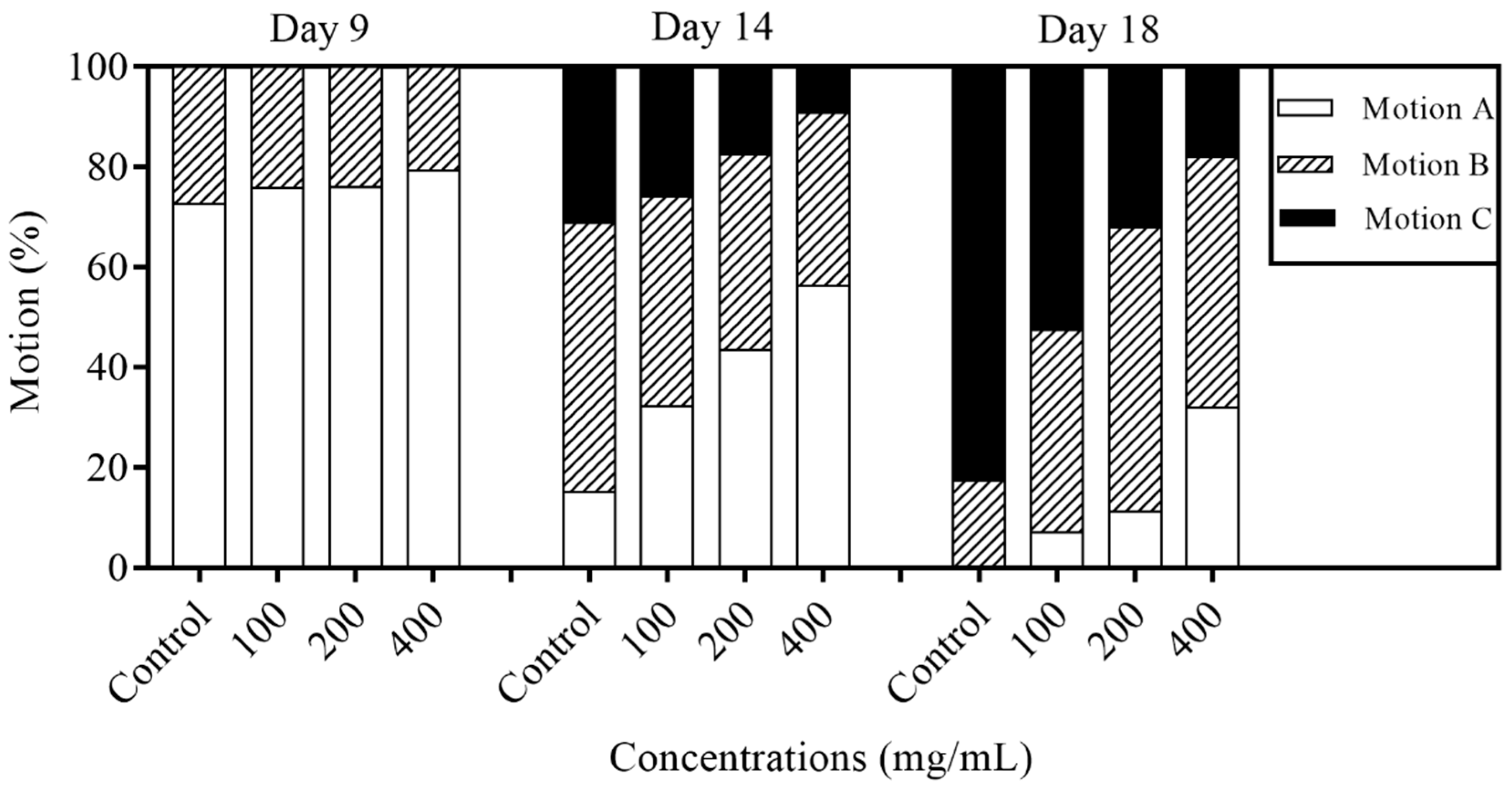
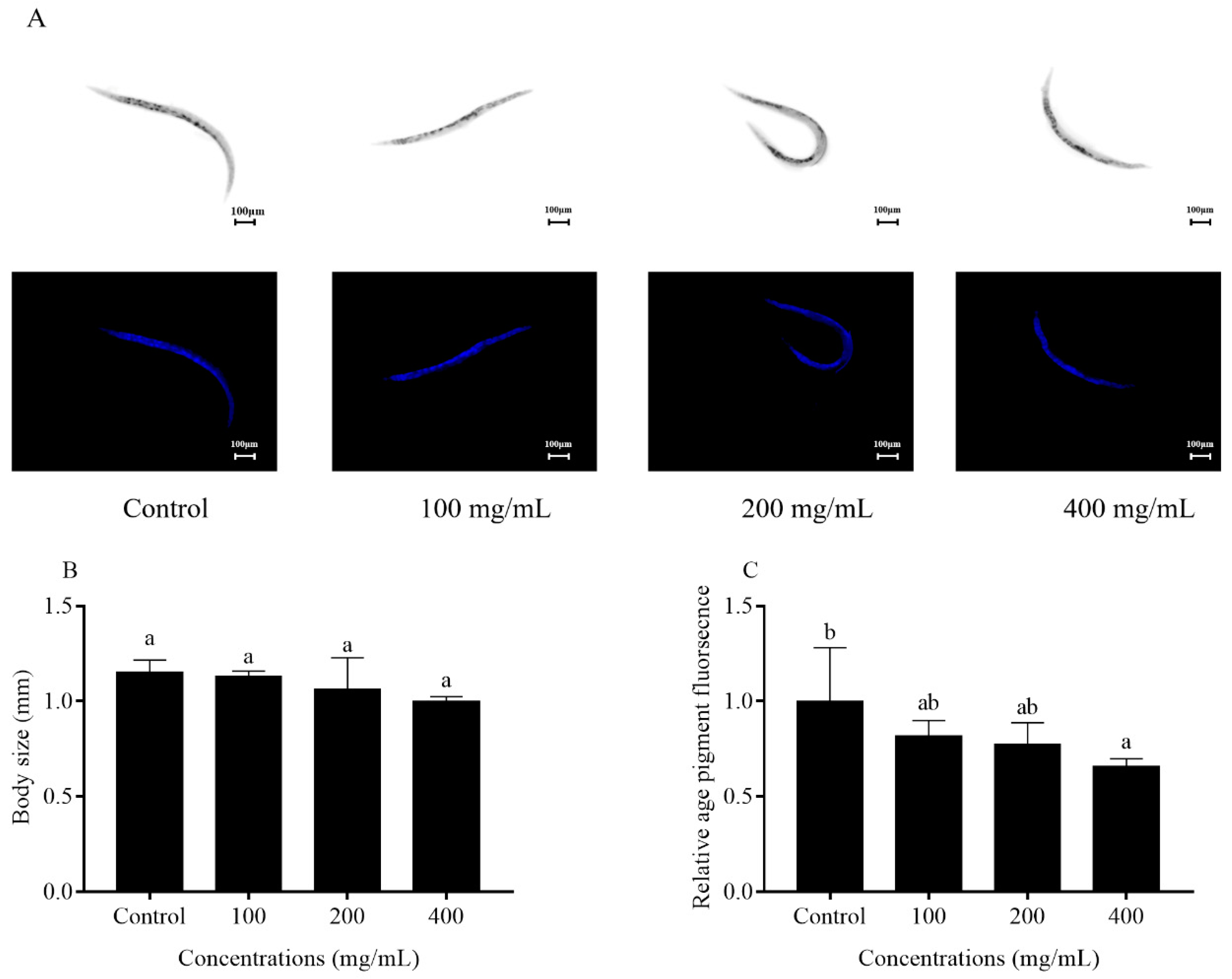
2.4. Effects of Orange Extracts on Physiological Responses of C. elegans
2.5. Orange Extracts-Treated C. elegans Exhibited Enhanced Resistance of Heat Shock and UV-B Radiation
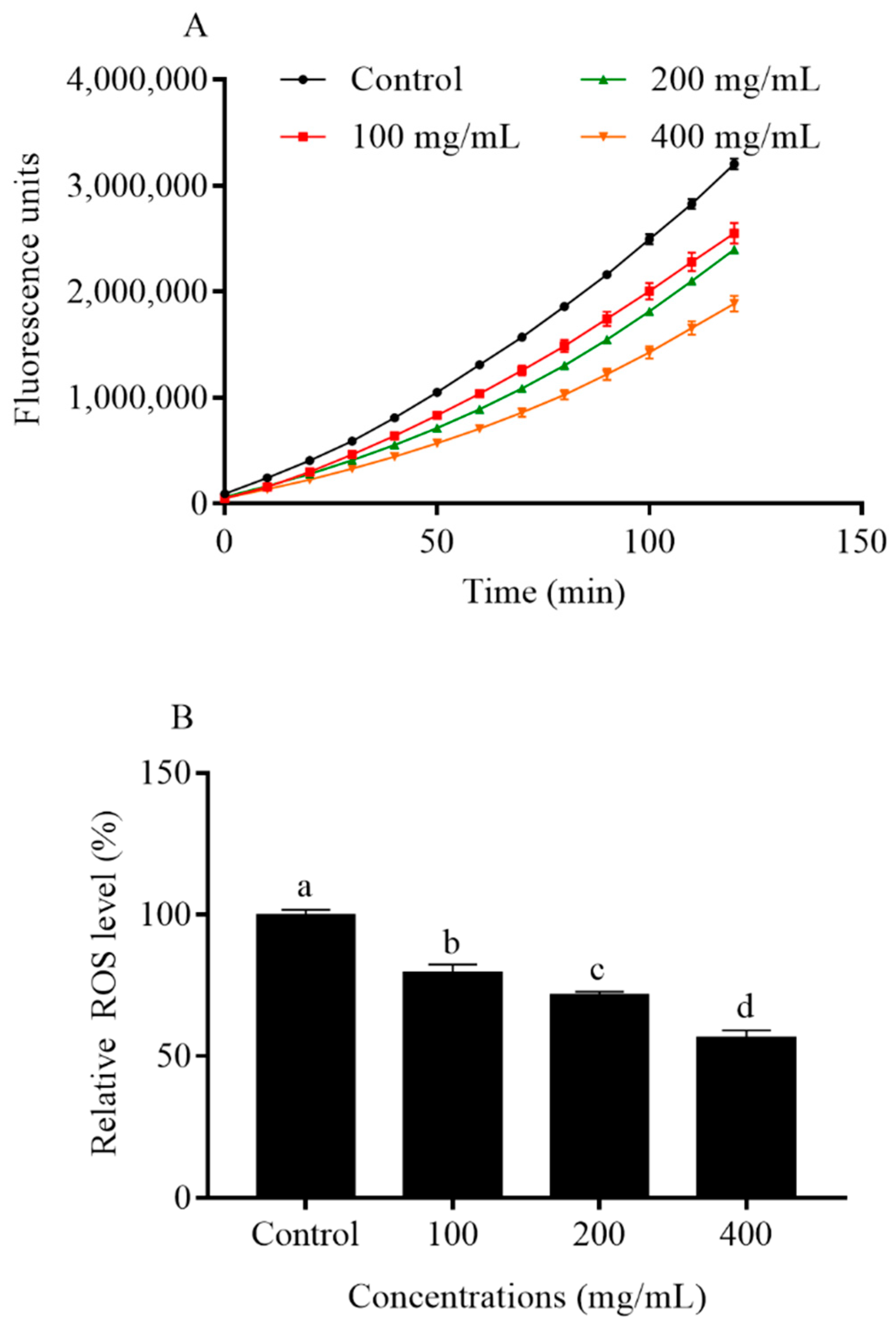
2.6. Orange Extracts Decreased Intracellular ROS Accumulation in C. elegans
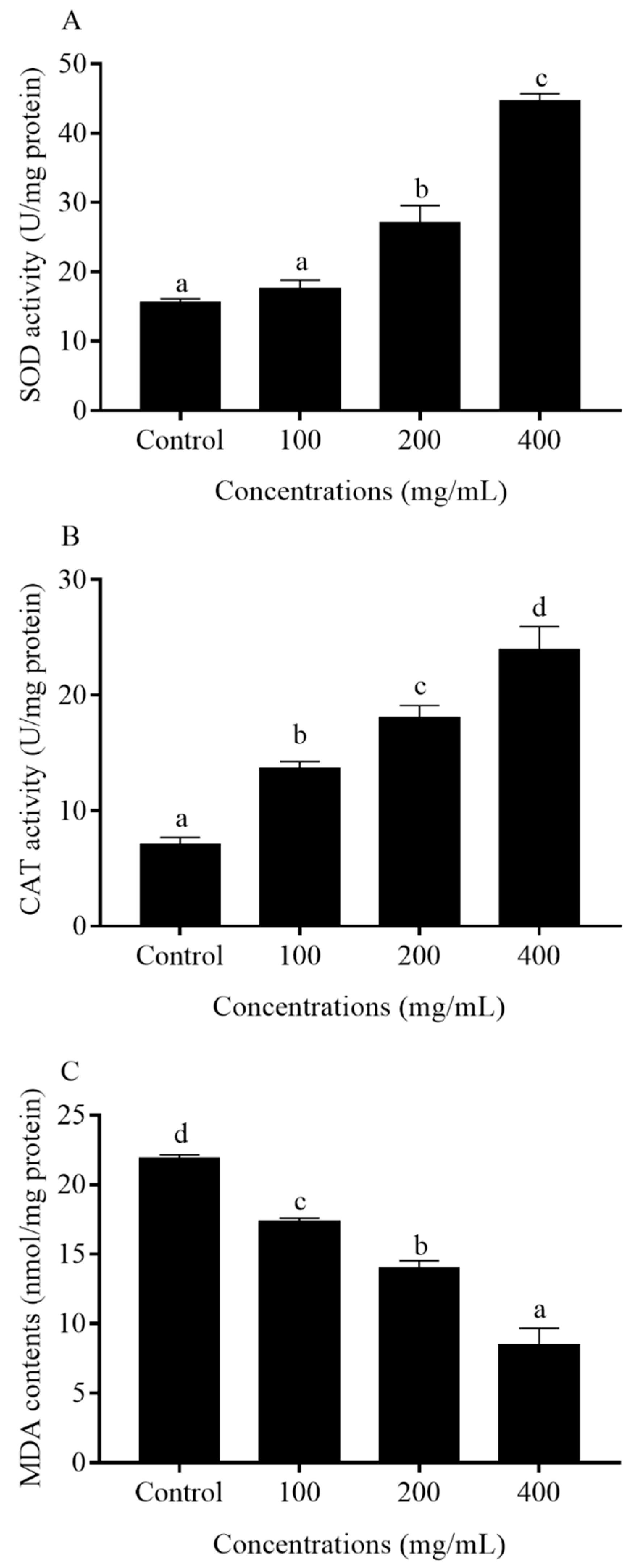
2.7. Antioxidant Enzyme Activities and MDA Contents in C. elegans
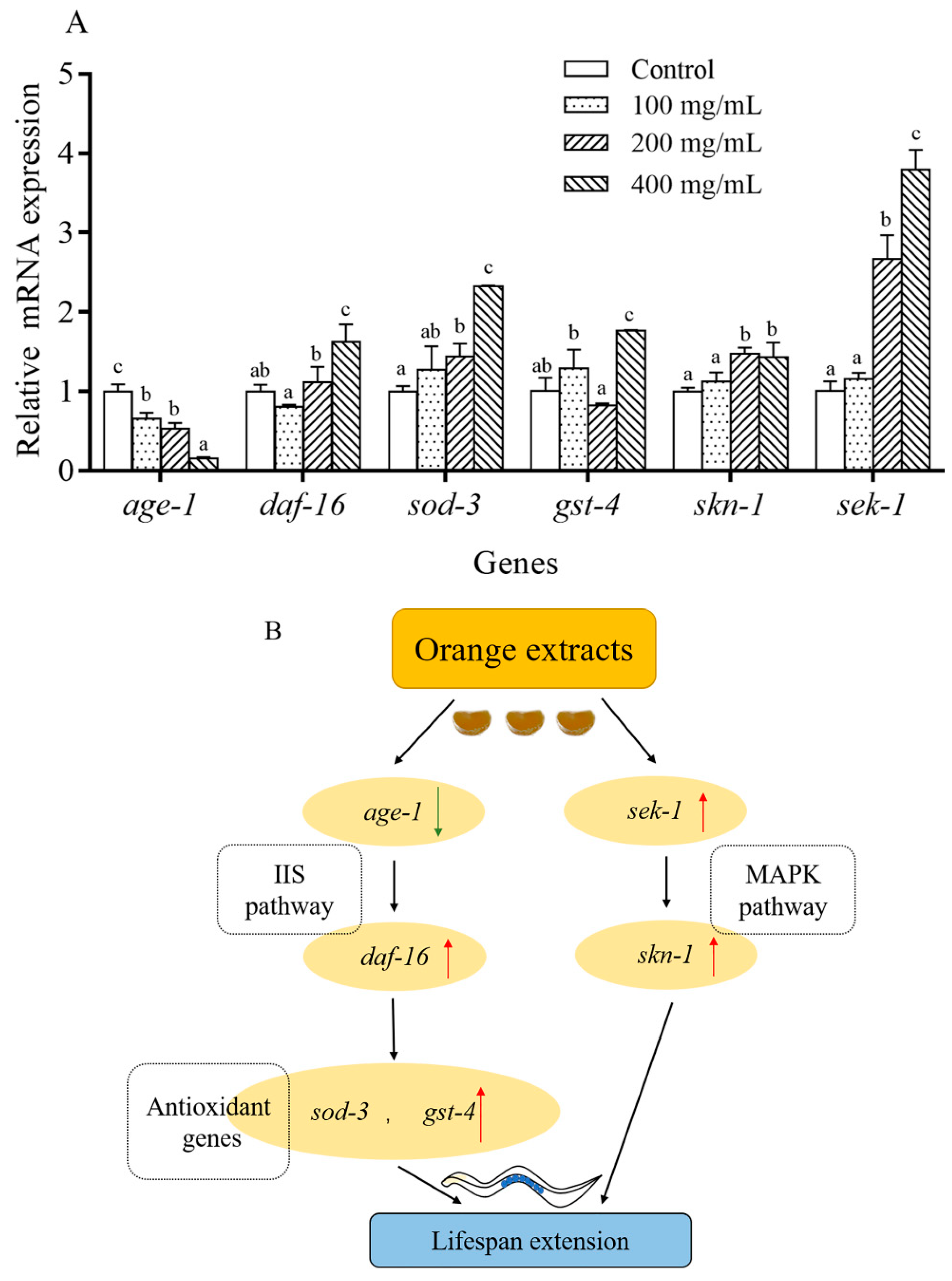
2.8. Orange Extract Treatment Regulated the Expression of Messenger RNA (mRNA) in C. elegans
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Sample Preparation
4.3. C. elegans Cultivation
4.4. The Lifespan Assay of C. elegans
4.5. Motility Assay
4.6. Reproduction Assay
4.7. Body Length and Fluorescence Quantification of Age Pigment
4.8. Stress Resistance Assay
4.8.1. Thermotolerance Assay
4.8.2. Resistance to UV-B Radiation Assay
4.9. Intracellular ROS Levels
4.10. Determination of the Antioxidant Enzyme Activities and MDA Levels
4.11. Real-Time RT-PCR
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Wink, M.; Tencomnao, T. Lifespan extending and oxidative stress resistance properties of a leaf extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019, 2019, 9012396. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Consortium, C.E.S. Genome sequence of the nematode C-elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, R.; Ge, L.M. The worm in us-Caenorhabditis elegans as a model of human disease. Trends Biotechnol. 2002, 20, 147–148. [Google Scholar] [CrossRef]
- Lin, C.; Xiao, J.; Xi, Y.; Zhang, X.; Zhong, Q.; Zheng, H.; Cao, Y.; Chen, Y. Rosmarinic acid improved antioxidant properties and healthspan via the IIS and MAPK pathways in Caenorhabditis elegans. BioFactors 2019, 45, 1–14. [Google Scholar] [CrossRef]
- Herndon, L.A.; Schmeissner, P.J.; Dudaronek, J.M.; Brown, P.A.; Listner, K.M.; Sakano, Y.; Paupard, M.C.; Hall, D.H.; Driscoll, M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 2002, 419, 808–814. [Google Scholar] [CrossRef]
- Zhang, L.; Jie, G.; Zhang, J.; Zhao, B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic. Biol. Med. 2009, 46, 414–421. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, J.; Li, T.; Liu, R.H. Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Food Funct. 2018, 9, 5273–5282. [Google Scholar] [CrossRef]
- Kim, D.K.; Jeon, H.; Cha, D.S. 4-Hydroxybenzoic acid-mediated lifespan extension in Caenorhabditis elegans. J. Funct. Foods 2014, 7, 630–640. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Guan, Y.; Rui, X.; Zhang, Y.; Dong, M.; Ma, W. An aqueous polyphenol extract from Rosa rugosa tea has antiaging effects on Caenorhabditis elegans. J. Food Biochem. 2019, 43, e12796. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Extraction, characterization and biological activity of citrus flavonoids. Rev. Chem. Eng. 2019, 35, 265–284. [Google Scholar] [CrossRef]
- Da Pozzo, E.; Costa, B.; Cavallini, C.; Testai, L.; Martelli, A.; Calderone, V.; Martini, C. The citrus flavanone naringenin protects myocardial cells against age-associated damage. Oxid. Med. Cell. Longev. 2017, 2017, 9536148. [Google Scholar] [CrossRef] [Green Version]
- Da Pozzo, E.; De Leo, M.; Faraone, I.; Milella, L.; Cavallini, C.; Piragine, E.; Testai, L.; Calderone, V.; Pistelli, L.; Braca, A.; et al. Antioxidant and antisenescence effects of bergamot juice. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, H.; Xu, Z.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Zhou, L.; Ding, C. Panax notoginseng polysaccharide increases stress resistance and extends lifespan in Caenorhabditis elegans. J. Funct. Foods 2018, 45, 15–23. [Google Scholar] [CrossRef]
- Chhikara, N.; Kour, R.; Jaglan, S.; Gupta, P.; Gat, Y.; Panghal, A. Citrus medica: Nutritional, phytochemical composition and health benefits—A review. Food Funct. 2018, 9, 1978–1992. [Google Scholar] [CrossRef]
- Wang, H.; Chen, G.; Guo, X.; Abbasi, A.M.; Liu, R.H. Influence of the stage of ripeness on the phytochemical profiles, antioxidant and antiproliferative activities in different parts of Citrus reticulata Blanco cv. Chachiensis. LWT-Food Sci. Technol. 2016, 69, 67–75. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N.; Grohmann, K. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr. Med. Chem. 2001, 8, 135–153. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Sun, K.; Xiang, L.; Ishihara, S.; Matsuura, A.; Sakagami, Y.; Qi, J. Anti-aging effects of hesperidin on Saccharomyces cerevisiae via inhibition of reactive oxygen species and UTH-1 gene expression. Biosci. Biotechnol. Biochem. 2012, 76, 640–645. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Bedmar, Z.; Anter, J.; de La Cruz-Ares, S.; Munoz-Serrano, A.; Alonso-Moraga, A.; Perez-Guisado, J. Role of citrus juices and distinctive components in the modulation of degenerative processes: Genotoxicity, antigenotoxicity, cytotoxicity, and longevity in drosophila. J. Toxicol. Environ. Health A 2011, 74, 1052–1066. [Google Scholar] [CrossRef]
- Shimizu, C.; Wakita, Y.; Inoue, T.; Hiramitsu, M.; Okada, M.; Mitani, Y.; Segawa, S.; Tsuchiya, Y.; Nabeshima, T. Effects of lifelong intake of lemon polyphenols on aging and intestinal microbiome in the senescence-accelerated mouse prone 1 (SAMP1). Sci. Rep. 2019, 9, 3671. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.J.; Evason, K.; Kornfeld, K. Pharmacology of delayed aging and extended lifespan of Caenorhabditis elegans. Exp. Gerontol. 2006, 41, 1032–1039. [Google Scholar] [CrossRef]
- De Oliveira Caland, R.B.; Munoz Cadavid, C.O.; Carmona, L.; Pena, L.; Oliveira, R.d.P. Pasteurized orange juice rich in carotenoids protects Caenorhabditis elegans against oxidative stress and beta-amyloid toxicity through direct and indirect mechanisms. Oxid. Med. Cell. Longev. 2019, 2019, 5046280. [Google Scholar] [CrossRef] [Green Version]
- Vayndorf, E.M.; Lee, S.S.; Liu, R.H. Whole apple extracts increase lifespan, healthspan and resistance to stress in Caenorhabditis elegans. J. Funct. Foods 2013, 5, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Jiang, Y.; Wan, L.; Wang, Q.; Huang, Z.; Liu, Y.; Wu, Y.; Chen, Z.; Liu, X. Feeding recombinant E-coli with GST-mBmKTX fusion protein increases the fecundity and lifespan of Caenorhabditis elegans. Peptides 2017, 89, 1–8. [Google Scholar] [CrossRef]
- Pandey, S.; Tiwari, S.; Kumar, A.; Niranjan, A.; Chand, J.; Lehri, A.; Chauhan, P.S. Antioxidant and anti-aging potential of Juniper berry (Juniperus communis L.) essential oil in Caenorhabditis elegans model system. Ind. Crops Prod. 2018, 120, 113–122. [Google Scholar] [CrossRef]
- Shen, L.; Xiao, J.; Ye, H.; Wang, D. Toxicity evaluation in nematode Caenorhabditis elegans after chronic metal exposure. Environ. Toxicol. Pharmacol. 2009, 28, 125–132. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Q.; Zhou, X.; Song, S.; Zhu, B. Stress resistance and lifespan extension of Caenorhabditis elegans enhanced by peptides from mussel (Mytilus edulis) protein hydrolyzate. Food Funct. 2018, 9, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Hader, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Henning, S.M.; Niu, Y.T.; Youssefian, A.A.; Seeram, N.P.; Xu, A.L.; Heber, D. The citrus flavonoid naringenin stimulates DNA repair in prostate cancer cells. J. Nutr. Biochem. 2006, 17, 89–95. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, L.; Qi, Y.; Li, M.; Zhou, L. Emodin extends lifespan of Caenorhabditis elegans through insulin/IGF-1 signaling pathway depending on DAF-16 and SIR-2.1. Biosci. Biotechnol. Biochem. 2017, 81, 1908–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, K.A.; Johnson, G.L. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 2002, 3, 663–672. [Google Scholar] [CrossRef]
- Park, S.-K.; Tedesco, P.M.; Johnson, T.E. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell 2009, 8, 258–269. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Liu, H.; Cao, Y. Antioxidation and anti-aging activities of astaxanthin geometrical isomers and molecular mechanism involved in Caenorhabditis elegans. J. Funct. Foods 2018, 44, 127–136. [Google Scholar] [CrossRef]
- Golden, T.R.; Hubbard, A.; Dando, C.; Herren, M.A.; Melov, S. Age-related behaviors have distinct transcriptional profiles in Caenorhabditis elegans. Aging Cell 2008, 7, 850–865. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Zhang, X.; Xiao, J.; Zhong, Q.; Kuang, Y.; Cao, Y.; Chen, Y. Effects on longevity extension and mechanism of action of carnosic acid in Caenorhabditis elegans. Food Funct. 2019, 10, 1398–1410. [Google Scholar] [CrossRef]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Rieckher, M.; Bujarrabal, A.; Doll, M.A.; Soltanmohammadi, N.; Schumacher, B. A simple answer to complex questions: Caenorhabditis elegans as an experimental model for examining the DNA damage response and disease genes. J. Cell. Physiol. 2018, 233, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, Q.; Peng, Q.; Yu, X.; Yin, X.; Liang, M.; Ma, C.W.; Huang, Z.; Jia, W. Antioxidant peptides derived from the hydrolyzate of purple sea urchin (Strongylocentrotus nudus) gonad alleviate oxidative stress in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 594–604. [Google Scholar] [CrossRef]
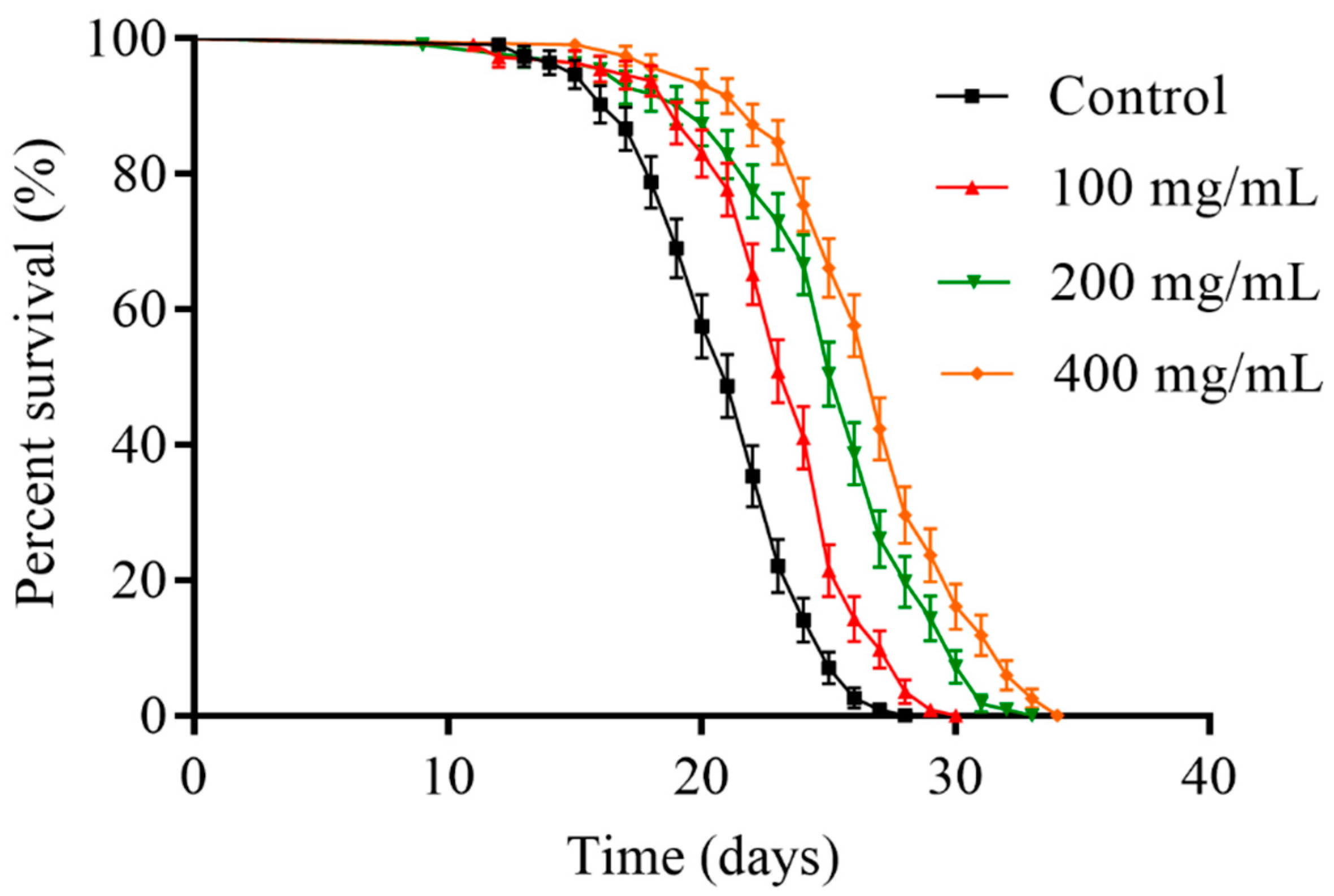
| Group | Number | Mean Lifespan (Days) | % of Control | Maximum Lifespan (Days) |
|---|---|---|---|---|
| Control | 113 ± 3 | 20.91 ± 1.60 a | 100.0 ± 7.6 a | 25.67 ± 2.08 a |
| 100 mg/mL | 110 ± 5 | 23.10 ± 1.95 b | 110.5 ± 9.3 b | 28.00 ± 2.00 a,b |
| 200 mg/mL | 106 ± 5 | 24.67 ± 2.26 b | 118.0 ± 10.8 b | 30.00 ± 2.65 a,b |
| 400 mg/mL | 114 ± 3 | 26.38 ± 2.06 c | 126.2 ± 9.8 c | 32.33 ± 2.87 b |
| Group | Number | Mean Lifespan (h) | % of Control | Maximum Lifespan (h) |
|---|---|---|---|---|
| Control | 94 ± 3 | 11.58 ± 1.02 a | 100.0 ± 9.3 a | 15.33 ± 1.15 a |
| 100 mg/mL | 95 ± 2 | 12.71 ± 0.99 b | 109.8 ± 9.1 b | 19.33 ± 1.15 b |
| 200 mg/mL | 97 ± 2 | 13.81 ± 1.24 b | 119.3 ± 11.4 b | 21.33 ± 1.15 b |
| 400 mg/mL | 96 ± 6 | 15.68 ± 1.09 c | 135.5 ± 10.0 c | 21.33 ± 1.15 b |
| Group | Number | Mean Lifespan (Days) | % of Control | Maximum Lifespan (Days) |
|---|---|---|---|---|
| Control | 101 ± 5 | 4.53 ± 0.52 a | 100.0 ± 5.1 a | 6.67 ± 0.58 a |
| 100 mg/mL | 92 ± 1 | 4.94 ± 0.71 a,b | 109.1 ± 9.7 a,b | 7.67 ± 0.58 a |
| 200 mg/mL | 93 ± 1 | 5.52 ± 0.77 b,c | 122.0 ± 10.7 b,c | 9.00 ± 1.00 b |
| 400 mg/mL | 93 ± 7 | 6.16 ± 0.90 b | 136.1 ± 13.6 b | 10.33 ± 0.58 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Deng, N.; Wang, H.; Li, T.; Chen, L.; Zheng, B.; Liu, R.H. Effects of Orange Extracts on Longevity, Healthspan, and Stress Resistance in Caenorhabditis elegans. Molecules 2020, 25, 351. https://doi.org/10.3390/molecules25020351
Wang J, Deng N, Wang H, Li T, Chen L, Zheng B, Liu RH. Effects of Orange Extracts on Longevity, Healthspan, and Stress Resistance in Caenorhabditis elegans. Molecules. 2020; 25(2):351. https://doi.org/10.3390/molecules25020351
Chicago/Turabian StyleWang, Jing, Na Deng, Hong Wang, Tong Li, Ling Chen, Bisheng Zheng, and Rui Hai Liu. 2020. "Effects of Orange Extracts on Longevity, Healthspan, and Stress Resistance in Caenorhabditis elegans" Molecules 25, no. 2: 351. https://doi.org/10.3390/molecules25020351