Tunable Aryl Imidazolium Recyclable Ionic Liquid with Dual Brønsted–Lewis Acid as Green Catalyst for Friedel–Crafts Acylation and Thioesterification
Abstract
1. Introduction
2. Results and Discussion
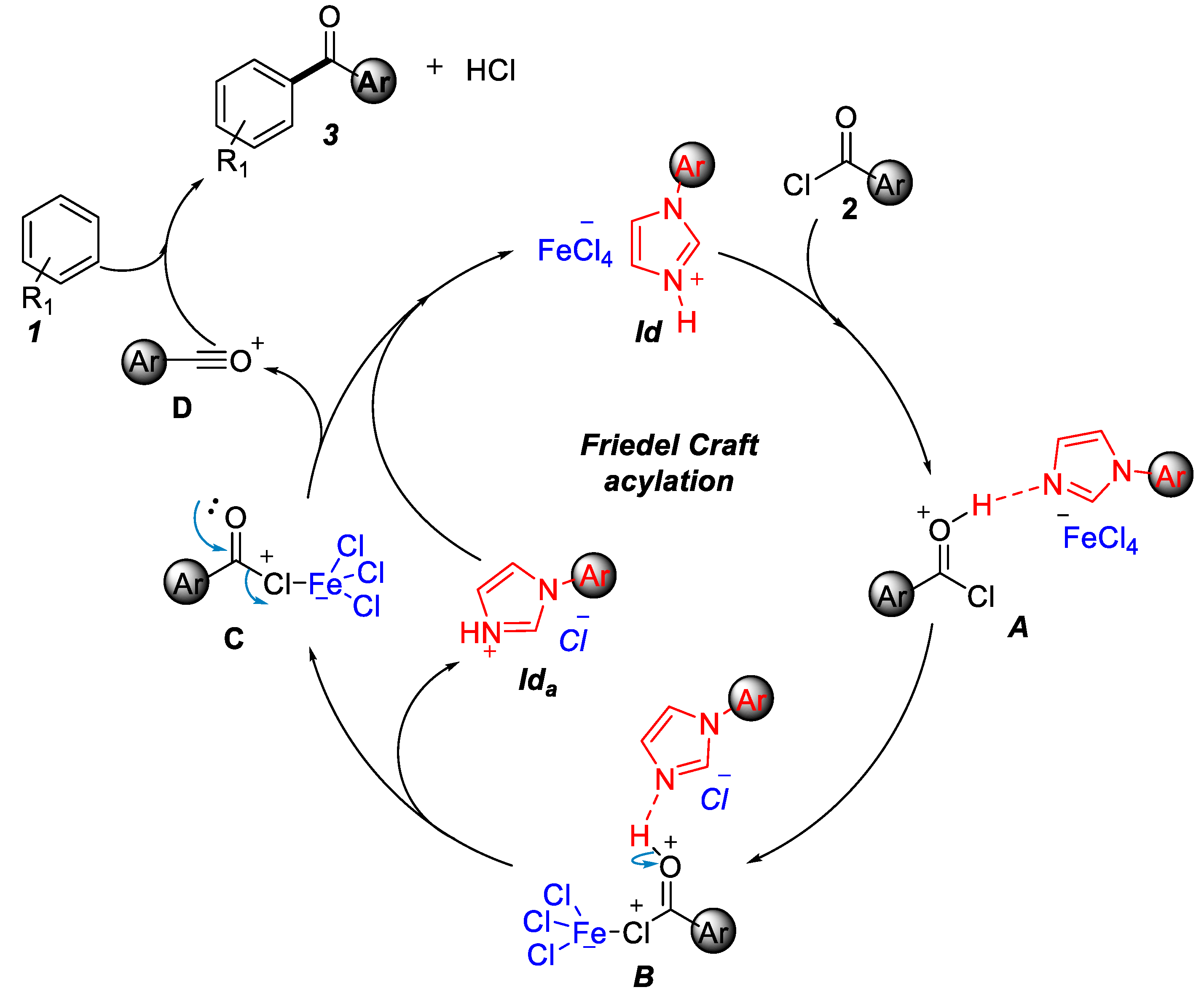
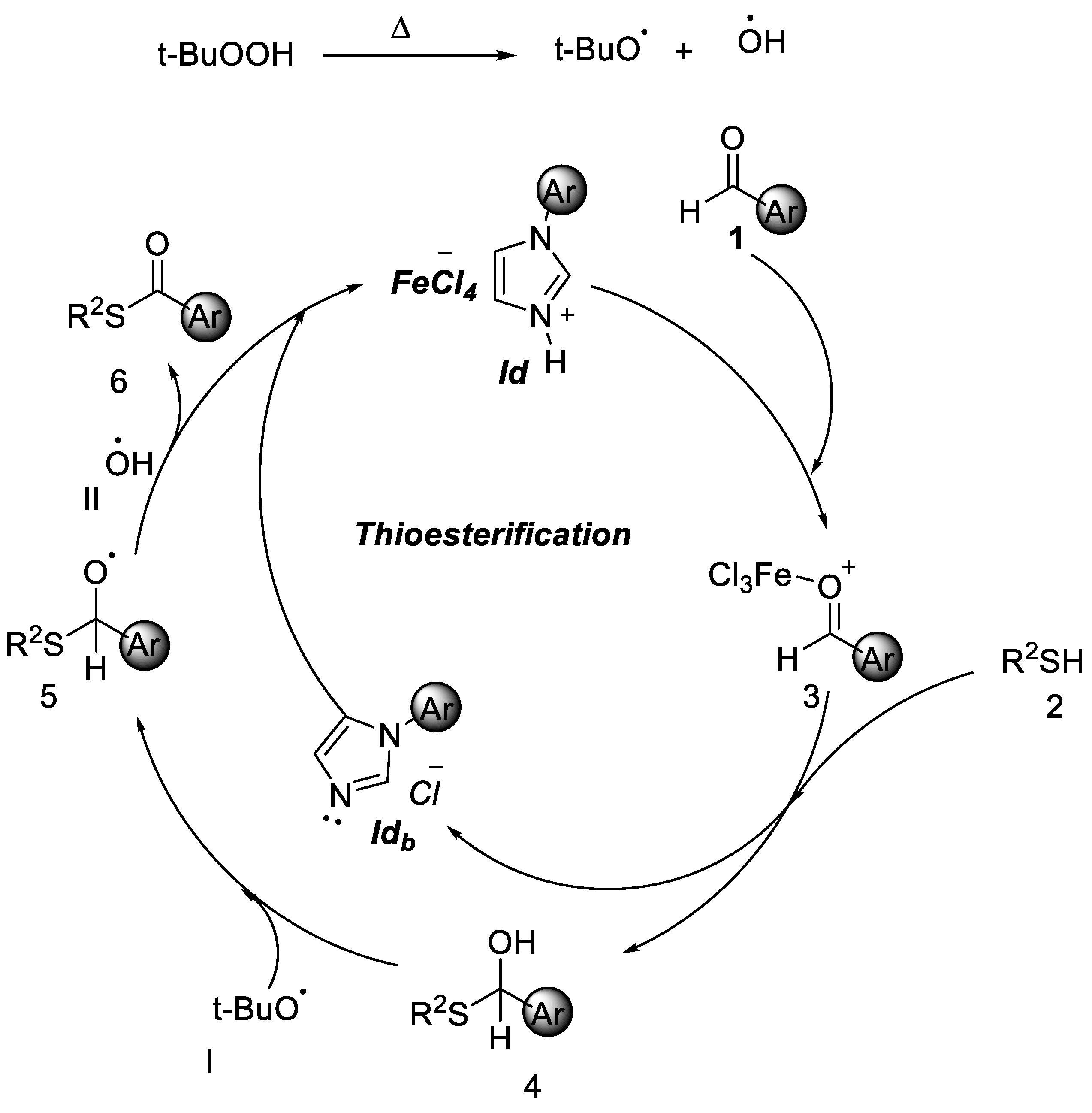
2.1. Friedel–Crafts Acylation
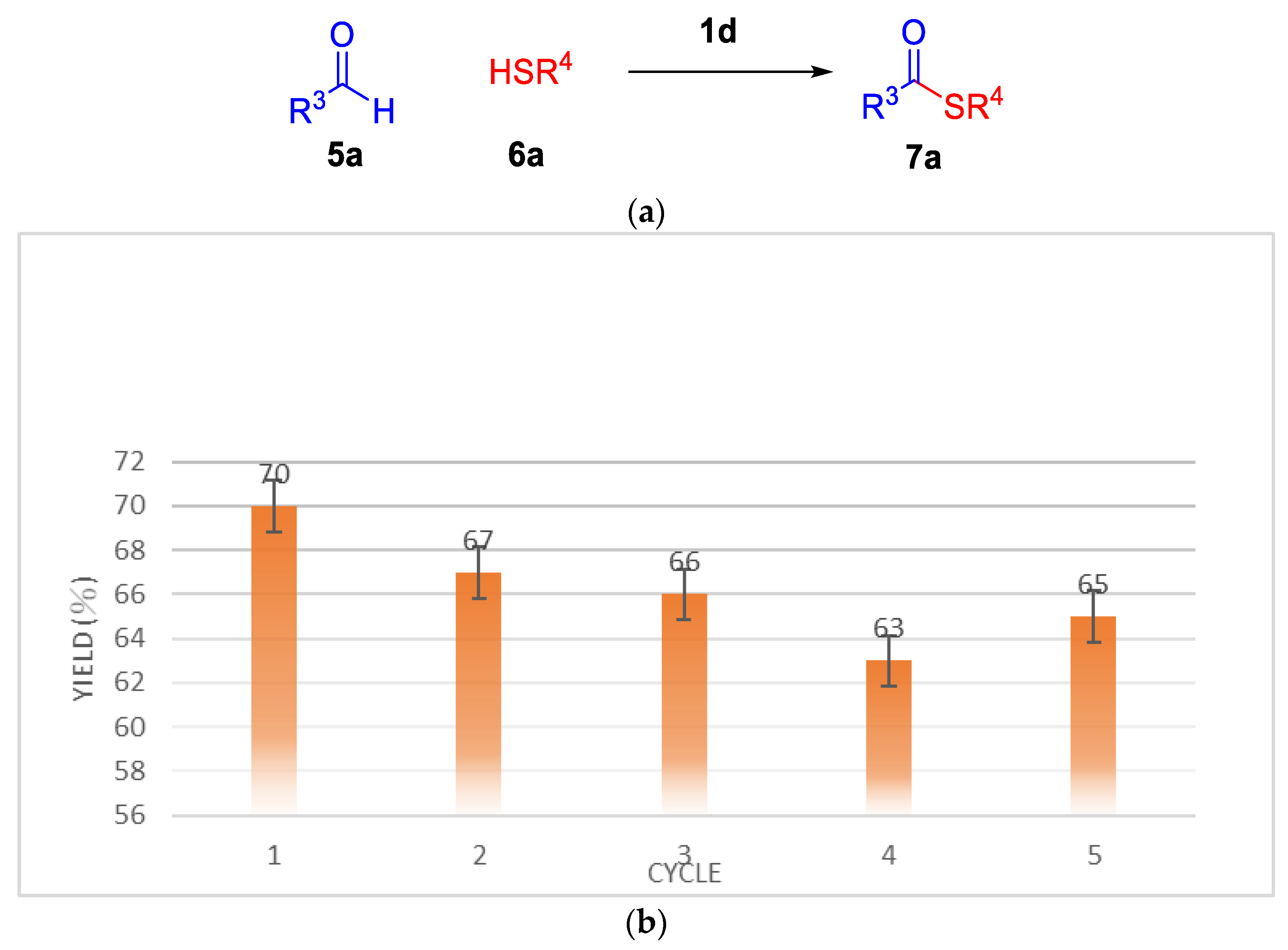
2.2. Thioesterification
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natalia, V.P.; Kenneth, R.S. Application of ionic liquids in chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar]
- Shi, R.; Wang, Y.-T. Dual ionic and organic nature of ionic liquids. Sci. Rep. 2016, 6, 19644. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, J.; Welton, T.; Armstrong, D.W. Characterizing ionic liquids on the basis of multiple solvation interactions. J. Am. Chem. Soc. 2002, 124, 14247–14254. [Google Scholar] [CrossRef] [PubMed]
- Weyershausen, B.; Lehmann, K. Industrial application of ionic liquids as performance additives. Green. Chem. 2005, 7, 15–19. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Creary, X.; Willis, E.D.; Gagnon, M. Carbocation-forming reactions in ionic liquids. J. Am. Chem. Soc. 2005, 127, 18114–18120. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Green Solvents II, Properties and Applications of Ionic Liquids; Inamuddin, A.M., Ed.; Springer: New York, NY, USA, 2012; pp. 1–32. [Google Scholar]
- Neves, C.M.S.S.; Freire, M.G.; Coutinho, J.A.P. Improved recovery of ionic liquids from contaminated aqueous streams using aluminium-based salts. RSC Adv. 2012, 2, 10882–10890. [Google Scholar] [CrossRef]
- Doorslaer, C.V.; Glas, D.; Peeters, A.; Odena, A.C.; Vankelecom, I.; Binnemans, K.; Mertensa, P.; Vos, D.D. Product recovery from ionic liquids by solvent-resistant nanofiltration; application to ozonation of acetals and methyl oleate. Green Chem. 2010, 12, 1726–1733. [Google Scholar] [CrossRef]
- Faßbach, T.A.; Kirchmann, R.; Behr, A.; Vorholt, A.J. Recycling of homogeneous catalysts in reactive ionic liquids-solvent-free amino functionalization of alkenes. Green Chem. 2017, 19, 5243–5249. [Google Scholar] [CrossRef]
- Ladnak, V.; Hofmann, N.; Brausch, N.; Wasserscheida, P. Continuous, ionic liquids-catalysed propylation of toluene in a liquid-liquid biphasic reaction mode using a loop reactor concept. Adv. Synth. Catal. 2007, 349, 719–726. [Google Scholar] [CrossRef]
- Canales, R.I.; Brennecke, J.F. Comparison of ionic liquids to conventional organic solvents for extraction of aromatics from aliphatics. J. Chem. Eng. Data 2016, 61, 1685–1699. [Google Scholar] [CrossRef]
- Dyson, P.J. Transition metal chemistry in ionic liquids. Transition Met. Chem. 2002, 27, 353–358. [Google Scholar] [CrossRef]
- Chinnappan, A.; Kim, H. Transition metal based ionic liquid (bulk and nanofiber composites) use as catalyst for reduction of aromatic nitro compounds under mild conditions. RSC Adv. 2013, 3, 3399–3406. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, M.; Zhang, A.P.; Prescher, S.; Antonietti, M.; Yuan, J. Helically structured nanoporous poly (ionic liquids) membranes: Facile preparation and application in fiber-optic pH sensing. J. Am. Chem. Soc. 2013, 135, 5549–5552. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Pollet, P.; Liotta, C.L.; Eckert, C.A. Reversible in situ catalyst formation. Acc. Chem. Res. 2008, 41, 458–467. [Google Scholar] [CrossRef] [PubMed]
- William, S.B.; Guillaume, P.; André, B.C. Chemoselective synthesis of ketones and ketimines by addition of organometallic reagents to secondary amides. Nat. Chem. 2012, 4, 228–234. [Google Scholar]
- Maji, A.; Dahiya, A.; Lu, G.; Bhattacharya, T.; Brochetta, M.; Zanoni, G.; Liu, P.; Maiti, D. H-bonded reusable template assisted para-selective ketonization using soft electrophilic vinyl ethers. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Li, M.-H.; Shang, M.; Xu, H.; Wang, X.; Dai, H.-X.; Yu, J.-Q. Remote Para-C–H acetoxylation of electron-deficient arenes. Org. Lett. 2019, 21, 540–544. [Google Scholar] [CrossRef]
- Gmouh, S.; Yang, H.; Vaultier, M. Activation of bismuth(III) derivatives in ionic liquids: Novel and recyclable catalytic systems for Friedel—Crafts acylation of aromatic compounds. Org. Lett. 2003, 5, 2219–2222. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Farzaneh, P.; Aditya, K.; Arjun, N.; Fang, W.; Golam, R.; Thomas, M.; George, A.O. Taming of superacids: PVP triflic acid as an effective solid triflic acid equivalent for Friedel—Crafts hydroxyalkylation and acylation. J. Fluor. Chem. 2014, 171, 102–112. [Google Scholar] [CrossRef]
- Tran, P.H.; Hansen, P.E.; Hoang, H.M.; Chau, D.K.N.; Le, T.N. Indium triflate in 1-isobutyl-3-methylimidazolium dihydrogenphosphate: An efficient and green catalytic system for Friedel—Crafts acylation. Tetrahedron Lett. 2015, 56, 2187–2192. [Google Scholar] [CrossRef]
- Olah, G.A.; Malhotra, R.; Narang, S.C.; Olah, J.A. Heterogeneous Catalysis by Solid Superacids. 14. Perfluorinated Resinsulfonic Acid Catalyzed Friedel—Crafts Acylation of Benzene and Substituted Benzenes. Synthesis 1978, 672–673. [Google Scholar] [CrossRef]
- Miles, W.H.; Nutaitis, C.F.; Anderton, C.A. Iron(III) Chloride as a Lewis Acid in the Friedel—Crafts Acylation Reaction. J. Chem. Educ. 1996, 73, 272. [Google Scholar] [CrossRef]
- Li, Z.; Li, G.; Jiang, L.; Li, J.; Sun, G.; Xia, C.; Li, F.-W. Ionic liquids as precursor for efficient mesoporous iron-nitrogen doped oxygen reduction electrocatalyst. Angew. Chem. Int. Ed. 2015, 54, 1494–1498. [Google Scholar] [CrossRef]
- Han, X.-X.; Du, H.; Hung, C.-T.; Liu, L.-L.; Wu, P.-H.; Ren, D.-H.; Huang, S.-J.; Liu, S.-B. Syntheses of novel halogen-free Brønsted—Lewis acidic ionic liquid catalysts and their applications for synthesis of methyl caprylate. Green Chem. 2015, 17, 499–508. [Google Scholar] [CrossRef]
- Wang, H.B.; Yao, N.; Wang, L.; Hu, Y.L. Brønsted—Lewis dual acidic ionic liquid immobilized on mesoporous silica materials as an efficient cooperative catalyst for Mannich reactions. New J. Chem. 2017, 41, 10528–10531. [Google Scholar] [CrossRef]
- Ross, J.; Xiao, J. Friedel—Crafts acylation reactions using metal triflates in ionic liquid. Green. Chem. 2002, 4, 129–133. [Google Scholar] [CrossRef]
- Earle, M.J.; Hakala, U.; Hardacre, C.; Karkkainen, J.; McAuley, B.J.; Rooney, D.W.; Seddon, K.R.; Thompson, J.M.; Wähälä, K. Chloroindate(III) ionic liquids: Recyclable media for Friedel—Crafts acylation reactions. Chem. Commun. 2005, 903–905. [Google Scholar] [CrossRef]
- Ahrens, S.; Peritz, A.; Strassner, T. Tunable aryl alkyl ionic liquids (TAAILs): The next generation of ionic liquids. Angew. Chem. Int. Ed. 2009, 48, 7908–7910. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Lu, S.-Y.; Yi, C.-L.; Lee, C.-F. Iron-catalyzed synthesis of thioesters from thiols and aldehydes in water. J. Org. Chem. 2014, 79, 4561–4568. [Google Scholar] [CrossRef]
- Nambu, H.; Hata, K.; Matsugi, M.; Kita, Y. The direct synthesis of thioesters using an intermolecular radical reaction of aldehydes with dipentafluorophenyl disulfide in water. Chem. Commun. 2002, 1082–1083. [Google Scholar] [CrossRef]
- Nambu, H.; Hata, K.; Matsugi, M.; Kita, Y. Efficient Synthesis of thioesters and amides from aldehydes by using an intermolecular radical reaction in water. Chem. Eur. J. 2005, 11, 719–727. [Google Scholar] [CrossRef]
- Uno, T.; Inokuma, T.; Takemoto, Y. NHC-catalyzed thioesterification of aldehydes by external redox activation. Chem. Commun. 2012, 48, 1901–1903. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Bandgar, S.B.; Korbad, B.L.; Sawant, S.S. Dess—Martin periodinane mediated synthesis of thioesters from aldehydes. Tetrahedron Lett. 2007, 48, 1287–1290. [Google Scholar] [CrossRef]
- Jhuang, H.-S.; Liu, Y.-W.; Reddy, D.M.; Tzeng, Y.-Z.; Lin, W.-Y.; Lee, C.-F. Microwave-assisted Synthesis of thioesters from aldehydes and thiols in water. Chin. Chem. Soc. 2018, 65, 24–27. [Google Scholar] [CrossRef]
- Chang, J.C.; Yang, C.H.; Sun, I.W.; Ho, W.Y.; Wu, T.-Y. Synthesis and properties of magnetic aryl-imidazolium ionic liquids with dual Brønsted/Lewis acidity. Materials 2018, 11, 2539. [Google Scholar] [CrossRef]
- Yang, C.H.; Chang, J.C.; Wu, T.Y.; Sun, I.W.; Wu, J.H.; Ho, W.Y. Novel aryl-imidazolium ionic liquids with dual Brønsted/Lewis acidity as both solvents and catalysts for Friedel—Crafts alkylation. Appl. Sci. 2019, 9, 4743. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
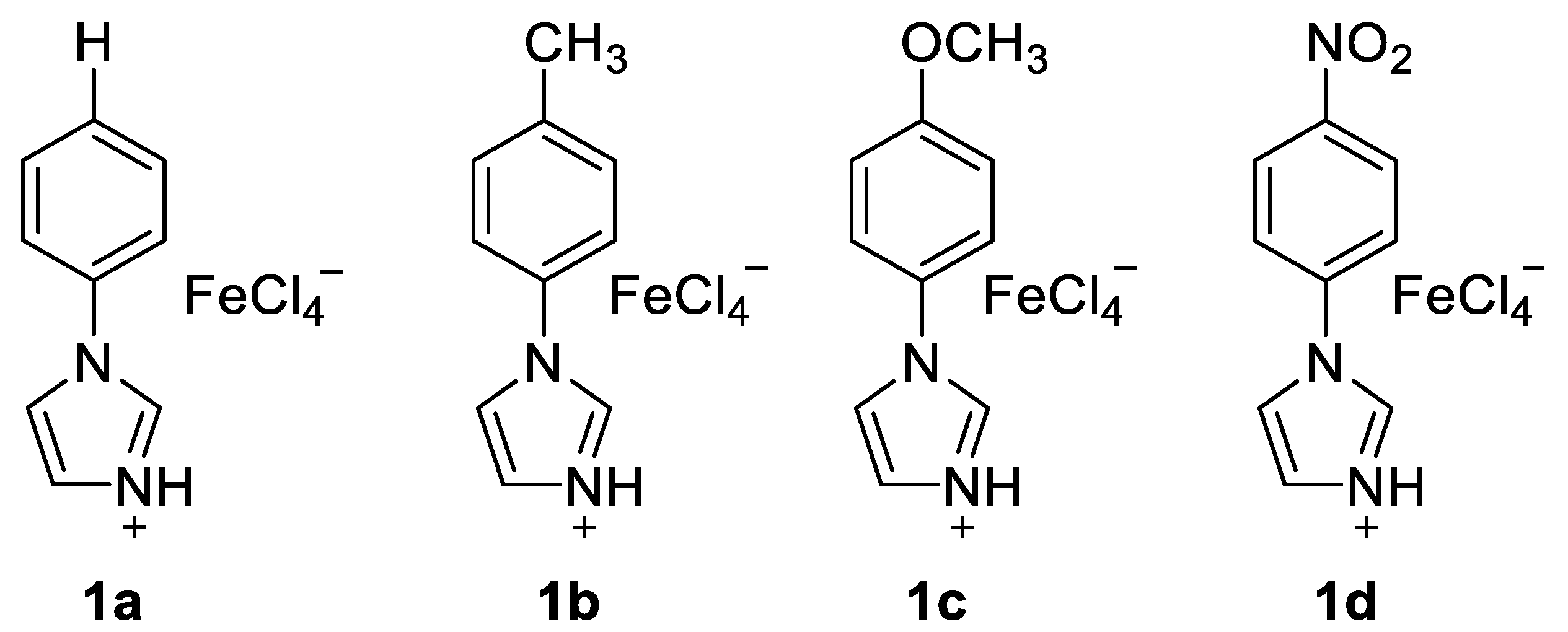
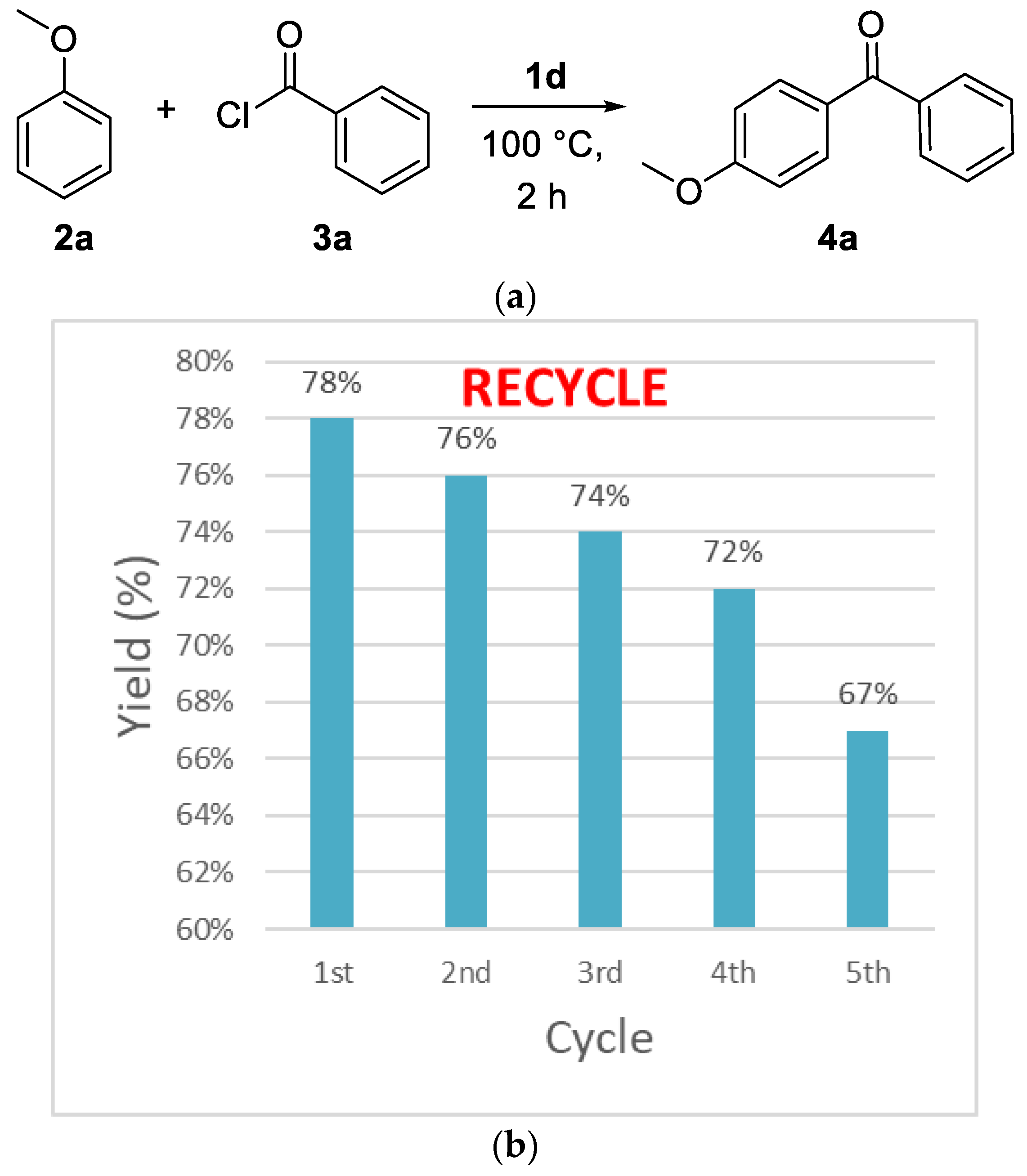
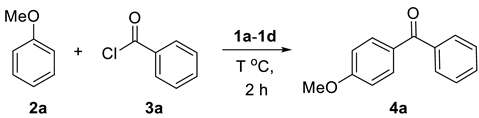
| Entry | IL (equiv.) a | T | Yield b |
|---|---|---|---|
| 1 | 1a (1.0) | 80 °C | 57% |
| 2 | 1b (1.0) | 80 °C | 32% |
| 3 | 1c (1.0) | 80 °C | 72% |
| 4 | 1d (1.0) | 80 °C | 74% |
| 5 | 1d (1.0) | 100 °C | 74% |
| 6 | 1d (1.0) | 120 °C | 71% |
| 7 | 1d (0.9) | 100 °C | 78% |
| 8 | 1d (0.8) | 100 °C | 73% |
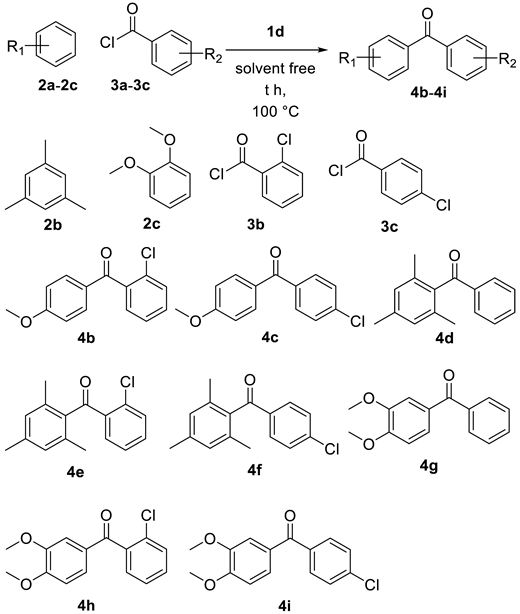
| Entry | Aryl Alkane | Acyl Chloride | t (h) | P (yield) |
|---|---|---|---|---|
| 1 | 2a | 3b | 4.0 | 4b (73%) |
| 2 | 2a | 3c | 4.0 | 4c (68%) |
| 3 | 2b | 3a | 2.0 | 4d (83%) |
| 4 | 2b | 3b | 3.5 | 4e (79%) |
| 5 | 2b | 3c | 2.0 | 4f (89%) |
| 6 | 2c | 3a | 3.0 | 4g (81%) |
| 7 | 2c | 3b | 3.5 | 4h (71%) |
| 8 | 2c | 3c | 3.0 | 4i (70%) |
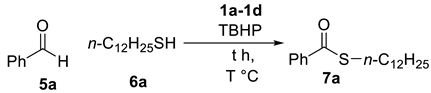
| Entry | IL (equiv.) a | T | t (h) | Yield b |
|---|---|---|---|---|
| 1 | 1a (0.025) | 120 °C | 2 h | 33% |
| 2 | 1b (0.025) | 120 °C | 2 h | 43% |
| 3 | 1c (0.025) | 120 °C | 2 h | 56% |
| 4 | 1d (0.025) | 120 °C | 2 h | 70% |
| 5 | 1d (0.025) | 100 °C | 2 h | 68% |
| 6 | 1d (0.025) | 140 °C | 2 h | 69% |
| 7 | 1d (0.010) | 120 °C | 2 h | 68% |
| 8 | 1d (0.030) | 120 °C | 2 h | 61% |
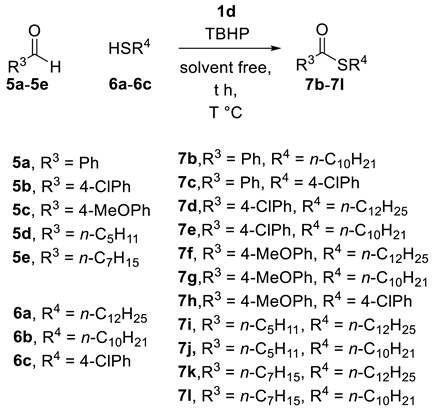
| Entry | IL (eq) a | RCHO | Thiol | T | Yield b |
|---|---|---|---|---|---|
| 1 | 1d (0.025) | 5a | 6b | 120 °C | 7b (88%) |
| 2 | 1d (0.025) | 5a | 6c | 120 °C | 7c (55%) |
| 3 | 1d (0.025) | 5b | 6a | 120 °C | 7d (73%) |
| 4 | 1d (0.025) | 5b | 6b | 120 °C | 7e (73%) |
| 5 | 1d (0.025) | 5c | 6a | 120 °C | 7f (76%) |
| 6 | 1d (0.025) | 5c | 6b | 120 °C | 7g (73%) |
| 7 | 1d (0.025) | 5c | 6c | 120.°C | 7h (51%) |
| 8 | 1d (0.025) | 5d | 6a | 120 °C | 7i (80%) |
| 9 | 1d (0.025) | 5d | 6b | 120 °C | 7j (71%) |
| 10 | 1d (0.025) | 5e | 6a | 120 °C | 7k (73%) |
| 11 | 1d(0.025) | 5e | 6b | 120 °C | 7l (64%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-J.; Wu, Y.-P.; Thul, M.; Hung, M.-W.; Chou, S.-H.; Chen, W.-T.; Lin, W.; Lin, M.; Reddy, D.M.; Wu, H.-R.; et al. Tunable Aryl Imidazolium Recyclable Ionic Liquid with Dual Brønsted–Lewis Acid as Green Catalyst for Friedel–Crafts Acylation and Thioesterification. Molecules 2020, 25, 352. https://doi.org/10.3390/molecules25020352
Lin Y-J, Wu Y-P, Thul M, Hung M-W, Chou S-H, Chen W-T, Lin W, Lin M, Reddy DM, Wu H-R, et al. Tunable Aryl Imidazolium Recyclable Ionic Liquid with Dual Brønsted–Lewis Acid as Green Catalyst for Friedel–Crafts Acylation and Thioesterification. Molecules. 2020; 25(2):352. https://doi.org/10.3390/molecules25020352
Chicago/Turabian StyleLin, Yi-Jyun, Yao-Peng Wu, Mayur Thul, Ming-Wei Hung, Shih-Huan Chou, Wen-Tin Chen, Wesley Lin, Michelle Lin, Daggula Mallikarjuna Reddy, Hsin-Ru Wu, and et al. 2020. "Tunable Aryl Imidazolium Recyclable Ionic Liquid with Dual Brønsted–Lewis Acid as Green Catalyst for Friedel–Crafts Acylation and Thioesterification" Molecules 25, no. 2: 352. https://doi.org/10.3390/molecules25020352
APA StyleLin, Y.-J., Wu, Y.-P., Thul, M., Hung, M.-W., Chou, S.-H., Chen, W.-T., Lin, W., Lin, M., Reddy, D. M., Wu, H.-R., Ho, W.-Y., & Luo, S.-Y. (2020). Tunable Aryl Imidazolium Recyclable Ionic Liquid with Dual Brønsted–Lewis Acid as Green Catalyst for Friedel–Crafts Acylation and Thioesterification. Molecules, 25(2), 352. https://doi.org/10.3390/molecules25020352






