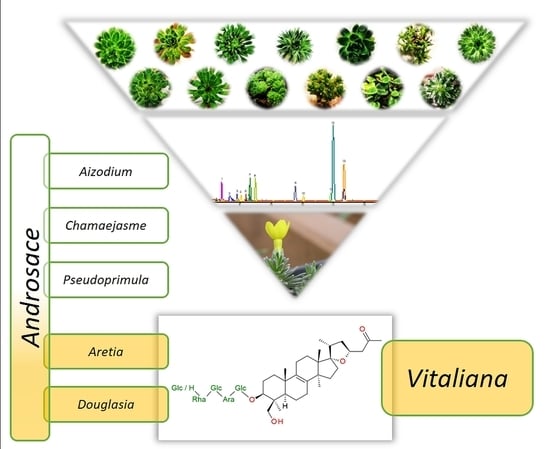
Lanostane-Type Saponins from Vitaliana primuliflora
Abstract
1. Introduction
2. Results
Structure Elucidation of New Saponins
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Instrumental Equipment
4.4. Analytical Samples Preparation
4.5. UHPLC-MS and UHPLC-MS/MS Analysis
4.6. Isolation of Compounds 12 and 13
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Taxon | Acronym | Taxon | Acronym |
|---|---|---|---|
| genus Androsace L. | genus Cortusa L. | ||
| sct. Aretia, ssct. Aretia | Cortusa matthioli | CMAT_P_2013 | |
| Androsace cylindrica | ACYL_K_2015 | Cortusa matthioli ssp. matthioli | CMAT_K_2015 |
| Androsace lehmannii | ALEH_P_2014 | Cortusa matthioli ssp. caucasica | CCAU_K_2015 |
| Androsace mathildae | AMTH_TK_2015 | Cortusa matthioli ssp. sachalinensis | CSAC_K_2015 |
| sct. Aretia, ssct. Dicranothrix | Cortusa matthioli var. sachalinensis | CSAC_P_2013 | |
| Androsace lactea | ALAC_TK_2015 | Cortusa matthioli ssp. turkestanica | CTUR_P_2013 |
| Androsace laggeri (= A. carnea var. laggeri) | ACAL_P_2014 | ||
| Androsace obtusifolia | AOBT_P_2014 | genus Dionysia Fenzl. | |
| ct. Chamaejasme, ssct. Hookerianae | Dionysia khatamii | DKHA_F_2015 | |
| Androsace limprichtii | ALIM_P_2014 | Dionysia zschummelii | DZSH_F_2015 |
| sct. Chamaejasme, ssct. Mucronifoliae | |||
| Androsace mariae var. tibetica | AMAT_P_2014 | genus Hottonia L. | |
| Androsace mucronifolia | AMUC_TK_2015 | Hottonia inflata hb | HOIN_MO_2014 |
| Androsace sempervivoides | ASPV_B_2015 | Hottonia palustris hb | HOPA_PG_2014 |
| sct. Chamaejasme, ssct. Strigillosae | |||
| Androsace spinulifera | ASPI_P_2015 | genus Soldanella L. | |
| Androsace strigillosa | ASTR_P_2014 | sct. Crateriflorae | |
| sct. Chamaejasme, ssct. Sublanatae | Soldanella alpina | SALP_K_2016 | |
| Androsace adenocephala | AADE_F_2015 | Soldanella carpatica | SCAR_K_2014 |
| Androsace nortonii | ANOR_P_2014 | Soldanella cyanaster | SCYA_K_2014 |
| sct. Chamaejasme, ssct. Villosae, series Chamaejasmoidae | Soldanella dimoniei | SDIM_K_2014 | |
| Androsace brachystegia | ABRA_P_2014 | Soldanella villosa | SVIL_K_2014 |
| Androsace chamaejasme ssp. carinata | ACHC_P_2014 | sct. Tubiflorae | |
| Androsace zambalensis | AZAM_P_2014 | Soldanella minima | SMIN_F_2015 |
| sct. Chamaejasme, ssct. Villosae, series Euvillosae | Soldanella minima | SMIN_TK_2015 | |
| Androsace dasyphylla | ADAS_P_2014 | ||
| Androsace robusta ssp. purpurea | AROP_P_2014 | genus Vitaliana Sesl. | |
| Androsace sarmentosa | ASAR_K_2015 | Vitaliana primuliflora | VPRI_B_2015 |
| sct. Pseudoprimula | Vitaliana primuliflora ssp. assoana | VPAS_TK_2015 | |
| Androsace elatior | AELA_F_2015 | Vitaliana primuliflora ssp. praetutiana | VPPR_B_2015 |
| sct. Douglasia | Vitaliana primuliflora ssp. praetutiana | VPPR_B_2017 | |
| Androsacemontana (= Douglasia montana) | AMON_F_2015 | ||
| sct. Aizodium | |||
| Androsace bulleyana | ABUL_F_2015 |
| Sample Abbreviations | VPPR_B_15 | VPAS_TK_15 | VPRI_B_15 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | RT ± RTSD [min] | Proposed Neutral Formula | calcd. m/z [for Neut. Form.–H]− | |Error| [ppm] | BPC Fragments; Collision Energy 30 eV (Relative Intensity %) | MS2 Fragments /Collision Energy/ (Relative Intensity %) | Rel. Area % | Rel. Area % | Rel. Area % |
| 1 glycoside 2 aglycone | [suggested interpretation] | [suggested interpretation] | |||||||
| 1. | 6.82 ± 0.01 | C58H98O27 C29H50O4 | 1225.6223 461.3636 | 2.8 2.7 | 1339.6070 (2.1) [M+FNa+FA–H]− 1293.6067 (8.3) [M+FNa–H]− 1225.6188 (100) [M–H]− | /96.0 eV/ 1079.5619 (49.7) [M–dxHex–H]− 917.5039 (100) [M–dxHex–Hex–H]− 785.4706 (38.5) [M–dxHex–Hex–Pen–H]− 623.4139 (98.1) [M–dxHex–Hex–Pen–Hex–H]− 461.3649 (4.7) [M–dxHex–Hex–Pen–Hex–Hex–H]− 367.1239 (29.5) [M–dxHex–Hex–Pen–Hex–256–H]− | 14.31 | 9.60 | 0.00 |
| 2. | 7.38 ± 0.01 | C53H86O24 C37H44O | 1105.5436 503.3319 | 2.4 13.7 | 1173.5263 (4.7) [M+FNa–H]− 1105.5410 (100) [M–H]− | /86.4 eV/ 1105.54 (100) [M–H]− 959.4814 (21.9) [M–dxHex–H]− 941.4744 (4.2) [M–dxHex–18–H]− 797.4326 (8.4) [M–dxHex–Hex–H]− 779.4224 (4.6) [M–dxHex–Hex–18–H]− 665.3906 (1.6) [M–dxHex–Hex–Pen–H]− 647.383 (1) [M–dxHex–Hex–Pen–18–H]− 503.3388 (2.1) [M–dxHex–Hex–Pen–Hex–H]− | 10.52 | 8.06 | 0.00 |
| 3. | 7.82 ± 0.01 | C58H94O28 C29H46O5 | 1237.5859 473.3272 | 1.1 5.2 | 1351.5710 (6.3) [M+FA+FNa–H]− 1305.5669 (7.2) [M+FNa–H]− 1283.5881 (30.1) [M+FA–H]− 1237.5845 (100) [M–H]− | /97.0 eV/ 1237.5700 (5.0) [M–H]− 1075.5204 (15.4) [M–Hex–H]− 929.4692 (57.0) [M–Hex–dxHex–H]− 767.4243 (80.9) [M–Hex–dxHex–Hex–H]− 655.3349 (11.8) 635.3776 (19.0) [M–Hex–dxHex–Hex–Pen–H]− 529.1791 (20.9) 473.3248 (14.9) [M–Hex–dxHex–Hex–Pen–Hex–H]− 395.1227 (23.2) [M–Hex–dxHex–Hex–Pen–240–H]− 367.1257 (100) [M–Hex–dxHex–Hex–Pen–268–H]− 293.0879 (20.7) | 7.51 | 8.01 | 9.00 |
| 4. | 8.07 ± 0.00 | C52H84O23 C29H46O5 | 1075.533 1473.3272 | 0.9 10.2 | 1189.5237 (7.5) [M+FA+FNa–H]− 1143.5171 (5.8) [M+FNa–H]− 1121.5363 (27.7) [M+FA–H]− 1075.5321 (100) [M–H]− | /84.0 eV/ 1075.5673 (3.1) [M–H]− 929.4713 (55.5) [M–dxHex–H]− 767.4235 (100) [M–dxHex–Hex–H]− 655.3402 (15.7) 635.3733 (19.6) [M–dxHex–Hex–Pen–H]− 541.1746 (10.0) 473.3321 (24.7) [M–dxHex–Hex–Pen–Hex–H]− 395.1206 (21.8) 367.1247 (43.1) [M–dxHex–Hex–Pen–Hex–106–H]− 361.237 (18.2) | 6.32 | 7.55 | 6.11 |
| 5. | 8.38 ± 0.01 | C53H84O24 C29H44O4 | 1103.5280 455.3167 | 1.0 3.1 | 1171.5138 (7.5) [M+FA+FNa–H]− 1103.5269 (100) [M+FA–H]− | /86.3 eV/ 1105.5379 (66.1) [M+FA+2–H]− 1103.5269 (30.8) [M+FA–H]− 1057.5203 (100) [M–H]− 959.4818 (14.1) 957.4602 (11.6) [911+FA]− 913.4669 (22.6) [911+2]− 911.4629 (46.5) [M–dxHex–H]− 893.455 (8.1) 751.4255 (32.4) [749+2]− 749.4088 (23.1) [M–dxHex–Hex–H]− 731.4039 (7.5) 705.4239 (9.4) 639.3456 (13.7) 617.3689 (10) [M–dxHex–Hex–Pen–H]− 541.1791 (5.3) 457.3345 (13.3) [455+2]− 455.3181 (17.7) [M–dxHex–Hex–Pen–Hex–H]− 395.1199 (12.1) 367.1246 (22.6) [M–dxHex–Hex–Pen–Hex–88–H]− 353.1113 (5.3) 345.244 (9.6) 293.0849 (10.4) | 6.58 | 5.41 | 0.00 |
| 6. | 8.57 ± 0.00 | C57H90O28 n.i. | 1221.5546 | 3.2 | 1335.5407 (5.1) [M+FA+FNa–H]− 1289.5337 (7.0) [M+FNa–H]− 1267.5545 (12.8) [M+FA–H]− 1221.5507 (100) [M–H]− | not fragmented | 2.82 | 3.22 | 3.35 |
| 7. | 8.65 ± 0.00 | C58H92O28 C29H44O5 | 1235.5702 471.3116 | 0.6 1.3 | 1349.5618 (6.3) [M+FA+FNa–H]− 1303.5527 (5.3) [M+FNa–H]− 1281.5739 (37.0) [M+FA–H]− 1235.5695 (100) [M–H]− | /96.8 eV/ 1235.5841 (1.7) [M–H]− 1217.5749 (4.6) [M–18–H]− 1177.5321 (3.8) [M–18–40–H]− 1073.5132 (3.9) [M–Hex–H]− 1055.5035 (8.7) [M–Hex–18–H]− 1015.4795 (10.2) [M–Hex–18–40–H]− 927.4543 (31.3) [M–Hex–dxHex–H]− 909.4439 (49.5) [M–Hex–dxHex–18–H]− 869.4148 (36.8) [M–Hex–dxHex–18–40–H]− 765.4053 (67.7) [M–Hex–dxHex–Hex–H]− 747.3951 (78.8) [M–Hex–dxHex–Hex–18–H]− 707.3648 (46.1) [M–Hex–dxHex–Hex–18–40–H]− 633.3663 (26.1) [M–Hex–dxHex–Hex–Pen–H]− 615.3533 (19.5) [M–Hex–dxHex–Hex–Pen–18–H]− 575.3221 (12.7) [M–Hex–dxHex–Hex–Pen–18–40–H]− 529.1771 (17.2) 471.3122 (9.9) [M–Hex–dxHex–Hex–Pen–Hex–H]− 469.1568 (9.2) [M–Hex–dxHex–Hex–Pen–Hex–2–H]− 453.3017 (37.1) 413.2702 (29.1) 395.1191 (18.5) 367.1253 (100) 353.1075 (6.8) 307.1017 (5.4) 293.0873 (14.9) | 22.43 | 41.90 | 30.37 |
| 8. | 8.99 ± 0.01 | C52H82O23 C29H44O5 | 1073.5174 471.3116 | 3.1 0.4 | 1187.5045 (6.4) [M+FA+FNa–H]− 1141.5003 (4.3) [M+FNa–H]− 1119.5172 (21.1) [M+FA–H]− 1073.5141 (100) [M–H]− | /83.9 eV/ 1073.5027 (2.0) [M–H]− 1015.4776 (2.0) [M–18–40–H]− 927.4560 (29.9) [M–dxHex–H]− 909.4401 (17.3) [M–dxHex–18–H]− 869.4096 (19.0) [M–dxHex–18–40–H]− 765.4025 (100) [M–dxHex–Hex–H]− 747.3931 (44.1) [M–dxHex–Hex–18–H]− 707.3619 (34.7) [M–dxHex–Hex–18–40–H]− 633.3613 (47.8) [M–dxHex–Hex–Pen–H]− 615.3514 (17.2) [M–dxHex–Hex–Pen–18–H]− 575.3216 (8.7) [M–dxHex–Hex–Pen–18–40–H]− 471.3114 (15.2) [M–dxHex–Hex–Pen–Hex–H]− 453.3007 (27.3) [M–dxHex–Hex–Pen–Hex–18–H]− 413.2691 (25.6) [M–dxHex–Hex–Pen–Hex–18–40–H]− 395.1204 (12.5) 367.1240 (26.8) 353.1086 (7.9) 293.0858 (13.8) | 22.46 | 42.64 | 28.55 |
| 9. | 11.56 ± 0.00 | C58H94O28 C29H46O5 | 1237.5859 473.3272 | 2.5 1.0 | 1351.5736 (4.8) [M+FA+FNa–H]− 1305.5669 (7.2) [M+FNa–H]− 1283.5868 (18.2) [M+FA–H]− 1237.5828 (100) [M–H]− | /97.0 eV/ 1237.5901 (6) [M–H]− 1075.5277 (10.4) [M–Hex–H]− 929.4714 (50) [M–Hex–dxHex–H]− 767.4205 (58.6) [M–Hex–dxHex–Hex–H]− 703.2274 (7.7) 661.2146 (26.9) 635.3797 (17.4) [M–Hex–dxHex–Hex–Pen–H]− 541.1773 (9.4) 529.1758 (31.1) 499.166 (40.3) 473.3268 (18.6) [M–Hex–dxHex–Hex–Pen–Hex–H]− 395.1193 (18.3) 367.1245 (100) [M–Hex–dxHex–Hex–Pen–Hex–106–H]− 353.1087 (55.4) 293.0868 (9.4) | 17.30 | 0.00 | 3.56 |
| 10. | 12.08 ± 0.00 | C52H84O23 C29H46O5 | 1075.5331 473.3272 | 3.7 1.0 | 1189.5223 (8.8) [M+FA+FNa–H]− 1143.5168 (6.3) [M+FNa–H]− 1121.5355 (61.4) [M+FA–H]− 1075.5291 (100) [M–H]− | /84.0 eV/ 1075.5302 (5.3) [M–H]− 929.4737 (58.4) [M–dxHex–H]− 767.4192 (86.9) [M–dxHex–Hex–H]− 635.3783 (32.2) [M–dxHex–Hex–Pen–H]− 541.172 (13) 499.1677 (50.6) [M–dxHex–Hex–Pen–Pen+4–H]− 473.3265 (42.2) [M–dxHex–Hex–Pen–Hex–H]− 395.1217 (36.2) 367.1258 (68.6) [M–dxHex–Hex–Pen–Hex–106–H]− 353.1086 (100) [M–dxHex–Hex–Pen–Pen+4–dxHex–H]− 335.0984 (16) 293.087 (13.1) | 6.27 | 0.00 | 0.00 |
| 11. | 13.81 ± 0.01 | C57H92O27 n.i. | 1207.5753 | 4.8 | 1321.5589 (4.1) [M+FA+FNa–H]− 1275.5544 (7.6) [M+FNa–H]− 1253.5718 (11.6) [M+FA–H]− 1207.5695 (100) [M–H]− | /94.6 eV/ 913.4668 (100) [M–(Hex+Pen)–H]− | 10.26 | 4.89 | 8.53 |
| 12. | 13.98 ± 0.00 | C58H94O27 C29H26O4 | 1221.5910 457.3323 | 1.7 2.9 | 1335.5780 (4.3) [M+FA+FNa–H]− 1289.5731 (5.0) [M+FNa–H]− 1267.5928 (31.9) [M+FA–H]− 1221.5889 (100) [M–H]− | /95.7 eV/ 1221.5829 (7.7) [M–H]− 1059.5321 (15.7) [M–Hex–H]− 913.4762 (79.4) [M–Hex–dxHex–H]− 895.4661 (6.6) 751.4257 (100) [M–Hex–dxHex–Hex–H]− 619.3837 (20) [M–Hex–dxHex–Hex–Pen–H]− 541.1739 (4.7) 529.1764 (15.5) 469.1561 (8.1) 457.3310 (7.9) [M–Hex–dxHex–Hex–Pen–Hex–H]− 439.1443 (3.9) 395.1182 (16.7) 367.1242 (82.1) [M–Hex–dxHex–Hex–Pen–Hex–90–H]− 353.1066 (5.4) 345.2439 (3.8) 293.0871 (11.4) | 100.00 | 100.00 | 100.00 |
| 13. | 14.69 ± 0.02 | C52H84O22 C29H26O4 | 1059.5381 457.3323 | 2.1 1.7 | 1173.5257 (5.8) [M+FA+FNa–H]− 1127.5206 (4.2) [M+FNa–H]− 1105.5401 (31.4) [M+FA–H]− 1059.5359 (100) [M–H]− | /82.8 eV/ 1059.5390 (4.5) [M–H]− 913.4783 (50) [M–dxHex–H]− 751.427 (100) [M–dxHex–Hex–H]− 619.3838 (23.3) [M–dxHex–Hex–Pen–H]− 457.3331 (12.3) [M–dxHex–Hex–Pen–Hex–H]− 395.1209 (13.8) 367.1245 (24.6) [M–dxHex–Hex–Pen–Hex–90–H]− 293.0866 (13.2) | 50.19 | 30.06 | 30.30 |
References
- Challinor, V.L.; de Voss, J.J. Open-chain steroidal glycosides, a diverse class of plant saponins. Nat. Prod. Rep. 2013, 30, 429–454. [Google Scholar] [CrossRef] [PubMed]
- Vermaak, I.; Hamman, J.; Viljoen, A. Hoodia gordonii: An up-to-date review of a commercially important anti-obesity plant. Planta Med. 2011, 77, 1149–1160. [Google Scholar] [CrossRef]
- Gauthaman, K.; Ganesan, A.P. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction—an evaluation using primates, rabbit and rat. Phytomedicine 2008, 15, 44–54. [Google Scholar] [CrossRef]
- Adinolfi, M.; Corsaro, M.M.; Lanzetta, R.; Mancino, A.; Mangoni, L.; Parrilli, M. Triterpenoid oligoglycosides from Chionodoxa luciliae. Phytochemistry 1993, 34, 773–778. [Google Scholar] [CrossRef]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.-J.; Sathishkumar, N.; Yang, D.-U.; Yang, D.-C. Ginseng saponins and the treatment of osteoporosis: Mini literature review. J. Ginseng Res. 2013, 37, 261–268. [Google Scholar] [CrossRef]
- Keller, A.-C.; Keller, J.; Maillard, M.P.; Hostettmann, K. A lanostane-type steroid from the fungus Ganoderma carnosum. Phytochemistry 1997, 46, 963–965. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Saponins; Cambridge University Press: Cambridge, UK, 1995; ISBN 0-521-32970-1. [Google Scholar]
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef]
- Buckingham, J.; Cooper, C.M.; Purchase, R. Natural products desk reference; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1-4398-7361-5. [Google Scholar]
- Fan, M.-Y.; Wang, Y.-M.; Wamng, Z.-M.; Gao, H.-M. Advances on chemical constituents and pharmacological activity of genus Scilla. China J. Chinese Mater. Medica 2014, 39, 162–170. [Google Scholar]
- Wang, G.-W.; Lu, C.; Yuan, X.; Ye, J.; Jin, H.-Z.; Shan, L.; Xu, X.-K.; Shen, Y.-H.; Zhang, W.-D. Lanostane-type triterpenoids from Abies faxoniana and their DNA topoisomerase inhibitory activities. Phytochemistry 2015, 116, 221–229. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides of sea cucumbers (Holothuroidea, Echinodermata) as taxonomic markers. Nat. Prod. Commun. 2015, 10, 21–26. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dolmatov, I.Y.; Kalinin, V.I. Cladolosides C4, D1, D2, M, M1, M2, N and Q, new triterpene glycosides with diverse carbohydrate chains from sea cucumber Cladolabes schmeltzii. An uncommon 20,21,22,23,24,25,26,27-okta-nor-lanostane aglycone. The synergism of inhibitory action of non-toxic dose of the glycosides and radioactive irradiation on colony formation of HT-29 cancer cells. Carbohydr. Res. 2018, 468, 36–44. [Google Scholar]
- Ríos, J.-L.; Andújar, I.; Recio, M.-C.; Giner, R.-M. Lanostanoids from fungi: A group of potential anticancer compounds. J. Nat. Prod. 2012, 75, 2016–2044. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef]
- Colombo, P.S.; Flamini, G.; Rodondi, G.; Giuliani, C.; Santagostini, L.; Fico, G. Phytochemistry of European Primula species. Phytochemistry 2017, 143, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hagiwara, K.; Iguchi, K.; Suzuki, S.; Hsu, H. Isolation and structures of arvenins from Anagallis arvensis L. (Primulaceae). New cucurbitacin glucosides. Chem. Pharm. Bull. 1978, 26, 3107–3112. [Google Scholar] [CrossRef]
- Budzianowski, J.; Morozowska, M.; Wesołowska, M. Lipophilic flavones of Primula veris L. from field cultivation and in vitro cultures. Phytochemistry 2005, 66, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Valant-Vetschera, K.M.; Bhutia, T.D.; Wollenweber, E. Exudate flavonoids of Primula spp: Structural and biogenetic chemodiversity. Nat. Prod. Commun. 2009, 4, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Frankiewicz, A.N. The analysis of saponins in some species of the Primulaceae Batsch. Master’s Thesis, Wroclaw Medical University, Wrocław, Poland, 2016. [Google Scholar]
- Włodarczyk, M.; Matysik, G.; Cisowski, W.; Gleńsk, M. Rapid densitometric quantitative screening of the myricitrin content of crude methanolic extracts of leaves from a variety of Acer species. J. Planar Chromatogr. 2006, 19, 378–382. [Google Scholar] [CrossRef]
- Markham, K.R. Techniques of Flavonoids Identification; Academic Press: London, UK, 1982; ISBN 0-12-472680-1. [Google Scholar]
- Kuroda, M.; Mimaki, Y.; Ori, K.; Sakagami, H.; Sashida, Y. 27-norlanostane glycosides from the bulbs of Muscari paradoxum. J. Nat. Prod. 2004, 67, 2099–2103. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, M.; Barone, G.; Corsaro, M.M.; Mangoni, L.; Lanzetta, R.; Parrilli, M. Glycosides from Muscari armeniacum and Muscari botryoides. Isolation and structure of Muscarosides G-N. Can. J. Chem. 1988, 66, 2787–2793. [Google Scholar] [CrossRef]
- Sholichin, M.; Miyahara, K.; Kawasaki, T. Oligoglycosides of spirocyclic nortriterpenoids related to eucosterol. Chem. Pharm. Bull. 1985, 33, 1756–1759. [Google Scholar] [CrossRef][Green Version]
- Wang, L.-K.; Zheng, C.-J.; Li, X.-B.; Chen, G.-Y.; Han, C.-R.; Chen, W.-H.; Song, X.-P. Two new lanostane triterpenoids from the branches and leaves of Polyalthia oblique. Molecules 2014, 19, 7621–7628. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Lowe, D. The genus Androsace: A monograph for gardeners and botanists; Alpine Garden Society: Pershore, UK, 1997; ISBN 0-900048-67-0. [Google Scholar]
- Li, G.; Kusari, S.; Kusari, P.; Kayser, O.; Spiteller, M. Endophytic Diaporthe sp. LG23 produces a potent antibacterial tetracyclic triterpenoid. J. Nat. Prod. 2015, 78, 2128–2132. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 12 and 13 are available from the author (M.W.). |


| Position | δC (ppm) | δH (ppm) | δH (ppm) |
|---|---|---|---|
| C1 | 36.24 (t) | 1.68 (m, 1H) eq | 1.17 (td, J = 13.6, 3.5 Hz, 1H) ax |
| C2 | 27.97 (t) | 2.27 (m, 1H) eq | 2.00 (m, 1H) ax |
| C3 | 89.42 (d) | 3.57 (dd, J = 11.8, 4.6 Hz, 1H) ax | |
| C4 | 44.91 (s) | - | |
| C5 | 52.29 (d) | 1.28 (dd, J = 12.8, 4.6 Hz, 1H) ax | |
| C6 | 19.23 (t) | 1.83 (dd, J = 13.2, 6.6 Hz, 1H) eq | 1.51 (m, 1H) ax |
| C7 | 27.39 (t) | 2.02 (m, 2H) | |
| C8 | 135.78 (s) | - | |
| C9 | 135.11 (s) | - | |
| C10 | 37.28 (s) | - | |
| C11 | 21.54 (t) | 2.10 (m, 1H) eq | 1.94 (m, 1H) ax |
| C12 | 25.76 (t) | 2.35 (dt, J = 13.9, 9.0 Hz, 1H) ax | 1.41 (m, 1H) eq |
| C13 | 49.32 (s) | - | |
| C14 | 51.30 (s) | - | |
| C15 | 32.65 (t) | 1.67 (m, 1H) ax | 1.38 (m, 1H) eq |
| C16 | 42.26 (t) | 1.99 (m, 1H) ax | 1.74 (m, 1H) eq |
| C17 | 96.22 (s) | - | |
| C18 | 19.82 (q) | 0.90 (s, 3H) ax (β) | |
| C19 | 20.01 (q) | 0.94 (s, 3H) ax (β) | |
| C20 | 44.47 (d) | 2.01 (m, 1H) ax | |
| C21 | 17.97 (q) | 1.02 (d, J = 6.8 Hz, 3H) eq | |
| C22 | 40.99 (t) | 1.69 (m, 2H) | |
| C23 | 74.11 (d) | 4.62 (m, 1H) ax | |
| C24 | 53.30 (t) | 2.85 (dd, J = 15.3, 7.8 Hz, 1H) A | 2.61 (dd, J = 15.3, 5.3 Hz, 1H) B |
| C25 | 207.59 (s) | - | |
| C26 | 30.83 (q) | 2.21 (s, 3H) | |
| nor-C27 | - | - | |
| C28 | 23.64 (q) | 1.56 (s, 3H) eq | |
| C29 | 63.65 (t) | 4.44 (m, 1H) A | 3.66 (m, 1H) B |
| C30 | 26.84 (q) | 1.35 (s, 3H) ax (α) | |
| G′1(→C3) | 106.54 (d) | 4.97 (d, J = 7.9 Hz, 1H) | |
| G′2 | 75.84 (d) | 3.99 (m, 1H) | |
| G′3 a | 78.72 (d) | 4.18 (m, 1H) | |
| G′4 | 73.23 (d) | 4.19 (m, 1H) | |
| G′5 | 75.84 (d) | 3.99 (m, 1H) | |
| G′6 | 69.10 (t) | 4.50 (dd, J = 10.1, 4.1 Hz, 1H) A | 4.22 (m, 1H) B |
| A″1(→G′6) | 101.34 (d) | 5.33 (d, J = 3.1 Hz, 1H) | |
| A″2 | 78.83 (d) | 4.63 (m, 1H) | |
| A″3 | 72.00 (d) | 4.65 (m, 1H) | |
| A″4 | 66.89 (d) | 4.59 (m, 1H) | |
| A″5 | 62.72 (t) | 4.39 (dd, J = 11.0, 7.9 Hz, 1H) A | 3.92 (dd, J = 10.9, 3.8 Hz, 1H) B |
| G‴1(→A″2) | 103.46 (d) | 5.17 (d, J = 7.0 Hz, 1H) | |
| G‴2 | 78.93 (d) | 4.18 (m, 1H) | |
| G‴3 | 79.65 (d) | 4.16 (m, 1H) | |
| G‴4 b | 71.75 (d) | 4.17 (m, 1H) | |
| G‴5 | 78.69 (d) | 3.68 (m, 1H) | |
| G‴6 | 62.53 (t) | 4.35 (dd, J = 12.1, 2.5 Hz, 1H) A | 4.27 (dd, J = 13.2, 4.9 Hz, 1H) B |
| R″″1(→G‴2) | 101.41 (d) | 6.52 (d, J = 1.8 Hz, 1H) | |
| R″″2 | 83.14 (d) | 4.78 (m, 1H) | |
| R″″3 | 73.01 (d) | 4.66 (m, 1H) | |
| R″″4 | 75.11 (d) | 4.23 (m, 1H) | |
| R″″5 | 69.99 (d) | 4.85 (dd, J = 9.5, 6.3 Hz, 1H) | |
| R″″6 | 19.12 (q) | 1.74 (d, J = 6.1 Hz, 3H) | |
| G‴″1(→R″″2) | 107.82 (d) | 5.25 (d, J = 7.9 Hz, 1H) | |
| G‴″2 | 76.26 (d) | 4.06 (m, 1H) | |
| G‴″3 a | 78.74 (d) | 4.18 (m, 1H) | |
| G‴″4 b | 71.84 (d) | 4.17 (m, 1H) | |
| G‴″5 | 79.10 (d) | 3.85 (m, 1H) | |
| G‴″6 | 63.07 (t) | 4.43 (m, 1H) A | 4.25 (m, 1H) B |
| Position | δC (ppm) | δH (ppm) | δH (ppm) |
|---|---|---|---|
| C1 | 36.26 (t) | 1.68 (m, 1H) eq | 1.18 (td, J = 13.6, 3.6 Hz, 1H) ax |
| C2 | 27.99 (t) | 2.27 (m, 1H) eq | 2.02 (m, 1H) ax |
| C3 | 89.43 (d) | 3.57 (dd, J = 11.5, 4.6 Hz, 1H) ax | |
| C4 | 44.93 (s) | - | |
| C5 | 52.30 (d) | 1.28 (dd, J = 12.7, 2.0 Hz, 1H) ax | |
| C6 | 19.25 (t) | 1.83 (dd, J = 13.0, 6.5 Hz, 1H) eq | 1.52 (m, 1H) ax |
| C7 | 27.41 (t) | 2.02 (m, 2H) | |
| C8 | 135.79 (s) | - | |
| C9 | 135.12 (s) | - | |
| C10 | 37.30 (s) | - | |
| C11 | 21.56 (t) | 2.12 (m, 1H) eq | 1.95 (m, 1H) ax |
| C12 | 25.77 (t) | 2.35 (m, 1H) ax | 1.42 (m, 1H) eq |
| C13 | 49.34 (s) | - | |
| C14 | 51.32 (s) | - | |
| C15 | 32.67 (t) | 1.67 (m, 1H) ax | 1.40 (d, J = 2.6 Hz, 1H) eq |
| C16 | 42.28 (t) | 1.99 (m, 1H) ax | 1.74 (m, 1H) eq |
| C17 | 96.24 (s) | - | |
| C18 | 19.83 (q) | 0.90 (s, 3H) ax (β) | |
| C19 | 20.03 (q) | 0.94 (s, 3H) ax (β) | |
| C20 | 44.48 (d) | 2.01 (m, 1H) ax | |
| C21 | 17.98 (q) | 1.02 (d, J = 6.7 Hz, 3H) eq | |
| C22 | 41.01 (t) | 1.68 (m, 2H) | |
| C23 | 74.13 (d) | 4.61 (m, 1H) ax | |
| C24 | 53.31 (t) | 2.85 (dd, J = 15.4, 7.8 Hz, 1H) A | 2.61 (dd, J = 15.4, 5.7 Hz, 1H) B |
| C25 | 207.61 (s) | - | |
| C26 | 30.85 (q) | 2.21 (s, 3H) | |
| nor-C27 | - | - | |
| C28 | 23.66 (q) | 1.56 (s, 3H) eq | |
| C29 | 63.67 (t) | 4.44 (d, J = 11.2 Hz, 1H) A | 3.62 (m, 1H) B |
| C30 | 26.86 (q) | 1.35 (s, 3H) ax (α) | |
| G′1(→C3) | 106.55 (d) | 4.96 (d, J = 7.8 Hz, 1H) | |
| G′2 | 75.87 (d) | 3.99 (m, 1H) | |
| G′3 | 78.77 (d) | 4.21 (m, 1H) | |
| G′4 | 73.27 (d) | 4.21 (m, 1H) | |
| G′5 | 75.87 (d) | 4.02 (m, 1H) | |
| G′6 | 69.11 (t) | 4.51 (dd, J = 10.2, 4.2 Hz, 1H) A | 4.23 (dd, J = 10.4, 4.7 Hz, 1H) B |
| A″1(→G′6) | 101.37 (d) | 5.36 (d, J = 3.2 Hz, 1H) | |
| A″2 | 78.79 (d) | 4.65 (m, 1H) | |
| A″3 | 71.97 (d) | 4.68 (m, 1H) | |
| A″4 | 66.88 (d) | 4.64 (m, 1H) | |
| A″5 | 62.66 (t) | 4.41 (dd, J = 11.3, 8.1 Hz, 1H) A | 3.95 (dd, J = 11.0, 4.0 Hz, 1H) B |
| G‴1(→A″2) | 103.56 (d) | 5.17 (d, J = 7.8 Hz, 1H) | |
| G‴2 | 78.10 (d) | 4.27 (m, 1H) | |
| G‴3 | 79.92 (d) | 4.18 (m, 1H) | |
| G‴4 | 71.84 (d) | 4.21 (m, 1H) | |
| G‴5 | 78.74 (d) | 3.70 (ddt, J = 6.9, 4.5, 2.3 Hz, 1H) | |
| G‴6 | 62.56 (t) | 4.33 (m, 1H) A | 4.26 (dd, J = 9.1, 7.5 Hz, 1H) B |
| R″″1(→G‴2) | 102.43 (d) | 6.39 (d, J = 1.8 Hz, 1H) | |
| R″″2 | 72.81 (d) | 4.77 (dd, J = 3.5, 1.6 Hz, 1H) | |
| R″″3 | 73.12 (d) | 4.66 (m, 1H) | |
| R″″4 | 74.72 (d) | 4.31 (m, 1H) | |
| R″″5 | 70.17 (d) | 4.92 (dd, J = 9.6, 6.2 Hz, 1H) | |
| R″″6 | 19.23 (q) | 1.77 (d, J = 6.2 Hz, 3H) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, M.; Szumny, A.; Gleńsk, M. Lanostane-Type Saponins from Vitaliana primuliflora. Molecules 2019, 24, 1606. https://doi.org/10.3390/molecules24081606
Włodarczyk M, Szumny A, Gleńsk M. Lanostane-Type Saponins from Vitaliana primuliflora. Molecules. 2019; 24(8):1606. https://doi.org/10.3390/molecules24081606
Chicago/Turabian StyleWłodarczyk, Maciej, Antoni Szumny, and Michał Gleńsk. 2019. "Lanostane-Type Saponins from Vitaliana primuliflora" Molecules 24, no. 8: 1606. https://doi.org/10.3390/molecules24081606
APA StyleWłodarczyk, M., Szumny, A., & Gleńsk, M. (2019). Lanostane-Type Saponins from Vitaliana primuliflora. Molecules, 24(8), 1606. https://doi.org/10.3390/molecules24081606