Evaluation of Melatonin Secretion and Metabolism Exponents in Patients with Ulcerative and Lymphocytic Colitis
Abstract
1. Introduction
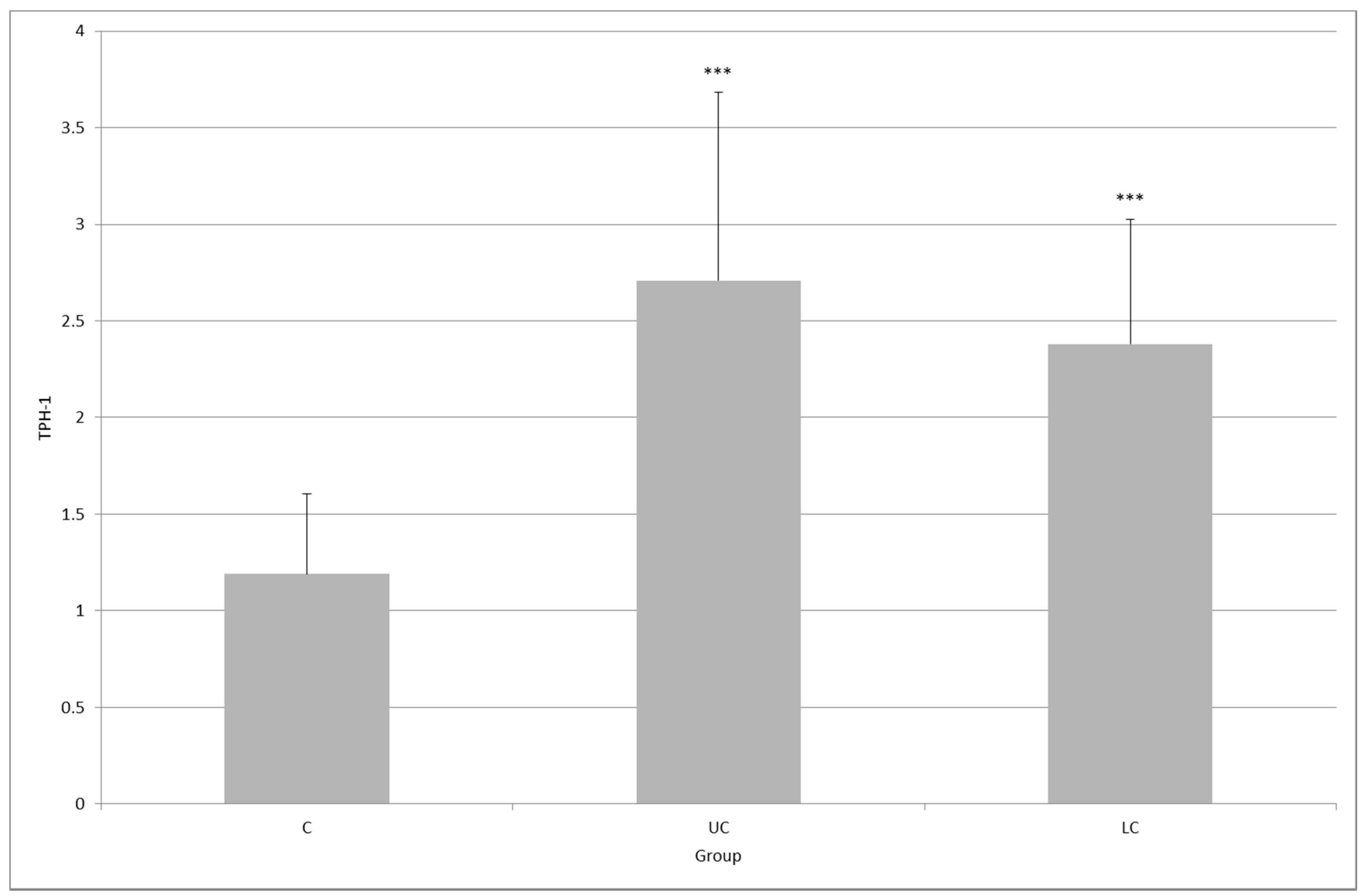
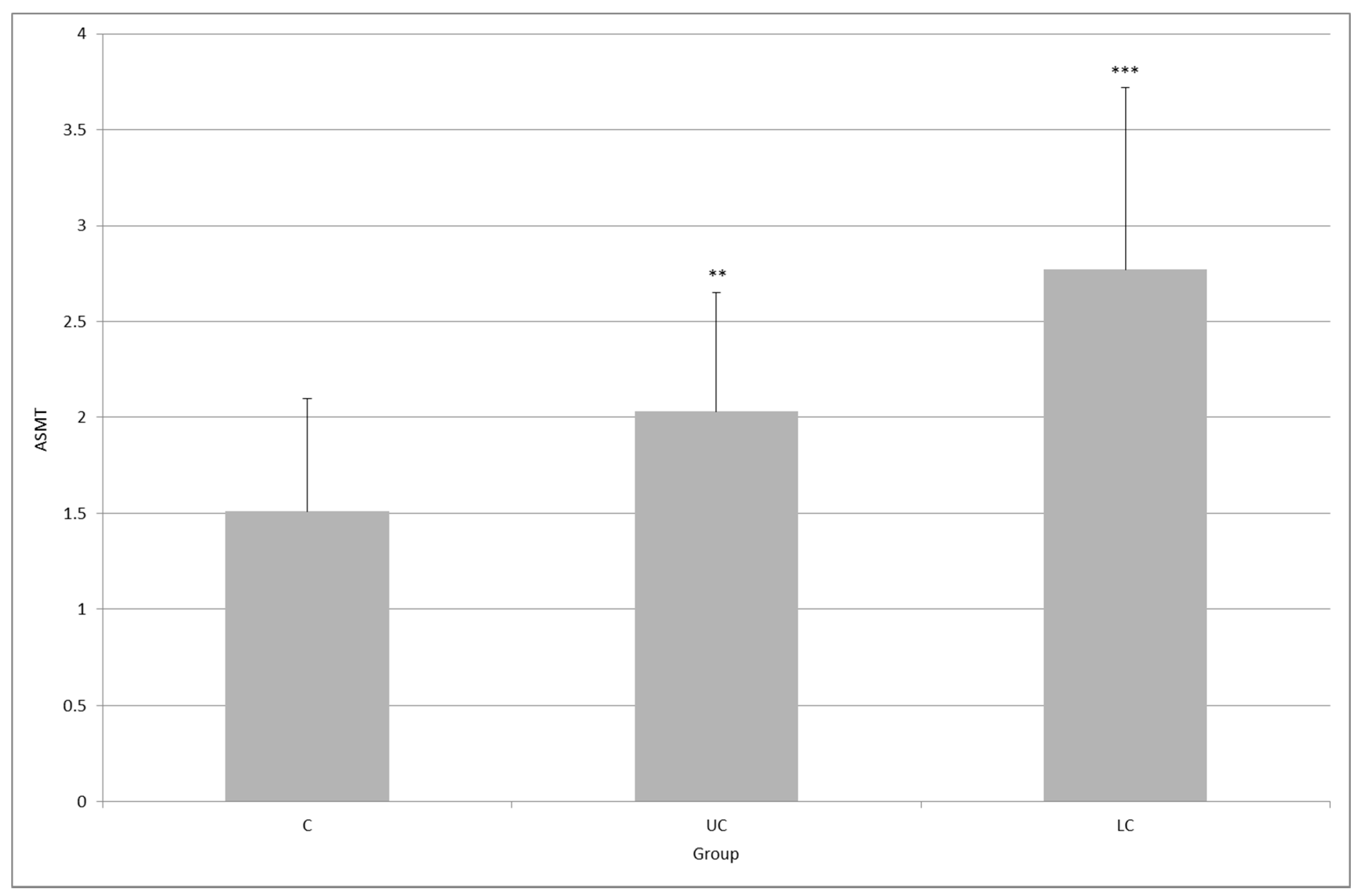
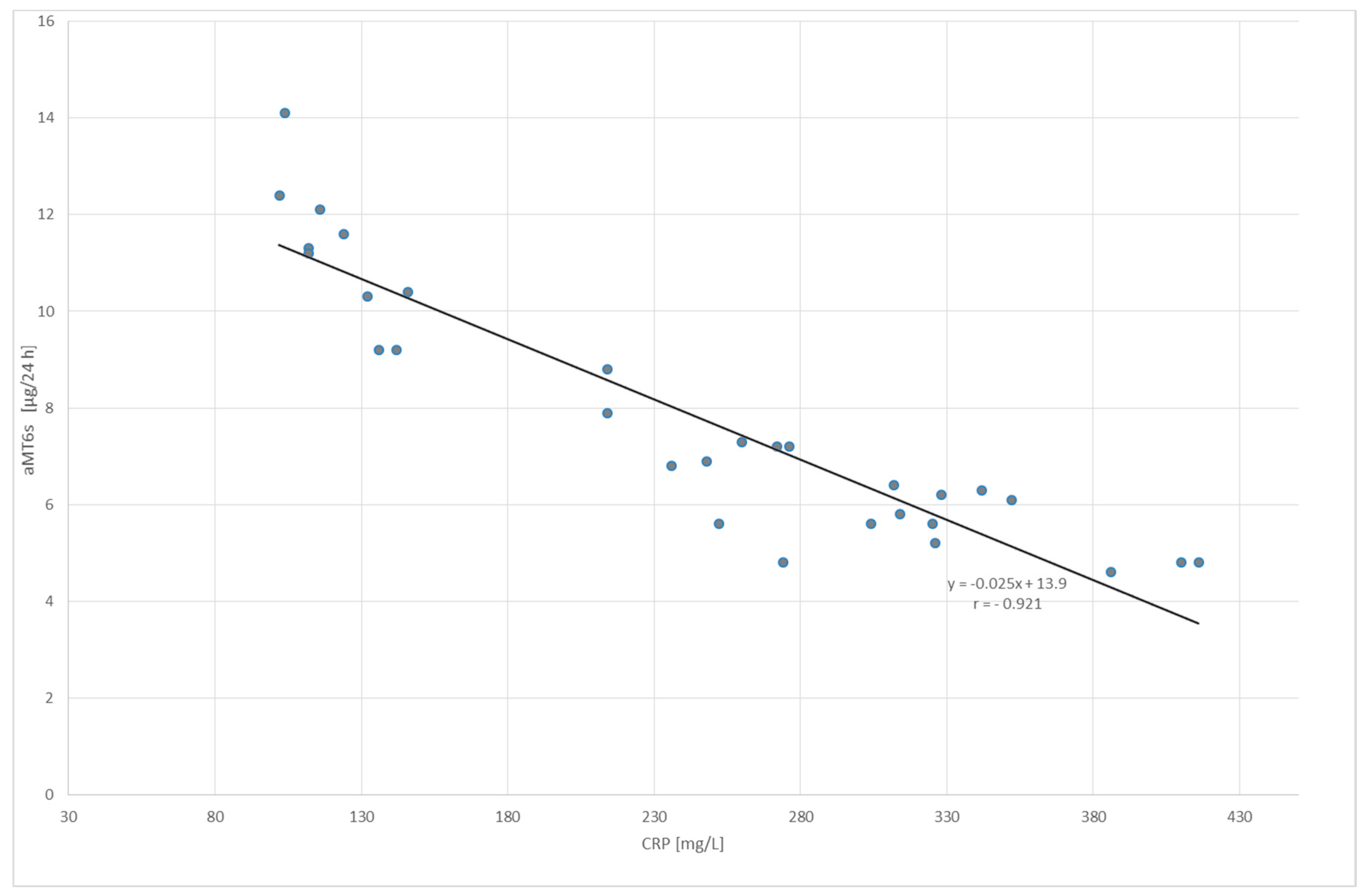
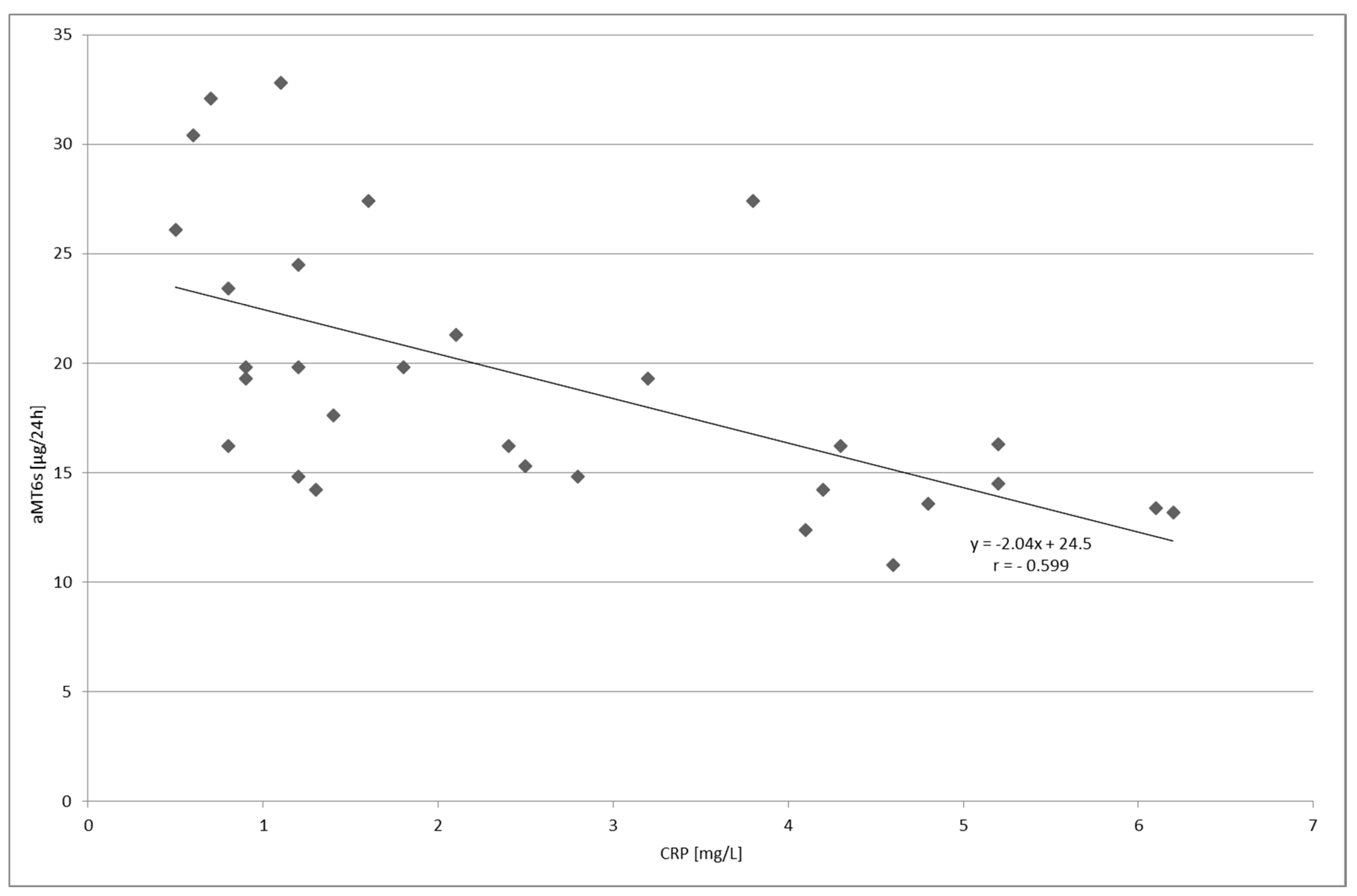
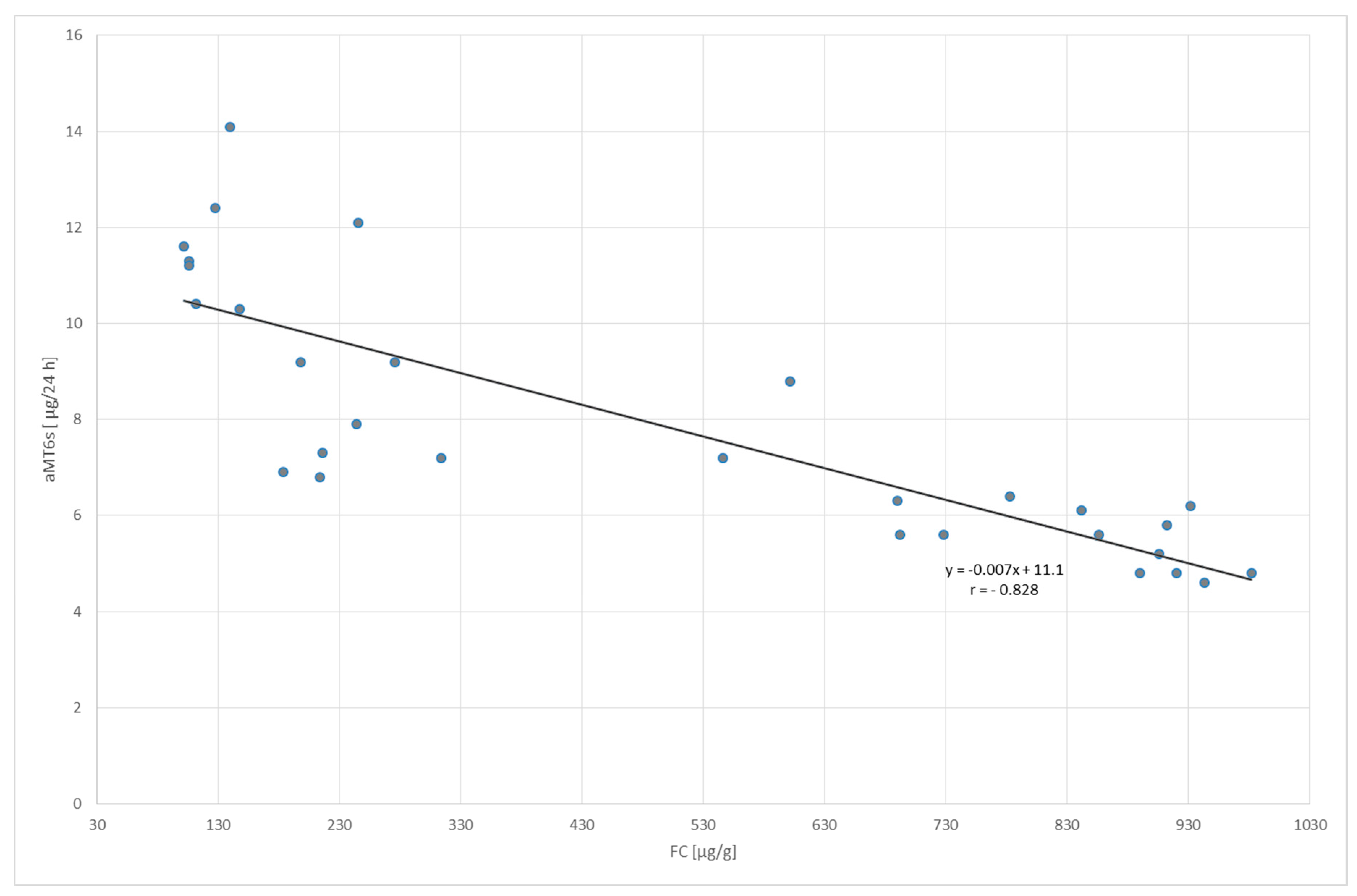
2. Results
3. Discussion
4. Material and methods
4.1. Patients
4.2. Study Design and Procedures
4.3. Ethics
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bubenik, G.A. Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol. Signals Recept. 2001, 10, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.-X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Bandyopadhyay, D. Neurally-mediated and neurally-independent beneficial actions of melatonin in the gastrointestinal tract. J. Physiol. Pharmacol. 2003, 54 (Suppl. 4), 113–125. [Google Scholar] [PubMed]
- Chen, C.-Q.; Fichna, J.; Bashashati, M.; Li, Y.-Y.; Storr, M. Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 2011, 17, 3888–3898. [Google Scholar] [CrossRef] [PubMed]
- Velarde, E.; Cerdá-Reverter, J.M.; Alonso-Gómez, A.L.; Sánchez, E.; Isorna, E.; Delgado, M.J. Melatonin-synthesizing enzymes in pineal, retina, liver, and gut of the goldfish (carassius): MRNA expression pattern and regulation of daily rhythms by lighting conditions. Chronobiol. Int. 2010, 27, 1178–1201. [Google Scholar] [CrossRef] [PubMed]
- Zagajewski, J.; Drozdowicz, D.; Brzozowska, I.; Hubalewska-Mazgaj, M.; Stelmaszynska, T.; Laidler, P.M.; Brzozowski, T. Conversion l-tryptophan to melatonin in the gastrointestinal tract: The new high performance liquid chromatography method enabling simultaneous determination of six metabolites of l-tryptophan by native fluorescence and UV-VIS detection. J. Physiol. Pharmacol. 2012, 63, 613–621. [Google Scholar] [PubMed]
- Ceinos, R.M.; Chansard, M.; Revel, F.; Calgari, C.; Míguez, J.M.; Simonneaux, V. Analysis of adrenergic regulation of melatonin synthesis in Siberian hamster pineal emphasizes the role of HIOMT. Neurosignals 2004, 13, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.; Tamura, E.K.; D’Argenio-Garcia, L.; Muxel, S.M.; da Silveira Cruz-Machado, S.; Marçola, M.; Carvalho-Sousa, C.E.; Cecon, E.; Ferreira, Z.S.; Markus, R.P. Dual Effect of Catecholamines and Corticosterone Crosstalk on Pineal Gland Melatonin Synthesis. Neuroendocrinology 2017, 104, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.A.; Moss, H.B. Pharmacokinetics of Melatonin in Man: First Pass Hepatic Metabolism. J. Clin. Endocrinol. Metab. 1985, 61, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Facciolá, G.; Hidestrand, M.; von Bahr, C.; Tybring, G. Cytochrome P450 isoforms involved in melatonin metabolism in human liver microsomes. Eur. J. Clin. Pharmacol. 2001, 56, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Baskett, J.J.; Cockrem, J.F.; Antunovich, T.A. Sulphatoxymelatonin excretion in older people: Relationship to plasma melatonin and renal function. J. Pineal Res. 1998, 24, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Kovács, J.; Brodner, W.; Kirchlechner, V.; Arif, T.; Waldhauser, F. Measurement of urinary melatonin: A useful tool for monitoring serum melatonin after its oral administration. J. Clin. Endocrinol. Metab. 2000, 85, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.M.; Tamura, E.K.; Macedo, S.M.D.; Cecon, E.; Bueno-Alves, L.; Farsky, S.H.P.; Ferreira, Z.S.; Markus, R.P. Melatonin inhibits nitric oxide production by microvascular endothelial cells in vivo and in vitro. Br. J. Pharmacol. 2007, 151, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Manikonda, P.K.; Jagota, A. Melatonin administration differentially affects age-induced alterations in daily rhythms of lipid peroxidation and antioxidant enzymes in male rat liver. Biogerontology 2012, 13, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, J.; Śliwiński, T.; Wiśniewska-Jarosińska, M.; Turant, M.; Błasiak, J.; Kulig, A.; Majsterek, I.; Chojnacki, C. Evaluation of the number of enterochromaffin cells and melatonin metabolism exponents in subjects with ulcerative colitis. Clin. Exp. Med. Lett. 2011, 52, 19–23. [Google Scholar]
- Neurath, M.F.; Finotto, S. The many roads to inflammatory bowel diseases. Immunity 2006, 25, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of Disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Calpena, A.; Clares, B. Evaluating the Oxidative Stress in Inflammation: Role of Melatonin. Int. J. Mol. Sci. 2015, 16, 16981–17004. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, Z.H.; Bogdanovski, D.A.; Barratt-Stopper, P.; Paglinco, S.R.; Antonioli, L.; Rolandelli, R.H. Crohn’s Disease and Ulcerative Colitis Show Unique Cytokine Profiles. Cureus 2017, 9, e1177. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Wiśniewska-Jarosińska, M.; Kulig, G.; Majsterek, I.; Reiter, R.J.; Chojnacki, J. Evaluation of enterochromaffin cells and melatonin secretion exponents in ulcerative colitis. World J. Gastroenterol. 2013, 19, 3602–3607. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Danielsson, A.; Stenling, R.; Grimelius, L. Colonic endocrine cells in inflammatory bowel disease. J. Intern. Med. 1997, 242, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Rybakova, M.G.; Botina, A.V.; Solov’eva, O.I. Immunomorphological characteristics of mucosal and endocrine cells of the colon in patients with chronic ulcerative colitis. Arkhiv Patol. 2005, 67, 30–33. [Google Scholar]
- Ingle, S.B.; Adgaonkar, B.D.; Ingle, C.R.H. Microscopic colitis: Common cause of unexplained nonbloody diarrhea. World J. Gastrointest. Pathophysiol. 2014, 5, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zabana, Y.; Ferrer, C.; Aceituno, M.; Salas, A.; Fernández-Bañares, F. Advances for improved diagnosis of microscopic colitis in patients with chronic diarrhoea. Gastroenterol. Hepatol. 2017, 40, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bañares, F.; Esteve, M.; Espinós, J.C.; Rosinach, M.; Forné, M.; Salas, A.; Viver, J.M. Drug Consumption and the Risk of Microscopic Colitis. Am. J. Gastroenterol. 2007, 102, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Cave, D.; Marshall, C. Microscopic colitis: A review of etiology, treatment and refractory disease. World J. Gastroenterol. 2015, 21, 8804–8810. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.F.; Tontini, G.E.; Vecchi, M.; Pastorelli, L. Microscopic Colitis. Inflamm. Bowel Dis. 2016, 22, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Law, E.H.; Badowski, M.; Hung, Y.T.; Weems, K.; Sanchez, A.; Lee, T.A. Association between Proton Pump Inhibitors and Microscopic Colitis. Ann. Pharmacother. 2017, 51, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Macaigne, G.; Lahmek, P.; Locher, C.; Boivin, J.F.; Lesgourgues, B.; Yver, M.; Costes, L.; Alsamad, I.A.; Cucherousset, J.; Charpignon, C.; et al. Over 90% of cases of Microscopic Colitis can be diagnosed by performing a short colonoscopy. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Pardi, D.S. Diagnosis and management of microscopic colitis. Am. J. Gastroenterol. 2017, 112, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Platz-Baudin, C.; Katzenberger, T.; Eck, M. Microscopic colitis: Histopathological review with a clinicopathological correlation. Pathologe 2011, 32, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, D.; Mokrani, N.; Fléjou, J.F. Microscopic colitis: Collagenous colitis and lymphocytic colitis. Ann. Pathol. 2007, 27, 448–458. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. High densities of serotonin and peptide YY cells in the colon of patients with lymphocytic colitis. World J. Gastroenterol. 2012, 18, 6070–6075. [Google Scholar] [CrossRef] [PubMed]
- Boznańska, P.; Wichan, P.; Stepień, A.; Wiśniewska-Jarosńska, M.; Smigielski, J.; Szadkowski, K.; Stec-Michalska, K. 24-hour urinary 6-hydroxymelatonin sulfate excretion in patients with ulcerative colitis. Pol. Merkur. Lekarski 2007, 22, 369–372. [Google Scholar] [PubMed]
- Chen, M.; Mei, Q.; Xu, J.; Lu, C.; Fang, H.; Liu, X. Detection of melatonin and homocysteine simultaneously in ulcerative colitis. Clin. Chim. Acta 2012, 413, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Binder, M.; Loftus, E.V., Jr.; Abboud, R.; McNally, M.A.; Smyrk, T.C.; Tremaine, W.J.; Sandborn, W.J.; Pardi, D.S. Development of a Microscopic Colitis Disease Activity Index: A prospective cohort study. Gut 2016. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.S.; Sood, R.; Law, G.R.; Gracie, D.J.; To, N.; Gold, M.J.; Ford, A.C. Validation and modification of a diagnostic scoring system to predict microscopic colitis. Scand. J. Gastroenterol. 2016, 51, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Liszka, Ł.; Woszczyk, D.; Pajak, J. Histopathological diagnosis of microscopic colitis. J. Gastroenterol. Hepatol. 2006, 21, 792–997. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kim, T.K. Is microscopic colitis really microscopic? Gut Liver 2015, 9, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Han, D.S.; Ro, Y.O.; Eun, C.S.; Yoo, K.S. Does lymphocytic colitis always present with normal endoscopic findings? Gut Liver 2015, 9, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Aust, D.E. Histopathology of microscopic colitis. Pathologe 2012, 33 (Suppl. 2), 221–224. [Google Scholar] [CrossRef] [PubMed]
- Langner, C.; Aust, D.; Ensari, A.; Villanacci, V.; Becheanu, G.; Miehlke, S.; Geboes, K.; Münch, A. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology 2015, 66, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.F.; Tontini, G.E.; Marinoni, B.; Villanacci, V.; Bruni, B.; Vecchi, M.; Pastorelli, L. Biomarkers and Microscopic Colitis: An Unmet Need in Clinical Practice. Front. Med. 2017, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Lomholt-Beck, B.; Gundersen, T.D. High chromogranin A cell density in the colon of patients with lymphocytic colitis. Mol. Med. Rep. 2011, 4, 603–605. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Chromogranin A cell density as a diagnostic marker for lymphocytic colitis. Dig. Dis. Sci. 2012, 57, 3154–3159. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.C.; Jenkins, D.; Thornley, J.P.; Hebden, J.M.; Wright, T.; Skinner, M.; Neal, K.R. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000, 47, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, S.P.; Jenkins, D.; Neal, K.R.; Spiller, R.C. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 2003, 125, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; Garcia-Maurino, S.; Reiter, R.J.; Guerro, J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intacrine, autocrine, and/or paracrine substance. FASEB J. 2004, 18, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.J.; Huang, S.H.; Chen, S.J.; Wang, C.H.; Chang, D.M.; Sytwu, H.K. Modulation by melatonin of the pathogenesis of inflammatory autoimmune diseases. Int. J. Mol. Sci. 2013, 14, 11742–11766. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Wisniewska-Jarosinska, M.; Walecka-Kapica, E.; Klupinska, G.; Jaworek, J.; Chojnacki, J. Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J. Physiol. Pharmacol. 2011, 62, 327–334. [Google Scholar] [PubMed]
- Jena, G.; Trivedi, P.P. A review of the use of melatonin in ulcerative colitis: Experimental evidence and new approaches. Inflamm. Bowel Dis. 2014, 20, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Zubero, E.; López-Pingarrón, L.; Alatorre-Jiménez, M.A.; Ochoa-Moneo, P.; Buisac-Ramón, C.; Rivas-Jiménez, M.; Castán-Ruiz, S.; Antoñanzas-Lombarte, Á.; Tan, D.X.; García, J.J.; et al. Melatonin’s role as a co-adjuvant treatment in colonic diseases: A review. Life Sci. 2017, 170, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Khurshid, Z.; Zohaib, S.; Zafar, M.S. Therapeutic potential of melatonin in oral medicine and peridontology. Kaohsiung J. Med. Sci. 2016, 32, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Werbach, M.R. Melatonin for the treatment of gastroesophageal reflux disease. Altern. Ther. Health Med. 2008, 14, 534–558. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| Features | Control Group (n = 30) | Ulcerative Colitis (n = 30) | Lymphocytic Colitis (n = 30) | |
|---|---|---|---|---|
| Age—years | 38.9 ± 9.4 | 41.4 ± 10.2 | 43.7 ± 12.4 | |
| Gender | M | 16 | 13 | 14 |
| K | 14 | 17 | 16 | |
| CRP (mg/L) | 1.97 ± 1.05 | 242.9 ± 99.4 | 2.58 ± 1.81 | |
| FC (µ/g) | 25.8 ± 9.72 | 498.6 ± 338.3 | 40.8 ± 21.0 | |
| aMT6s (µg/24 h) | 13.4 ± 4.87 | 7.86 ± 2.69 | 19.2 ± 6.14 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnacki, C.; Błasiak, J.; Fichna, J.; Chojnacki, J.; Popławski, T. Evaluation of Melatonin Secretion and Metabolism Exponents in Patients with Ulcerative and Lymphocytic Colitis. Molecules 2018, 23, 272. https://doi.org/10.3390/molecules23020272
Chojnacki C, Błasiak J, Fichna J, Chojnacki J, Popławski T. Evaluation of Melatonin Secretion and Metabolism Exponents in Patients with Ulcerative and Lymphocytic Colitis. Molecules. 2018; 23(2):272. https://doi.org/10.3390/molecules23020272
Chicago/Turabian StyleChojnacki, Cezary, Janusz Błasiak, Jakub Fichna, Jan Chojnacki, and Tomasz Popławski. 2018. "Evaluation of Melatonin Secretion and Metabolism Exponents in Patients with Ulcerative and Lymphocytic Colitis" Molecules 23, no. 2: 272. https://doi.org/10.3390/molecules23020272
APA StyleChojnacki, C., Błasiak, J., Fichna, J., Chojnacki, J., & Popławski, T. (2018). Evaluation of Melatonin Secretion and Metabolism Exponents in Patients with Ulcerative and Lymphocytic Colitis. Molecules, 23(2), 272. https://doi.org/10.3390/molecules23020272






