Genomic Insights into Host Susceptibility to Periprosthetic Joint Infections: A Comprehensive Literature Review
Abstract
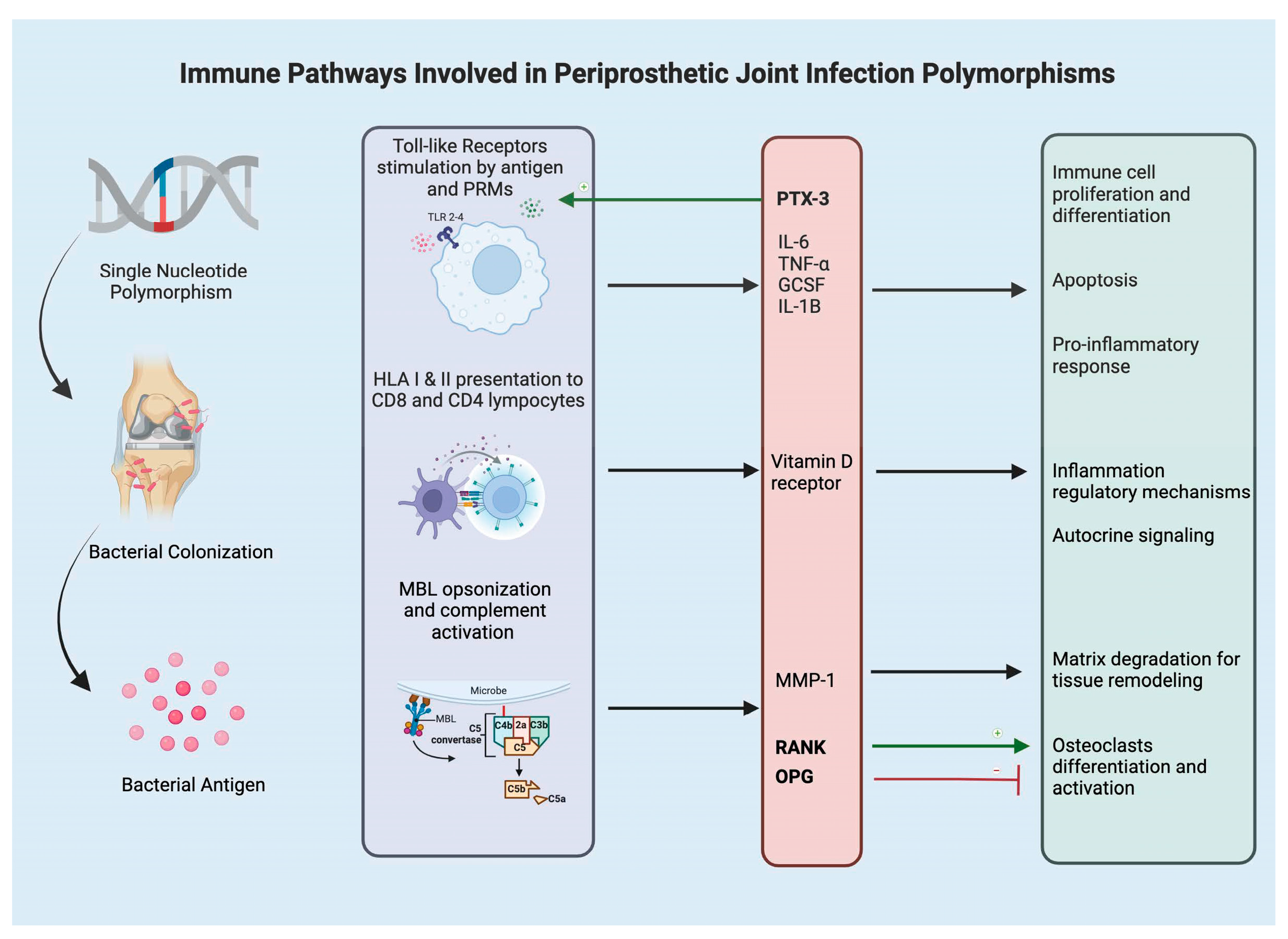
:1. Introduction
2. Toll Like Receptors and Humoral Pattern Recognition Molecules
3. Cytokines and Chemokines
4. Mannose-Binding Lectin
5. Bone Metabolism (VDR, OPG, MMP)
6. Human Leukocyte Antigen
7. Future of Genetic Testing and PJI
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- American Joint Replacement Registry (AJRR) 2023 Anual Report; American Academy of Orthopaedic Surgeons (AAOS): Rosemont, IL, USA, 2023; pp. 1–121.
- Siddiqi, A.; Warren, J.A.; Manrique-Succar, J.; Molloy, R.M.; Barsoum, W.K.; Piuzzi, N.S. Temporal Trends in Revision Total Hip and Knee Arthroplasty from 2008 to 2018: Gaps and Opportunities. J. Bone Jt. Surg. 2021, 103, 1335–1354. [Google Scholar] [CrossRef] [PubMed]
- Corona, P.S.; Vicente, M.; Carrera, L.; Rodríguez-Pardo, D.; Corró, S. Current Actual Success Rate of the Two-Stage Exchange Arthroplasty Strategy in Chronic Hip and Knee Periprosthetic Joint Infection: Insights into Non-Completed Second-Stage Cases. Bone Jt. J. 2020, 102-B, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.H.; Barbosa, T.A.; Esteves, J.; Abreu, M.; Soares, D.; Sousa, R. Early Debridement, Antibiotics and Implant Retention (DAIR) in Patients with Suspected Acute Infection after Hip or Knee Arthroplasty—Safe, Effective and without Negative Functional Impact. J. Bone Jt. Infect. 2019, 4, 300–305. [Google Scholar] [CrossRef]
- Jevnikar, B.E.; Khan, S.T.; Huffman, N.; Pasqualini, I.; Surace, P.A.; Deren, M.E.; Piuzzi, N.S. Advancements in Treatment Strategies for Periprosthetic Joint Infections: A Comprehensive Review. J. Clin. Orthop. Trauma 2024, 55, 102496. [Google Scholar] [CrossRef]
- Piuzzi, N.S.; Klika, A.K.; Lu, Q.; Higuera-Rueda, C.A.; Stappenbeck, T.; Visperas, A. Periprosthetic Joint Infection and Immunity: Current Understanding of Host–Microbe Interplay. J. Orthop. Res. 2024, 42, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Chisari, E.; Cho, J.; Wouthuyzen-Bakker, M.; Parvizi, J. Periprosthetic Joint Infection and the Trojan Horse Theory: Examining the Role of Gut Dysbiosis and Epithelial Integrity. J. Arthroplast. 2022, 37, 1369–1374. [Google Scholar] [CrossRef]
- Harmer, J.R.; Wyles, C.C.; Duong, S.Q.; Morgan Iii, R.J.; Maradit-Kremers, H.; Abdel, M.P. Depression and Anxiety Are Associated with an Increased Risk of Infection, Revision, and Reoperation Following Total Hip or Knee Arthroplasty. Bone Jt. J. 2023, 105, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Anis, H.K.; Warren, J.A.; Klika, A.K.; Navale, S.M.; Zhou, G.; Barsoum, W.K.; Higuera, C.A.; Piuzzi, N.S. Greater Prevalence of Mental Health Conditions in Septic Revision Total Knee Arthroplasty: A Call to Action. J. Knee Surg. 2022, 35, 190–197. [Google Scholar] [CrossRef]
- Scarcella, N.R.; Mills, F.B.; Seidelman, J.L.; Jiranek, W.A. The Effect of Nutritional Status in the Treatment of Periprosthetic Joint Infections in Total Hip Arthroplasty. J. Arthroplast. 2024, 39, S225–S228. [Google Scholar] [CrossRef]
- Emara, A.K.; Hadad, M.J.; Dube, M.; Klika, A.K.; Burguera, B.; Piuzzi, N.S. Team Approach: Nutritional Assessment and Interventions in Elective Hip and Knee Arthroplasty. JBJS Rev. 2022, 10, e21. [Google Scholar] [CrossRef]
- Magruder, M.L.; Yao, V.J.H.; Rodriguez, A.N.; Ng, M.K.; Sasson, V.; Erez, O. Does Semaglutide Use Decrease Complications and Costs Following Total Knee Arthroplasty? J. Arthroplast. 2023, 38, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Koks, S.; Wood, D.J.; Reimann, E.; Awiszus, F.; Lohmann, C.H.; Bertrand, J.; Prans, E.; Maasalu, K.; Märtson, A. The Genetic Variations Associated with Time to Aseptic Loosening After Total Joint Arthroplasty. J. Arthroplast. 2020, 35, 981–988. [Google Scholar] [CrossRef]
- Veronesi, F.; Tschon, M.; Fini, M. Gene Expression in Osteolysis: Review on the Identification of Altered Molecular Pathways in Preclinical and Clinical Studies. Int. J. Mol. Sci. 2017, 18, 499. [Google Scholar] [CrossRef] [PubMed]
- MacInnes, S.J.; Hatzikotoulas, K.; Fenstad, A.M.; Shah, K.; Southam, L.; Tachmazidou, I.; Hallan, G.; Dale, H.; Panoutsopoulou, K.; Furnes, O.; et al. The 2018 Otto Aufranc Award: How Does Genome-Wide Variation Affect Osteolysis Risk After THA? Clin. Orthop. Relat. Res. 2019, 477, 297–309. [Google Scholar] [CrossRef]
- Brüggemann, A.; Eriksson, N.; Michaëlsson, K.; Hailer, N.P. Risk of Revision After Arthroplasty Associated with Specific Gene Loci: A Genomewide Association Study of Single-Nucleotide Polymorphisms in 1,130 Twins Treated with Arthroplasty. J. Bone Jt. Surg. 2022, 104, 610. [Google Scholar] [CrossRef]
- Anderson, M.B.; Curtin, K.; Wong, J.; Pelt, C.E.; Peters, C.L.; Gililland, J.M. Familial Clustering Identified in Periprosthetic Joint Infection Following Primary Total Joint Arthroplasty: A Population-Based Cohort Study. J. Bone Jt. Surg. 2017, 99, 905–913. [Google Scholar] [CrossRef]
- Neufeld, M.E.; Sheridan, G.A.; MacDonell, T.; Howard, L.C.; Masri, B.A.; Keown, P.; Sherwood, K.; Garbuz, D.S. The John Charnley Award: The Impact of Human Leukocyte Antigen Genotype on Bacterial Infection Rates and Successful Eradication in Total Hip Arthroplasty. J. Arthroplast. 2024, 39, S17–S23.e4. [Google Scholar] [CrossRef]
- Granata, V.; Strina, D.; Possetti, V.; Leone, R.; Valentino, S.; Chiappetta, K.; Loppini, M.; Mantovani, A.; Bottazzi, B.; Asselta, R.; et al. Interleukin-1β Polymorphisms Are Genetic Markers of Susceptibility to Periprosthetic Joint Infection in Total Hip and Knee Arthroplasty. Genes 2024, 15, 596. [Google Scholar] [CrossRef]
- Hijazi, A.; Hasan, A.; Pearl, A.; Memon, R.; Debeau, M.; Roldan, M.; Awad, M.E.; Abdul-Kabir, E.; Saleh, K.J. Genetic Polymorphisms Associated with Perioperative Joint Infection Following Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1187. [Google Scholar] [CrossRef]
- Zhou, X.; Yishake, M.; Li, J.; Jiang, L.; Wu, L.; Liu, R.; Xu, N. Genetic Susceptibility to Prosthetic Joint Infection Following Total Joint Arthroplasty: A Systematic Review. Gene 2015, 563, 76–82. [Google Scholar] [CrossRef]
- El-Helou, O.; Berbari, E.F.; Brown, R.A.; Gralewski, J.H.; Osmon, D.R.; Razonable, R.R. Functional Assessment of Toll-like Receptor 2 and Its Relevance in Patients with Staphylococcus Aureus Infection of Joint Prosthesis. Hum. Immunol. 2011, 72, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mrazek, F.; Gallo, J.; Stahelova, A.; Petrek, M. Coding Variants of TLR2 and TLR4 Genes Do Not Substantially Contribute to Prosthetic Joint Infection. Inflamm. Res. 2013, 62, 483–487. [Google Scholar] [CrossRef]
- Stahelova, A.; Mrazek, F.; Smizansky, M.; Petrek, M.; Gallo, J. Variation in the IL1B, TNF and IL6 Genes and Individual Susceptibility to Prosthetic Joint Infection. BMC Immunol 2012, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Erdemli, B.; Özbek, E.A.; Başarir, K.; Karahan, Z.C.; Öcal, D.; Biriken, D. Proinflammatory Biomarkers’ Level and Functional Genetic Polymorphisms in Periprosthetic Joint Infection. Acta Orthop. Traumatol. Turc. 2018, 52, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.H.A.; Jury, F.; Bayat, A.; Ollier, W.E.R.; Kay, P.R. Genetic Susceptibility to Total Hip Arthroplasty Failure: A Preliminary Study on the Influence of Matrix Metalloproteinase 1, Interleukin 6 Polymorphisms and Vitamin D Receptor. Ann. Rheum Dis. 2007, 66, 1116–1120. [Google Scholar] [CrossRef]
- Malik, M.H.A.; Bayat, A.; Jury, F.; Kay, P.R.; Ollier, W.E.R. Genetic Susceptibility to Total Hip Arthroplasty Failure—Positive Association with Mannose-Binding Lectin. J. Arthroplast. 2007, 22, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, Z.; Gallo, J.; Mrazek, F.; Lostak, J.; Petrek, M. MBL2 Gene Variation Affecting Serum MBL Is Associated with Prosthetic Joint Infection in Czech Patients after Total Joint Arthroplasty. Tissue Antigens 2012, 80, 444–451. [Google Scholar] [CrossRef]
- Malik, M.H.A.; Bayat, A.; Jury, F.; Ollier, W.E.R.; Kay, P.R. Genetic Susceptibility to Hip Arthroplasty Failure—Association with the RANK/OPG Pathway. Int. Orthop. (SICO) 2006, 30, 177–181, Erratum in Int. Orthop. (SICO) 2009, 33, 297. [Google Scholar] [CrossRef]
- Navratilova, Z.; Gallo, J.; Smizansky, M.; Mrazek, F.; Petrek, M. Osteoprotegerin Gene Polymorphism Is Not Associated with Prosthetic Joint Infection after Total Joint Arthroplasty in the Czech Population. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2014, 158, 273–276. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef]
- Simpson, M.E.; Petri, W.A. TLR2 as a Therapeutic Target in Bacterial Infection. Trends Mol. Med. 2020, 26, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Hanzelmann, D.; Joo, H.-S.; Franz-Wachtel, M.; Hertlein, T.; Stevanovic, S.; Macek, B.; Wolz, C.; Götz, F.; Otto, M.; Kretschmer, D.; et al. Toll-like Receptor 2 Activation Depends on Lipopeptide Shedding by Bacterial Surfactants. Nat. Commun. 2016, 7, 12304. [Google Scholar] [CrossRef] [PubMed]
- Elson, G.; Dunn-Siegrist, I.; Daubeuf, B.; Pugin, J. Contribution of Toll-like Receptors to the Innate Immune Response to Gram-Negative and Gram-Positive Bacteria. Blood 2007, 109, 1574–1583. [Google Scholar] [CrossRef]
- Galliera, E.; Drago, L.; Vassena, C.; Romanò, C.; Gioia Marazzi, M.; Salcito, L.; Corsi Romanelli, M.M. Toll-Like Receptor 2 in Serum: A Potential Diagnostic Marker of Prosthetic Joint Infection? J. Clin. Microbiol. 2014, 52, 620–623. [Google Scholar] [CrossRef]
- Chen, M.-F.; Chang, C.-H.; Hu, C.-C.; Wu, Y.-Y.; Chang, Y.; Ueng, S.W.N. Periprosthetic Joint Infection Caused by Gram-Positive Versus Gram-Negative Bacteria: Lipopolysaccharide, but Not Lipoteichoic Acid, Exerts Adverse Osteoclast-Mediated Effects on the Bone. J. Clin. Med. 2019, 8, 1289. [Google Scholar] [CrossRef]
- Visperas, A.; Santana, D.; Klika, A.K.; Higuera-Rueda, C.A.; Piuzzi, N.S. Current Treatments for Biofilm-associated Periprosthetic Joint Infection and New Potential Strategies. J. Orthop. Res. 2022, 40, 1477–1491. [Google Scholar] [CrossRef]
- Porte, R.; Davoudian, S.; Asgari, F.; Parente, R.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Humoral Innate Immunity Functional Player and Biomarker of Infections and Sepsis. Front. Immunol. 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Loppini, M.; Di Maio, M.; Avigni, R.; Leone, R.; Inforzato, A.; Grappiolo, G.; Mantovani, A.; Bottazzi, B. Long Pentraxin 3 as a New Biomarker for Diagnosis of Hip and Knee Periprosthetic Joint Infections. J. Clin. Med. 2023, 12, 1055. [Google Scholar] [CrossRef]
- Gollwitzer, H.; Dombrowski, Y.; Prodinger, P.M.; Peric, M.; Summer, B.; Hapfelmeier, A.; Saldamli, B.; Pankow, F.; Von Eisenhart-Rothe, R.; Imhoff, A.B.; et al. Antimicrobial Peptides and Proinflammatory Cytokines in Periprosthetic Joint Infection. J. Bone Jt. Surg. 2013, 95, 644–651. [Google Scholar] [CrossRef]
- Deirmengian, C.; Hallab, N.; Tarabishy, A.; Valle, C.D.; Jacobs, J.J.; Lonner, J.; Booth, R.E. Synovial Fluid Biomarkers for Periprosthetic Infection. Clin. Orthop. Relat. Res. 2010, 468, 2017–2023. [Google Scholar] [CrossRef]
- Masters, T.L.; Bhagwate, A.V.; Dehankar, M.K.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Mandrekar, J.N.; Patel, R. Human Transcriptomic Response to Periprosthetic Joint Infection. Gene 2022, 825, 146400. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Greis, K.D. Granulocyte Colony Stimulating Factor Receptor (G-CSFR) Signaling in Severe Congenital Neutropenia, Chronic Neutrophilic Leukemia and Related Malignancies. Exp. Hematol. 2017, 46, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Winthrop, K.; Calabrese, L. The Role of IL-6 in Host Defence against Infections: Immunobiology and Clinical Implications. Nat. Rev. Rheumatol. 2017, 13, 399–409. [Google Scholar] [CrossRef]
- Van Loo, G.; Bertrand, M.J.M. Death by TNF: A Road to Inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Arvieux, C.; Common, H. New Diagnostic Tools for Prosthetic Joint Infection. Orthop. Traumatol. Surg. Res. 2019, 105, S23–S30. [Google Scholar] [CrossRef]
- Yeganeh, M.H.; Kheir, M.M.; Shahi, A.; Parvizi, J. Rheumatoid Arthritis, Disease Modifying Agents, and Periprosthetic Joint Infection: What Does a Joint Surgeon Need to Know? J. Arthroplast. 2018, 33, 1258–1264. [Google Scholar] [CrossRef]
- Qin, L.; Du, C.; Yang, J.; Wang, H.; Su, X.; Wei, L.; Zhao, C.; Chen, C.; Chen, H.; Hu, N.; et al. Synovial Fluid Interleukin Levels Cannot Distinguish between Prosthetic Joint Infection and Active Rheumatoid Arthritis after Hip or Knee Arthroplasty. Diagnostics 2022, 12, 1196. [Google Scholar] [CrossRef]
- Navratilova, Z.; Gallo, J.; Mrazek, F.; Petrek, M. Genetic Variation in Key Molecules of the Th-17 Immune Response Is Not Associated with Risk for Prosthetic Joint Infection in a Czech Population. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2012, 156, 248–252. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. The Ambiguous Role of Mannose-Binding Lectin (MBL) in Human Immunity. Open Med. 2021, 16, 299–310. [Google Scholar] [CrossRef]
- Takahashi, K.; Ezekowitz, R.A.B. The Role of the Mannose-Binding Lectin in Innate Immunity. Clin. Infect. Dis. 2005, 41, S440–S444. [Google Scholar] [CrossRef]
- Campbell, M.J.; Bustamante-Gomez, C.; Fu, Q.; Beenken, K.E.; Reyes-Pardo, H.; Smeltzer, M.S.; O’Brien, C.A. RANKL-Mediated Osteoclast Formation Is Required for Bone Loss in a Murine Model of Staphylococcus Aureus Osteomyelitis. Bone 2024, 187, 117181. [Google Scholar] [CrossRef]
- Takács, I.; Lazáry, Á.; Kósa, J.P.; Kiss, J.; Balla, B.; Nagy, Z.; Bácsi, K.; Speer, G.; Lakatos, P. Allelic Variations of RANKL/OPG Signaling System Are Related to Bone Mineral Density and in Vivo Gene Expression. Eur. J. Endocrinol. 2010, 162, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, K.; Tanabe, N.; Suzuki, N.; Mitsui, N.; Oka, H.; Ito, K.; Maeno, M. The Effect of IL-1α on the Expression of Matrix Metalloproteinases, Plasminogen Activators, and Their Inhibitors in Osteoblastic ROS 17/2.8 Cells. Life Sci. 2006, 78, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Konttinen, Y.T.; Kemppinen, P.; Sorsa, T.; Tschesche, H.; Bläser, J.; Suda, A.; Santavirta, S. Tissue Inhibitor of Metalloproteinase 1, Collagenolytic and Gelatinolytic Activity in Loose Hip Endoprostheses. J. Rheumatol. 1995, 22, 2285–2290. [Google Scholar] [PubMed]
- Godoy-Santos, A.L.; D’Elia, C.O.; Teixeira, W.J.; Cabrita, H.B.; Camanho, G.L. Aseptic Loosening of Total Hip Arthroplasty: Preliminary Genetic Investigation. J. Arthroplast. 2009, 24, 297–302. [Google Scholar] [CrossRef]
- Montes, A.H.; Valle-Garay, E.; Alvarez, V.; Pevida, M.; García Pérez, E.; Paz, J.; Meana, A.; Asensi, V. A Functional Polymorphism in MMP1 Could Influence Osteomyelitis Development. J. Bone Miner. Res. 2010, 25, 912–919. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, C.; Guo, Y. The Association of OPG Polymorphisms with Risk of Osteoporotic Fractures: A Systematic Review and Meta-Analysis. Medicine 2021, 100, e26716. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tang, K.; Quan, Z.; Zhao, Z.; Jiang, D. Association Between Seven Common OPG Genetic Polymorphisms and Osteoporosis Risk: A Meta-Analysis. DNA Cell Biol. 2014, 33, 29–39. [Google Scholar] [CrossRef]
- Pasqualini, I.; Huffman, N.; Keller, S.F.; McLaughlin, J.P.; Molloy, R.M.; Deren, M.E.; Piuzzi, N.S. Team Approach: Bone Health Optimization in Orthopaedic Surgery. JBJS Rev. 2023, 11, e23. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.J.; Van Leeuwen, H.; Pols, H.A.P. Vitamin D Receptor Gene Polymorphisms in Relation to Vitamin D Related Disease States. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 187–193. [Google Scholar] [CrossRef]
- Mohammadifard, N.; Sadeghian, L.; Hassannejad, R.; Khosravi, E.; Gharipour, M.; Karimi, S.; Hosseini, S.; Sepahifar, M.; Bahrami, G.; Haghighatdoost, F.; et al. Comparing Vitamin D Receptor Gene Polymorphisms in Rs11568820, Rs7970314, Rs4334089 between COVID-19 Patients with Mild and Severe Symptoms: A Case Control Study. Sci. Rep. 2024, 14, 10170. [Google Scholar] [CrossRef] [PubMed]
- Zhai, N.; Bidares, R.; Makoui, M.H.; Aslani, S.; Mohammadi, P.; Razi, B.; Imani, D.; Yazdchi, M.; Mikaeili, H. Vitamin D Receptor Gene Polymorphisms and the Risk of the Type 1 Diabetes: A Meta-Regression and Updated Meta-Analysis. BMC Endocr. Disord. 2020, 20, 121. [Google Scholar] [CrossRef]
- Smolders, J.; Peelen, E.; Thewissen, M.; Menheere, P.; Cohen Tervaert, J.W.; Hupperts, R.; Damoiseaux, J. The Relevance of Vitamin D Receptor Gene Polymorphisms for Vitamin D Research in Multiple Sclerosis. Autoimmun. Rev. 2009, 8, 621–626. [Google Scholar] [CrossRef]
- Emara, A.K.; Nageeb, E.; George, J.; Buttaro, M.A.; Higuera, C.; Piuzzi, N.S. Hypovitaminosis D in Lower Extremity Joint Arthroplasty: A Systematic Review and Meta-Analysis. J. Orthop. 2020, 21, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Piuzzi, N.S.; George, J.; Khlopas, A.; Klika, A.K.; Mont, M.A.; Muschler, G.F.; Higuera, C.A. High Prevalence and Seasonal Variation of Hypovitaminosis D in Patients Scheduled for Lower Extremity Total Joint Arthroplasty. Ann. Transl. Med. 2018, 6, 321. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Zacharioudaki, M.; Messaritakis, I.; Galanakis, E. Vitamin D Receptor, Vitamin D Binding Protein and CYP27B1 Single Nucleotide Polymorphisms and Susceptibility to Viral Infections in Infants. Sci. Rep. 2021, 11, 13835. [Google Scholar] [CrossRef]
- Roth, D.E.; Jones, A.B.; Prosser, C.; Robinson, J.L.; Vohra, S. Vitamin D Receptor Polymorphisms and the Risk of Acute Lower Respiratory Tract Infection in Early Childhood. J. Infect. Dis. 2008, 197, 676–680. [Google Scholar] [CrossRef]
- Wu, S.; Liao, A.P.; Xia, Y.; Chun Li, Y.; Li, J.-D.; Sartor, R.B.; Sun, J. Vitamin D Receptor Negatively Regulates Bacterial-Stimulated NF-κB Activity in Intestine. Am. J. Pathol. 2010, 177, 686–697. [Google Scholar] [CrossRef]
- Chisari, E.; D’Mello, D.; Sherman, M.B.; Parvizi, J. Inflammatory Bowel Diseases Increase the Risk of Periprosthetic Joint Infection. J. Bone Jt. Surg. 2022, 104, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Medhasi, S.; Chantratita, N. Human Leukocyte Antigen (HLA) System: Genetics and Association with Bacterial and Viral Infections. J. Immunol. Res. 2022, 2022, 9710376. [Google Scholar] [CrossRef] [PubMed]
- Crux, N.B.; Elahi, S. Human Leukocyte Antigen (HLA) and Immune Regulation: How Do Classical and Non-Classical HLA Alleles Modulate Immune Response to Human Immunodeficiency Virus and Hepatitis C Virus Infections? Front. Immunol. 2017, 8, 832. [Google Scholar] [CrossRef]
- Weiss, S.; Holtfreter, S.; Meyer, T.C.; Schmiedeke, F.; Cammann, C.; Dörr, M.; Felix, S.B.; Grabe, H.J.; Homuth, G.; Kohler, C.; et al. Toxin Exposure and HLA Alleles Determine Serum Antibody Binding to Toxic Shock Syndrome Toxin 1 (TSST-1) of Staphylococcus Aureus. Front. Immunol. 2023, 14, 1229562. [Google Scholar] [CrossRef]
- DeLorenze, G.N.; Nelson, C.L.; Scott, W.K.; Allen, A.S.; Ray, G.T.; Tsai, A.-L.; Charles, P.; Quesenberry, J.; Vance, G.; Fowler, J. Polymorphisms in HLA Class II Genes Are Associated with Susceptibility to Staphylococcus Aureus Infection in a White Population. J. Infect. Dis. 2015, 213, 816. [Google Scholar] [CrossRef] [PubMed]
- Cyr, D.D.; Allen, A.S.; Du, G.-J.; Ruffin, F.; Adams, C.; Thaden, J.T.; Maskarinec, S.A.; Souli, M.; Guo, S.; Dykxhoorn, D.M.; et al. Evaluating Genetic Susceptibility to Staphylococcus Aureus Bacteremia in African Americans Using Admixture Mapping. Genes Immun. 2017, 18, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Jaén, M.; Martín-Regalado, Á.; Bartolomé, R.A.; Robles, J.; Casal, J.I. Interleukin 13 Receptor Alpha 2 (IL13Rα2): Expression, Signaling Pathways and Therapeutic Applications in Cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188802. [Google Scholar] [CrossRef]
- Washington, A.; Varki, N.; Valderrama, J.A.; Nizet, V.; Bui, J.D. Evaluation of Interleukin-17D in Host Immunity to Group A Streptococcus Infection. J. Immunol. 2020, 205, 3122–3129. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Li, H.; Cheng, C.-K.; Zhang, J. Genetic and Modifiable Risk Factors for Postoperative Complications of Total Joint Arthroplasty: A Genome-Wide Association and Mendelian Randomization Study. Bioengineering 2024, 11, 797. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Wen, S.-H. Integrating Genome-Wide Polygenic Risk Scores with Nongenetic Models to Predict Surgical Site Infection After Total Knee Arthroplasty Using United Kingdom Biobank Data. J. Arthroplast. 2024, 39, 2471–2477.e1. [Google Scholar] [CrossRef]
| Risk Factor | Author | Reference Number | SNP/Genotype | Alleles | Number of Subjects Tested for SNP | ||
|---|---|---|---|---|---|---|---|
| PJI Group | Aseptic Control Group | Healthy Control Group | |||||
| TLR-2 | El-Helou (2011) | [22] | rs5743708 | G/A | 66 | - | 57 |
| Mrazek (2013) | [23] | rs5743708 | G/A | 98 | - | 252 | |
| TL-4 | Mrazek (2013) | [23] | rs4986790 | A/G,T | 98 | - | 252 |
| rs4986791 | C/T | 98 | - | 252 | |||
| PTX3 | Granata § (2014) | [19] | rs2305619 | A/G,T | 46 | - | 47 |
| rs3816527 | C/A,T | 46 | - | 47 | |||
| rs1840680 | A/C,G,T | 46 | - | 47 | |||
| IL-1B | Stahelova (2012) | [24] | rs16944 | A/G | 89 | 214 | 188 |
| rs1143634 | G/A | 89 | 214 | 188 | |||
| Granata (2014) | [19] | rs2853550 | A/G,T | 46 | - | 47 | |
| Erdemli (2018) | [25] | rs1143623 | C/A,G | 36 | 52 | - | |
| GCSF | Erdemli (2018) | [25] | rs3769817 | T/A,C,G | 36 | 52 | - |
| IL-6 | Erdemli (2018) | [25] | rs1800795 | C/G,T | 36 | 52 | - |
| Malik (2007) | [26] | rs1800795 | C/G,T | 63 | 88 | 188 | |
| Stahelova (2012) | [24] | rs1800795 | C/G,T | 89 | 214 | 188 | |
| [24] | rs1800796 | G/A,C | 89 | 214 | 188 | ||
| Granata (2024) | [19] | rs1800796 | G/A,C | 46 | - | 47 | |
| [19] | rs1800797 | A/C,G,T | 46 | - | 47 | ||
| TNF-α | Erdemli (2018) | [25] | rs361525 | G/A | 36 | 52 | - |
| Stahelova (2012) | [24] | rs361525 | G/A | 89 | 214 | 188 | |
| rs1800629 | G/A | 89 | 214 | 188 | |||
| MLB | Malik (2007) | [27] | rs11003125 | G/C | 144 | 91 | 62 |
| rs7096206 | G/A,C,T | 148 | 91 | 62 | |||
| rs5030737 | G/A,T | 145 | 91 | 62 | |||
| rs1800450 | C/T | 148 | 91 | 61 | |||
| Navratilova (2012) | [28] | rs11003125 | G/C | 112 | 245 | 196 | |
| rs7096206 | G/A,C,T | 112 | 245 | 196 | |||
| rs1800450 | G/A | 112 | 245 | 196 | |||
| OPG | Malik (2006-2009) | [29] | rs2073618 | G/C | 53 | 91 | 150 |
| rs2073617 | G/A,T | 62 | 91 | 149 | |||
| rs3102735 | A/C,G,T | 62 | 89 | 147 | |||
| Navratilova (2012) | [30] | rs3102735 | A/C,G,T | 185 | 251 | 98 | |
| RANK | Malik (2006-2009) | [29] | rs1805034 | C/T | 62 | 85 | 144 |
| MMP-1 | Malik ‡ (2007) | [26] | rs5854 | G/A | 62 | 87 | 148 |
| rs2397776 | T/C | 62 | 88 | 148 | |||
| rs470747 | A/G | 62 | 89 | 147 | |||
| VDR | Malik ‡ (2007) | [26] | rs731236 | A/G,T | 63 | 88 | 148 |
| HLA | Neufeld (2024) | [18] | - | HLA-C∗06:02 | 23 | - | 26 |
| - | HLA-DQA1∗04:01 | 23 | - | 26 | |||
| - | HLA-DQB1∗04:02 | 23 | - | 26 | |||
| - | HLA-C∗03:04 | 23 | - | 26 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizcano, J.D.; Visperas, A.; Piuzzi, N.S.; Abdelbary, H.; Higuera-Rueda, C.A. Genomic Insights into Host Susceptibility to Periprosthetic Joint Infections: A Comprehensive Literature Review. Microorganisms 2024, 12, 2486. https://doi.org/10.3390/microorganisms12122486
Lizcano JD, Visperas A, Piuzzi NS, Abdelbary H, Higuera-Rueda CA. Genomic Insights into Host Susceptibility to Periprosthetic Joint Infections: A Comprehensive Literature Review. Microorganisms. 2024; 12(12):2486. https://doi.org/10.3390/microorganisms12122486
Chicago/Turabian StyleLizcano, Juan D., Anabelle Visperas, Nicolas S. Piuzzi, Hesham Abdelbary, and Carlos A. Higuera-Rueda. 2024. "Genomic Insights into Host Susceptibility to Periprosthetic Joint Infections: A Comprehensive Literature Review" Microorganisms 12, no. 12: 2486. https://doi.org/10.3390/microorganisms12122486
APA StyleLizcano, J. D., Visperas, A., Piuzzi, N. S., Abdelbary, H., & Higuera-Rueda, C. A. (2024). Genomic Insights into Host Susceptibility to Periprosthetic Joint Infections: A Comprehensive Literature Review. Microorganisms, 12(12), 2486. https://doi.org/10.3390/microorganisms12122486







