Abstract
Hepatocrinology is defined as a bidirectional, complex relationship between hepatic physiology and endocrine function, hepatic disease and endocrine dysfunction, hepatotropic drugs and endocrine function, and endocrine drugs and hepatic health. The scope of hepatocrinology includes conditions of varied etiology (metabolic, infectious, autoimmune, and invasive) that we term as hepato-endocrine syndromes. This perspective shares the definition, concept, and scope of hepatocrinology and shares insight related to this aspect of medicine. It is hoped that this communication will encourage further attention and research in this critical field.
1. Introduction
The liver has long been recognized as the seat of the metabolism. Simultaneously, the endocrine system controls the homeostasis of the body. The subject ‘hepatocrinology’ is the field of medicine that studies the bidirectional relationship between hepatic and endocrine physiology, as well as dysfunction. The discipline of hepatocrinology explores the liver as an endocrine gland by describing its production of hormones and its role in hormonal modulation (by synthesizing transport proteins). The hepato-insular axis is a part of hepatocrine physiology []. Endocrine manifestations of liver insufficiency (cirrhosis) and malignancy, and hepatic complications of various endocrine disorders are included. Special attention is paid to hepato-endocrine syndromes in which hepatic and endocrine dysfunction co-occur. The possible hepatotropic effect of endocrine drugs, pleiotropic endocrine consequences of medicines used in the management of liver disease, and potential exaptation of endocrine agents for use in hepatology form part of this science.
2. The Liver as an Endocrine Organ
The liver secretes various hormones, which mediate glucose metabolism, blood pressure, growth, and hemorheological homeostasis. These include insulin-like growth factor (IGF)-1, betatrophin, and irisin, all of which mediate insulin sensitivity [,]. Angiotensinogen, produced by the liver, is the bedrock of the renin-angiotensin-aldosterone system, which contributes to blood pressure maintenance []. Hepcidin and thrombopoietin contribute to the regulation of iron metabolism and platelet production, respectively [,]. The hepato-insular axis is a well-researched contributor to glucose metabolism and has been described variously as the entero-insular or adipo-hepato-insular axis []. There are several other hormones or their precursors that are synthesized by the liver. Some of the important products are summarized in Table 1 and detailed below.

Table 1.
The liver as an endocrine organ.
2.1. Insulin-Like Growth Factor and Insulin-Like Growth Factor Binding Proteins
The IGF and IGF-binding proteins (IGFBPs) are primarily synthesized in the liver and constitute a complex system that plays a critical role in cellular proliferation and differentiation [,]. Growth hormone (GH), secreted from the somatotrophs in the anterior pituitary, drives the synthesis of IGF-1 in the liver. The IGF-1 is a crucial mediator of development during childhood, and the primary determinant of linear growth. In adults, it continues to exert an anabolic effect, and adult GH deficiency (GHD) portends to a negative cardiovascular (CV) outcome []. IGF-2, the other hormone responsible for growth-promoting effects, is widely expressed during fetal development, but synthesized in the liver and epithelial cell lining of the brain surface after birth []. The actions of IGF-1 and IGF-2 are modulated both systemically and locally by six different IGFBP subtypes designated IGFBP-1 through 6 [].
Serum IGF-1 levels are decreased in cirrhosis as the synthetic capacity of the liver is diminished [,]. Hepatic IGF-1 production is also lower in those with higher degrees of steatosis, non-alcoholic fatty liver disease (NAFLD) activity score (NAS), and hepatic fibrosis [,]. Conversely, NAFLD occurs more commonly in adult GHD. GH and IGF-1 prevent NAFLD by decreasing visceral fat, reducing lipogenesis in the hepatocytes, and improving fibrosis by inactivating stellate cells [].
The GH-IGF-1 axis is involved in the pathogenesis of several other endocrine and hepatic disorders. Notable among them is the development of hormone-sensitive cancers. There is emerging evidence that cross-talk between sex steroids and IGF-1 modulates the propensity for the development of breast and prostate cancers [,]. The various pathophysiological effects of the GH-IGF-1 axis are thus orchestrated through IGF and IGFBP synthesized in the liver.
2.2. Angiotensinogen
Hemodynamic homeostasis is governed by hepatic secretory products. A key component among them is angiotensinogen, an alpha-globulin synthesized in multiple tissues []. It is abundantly present in the plasma, and the serum levels are determined by hepatic secretion. Renin from the juxtaglomerular cells of the kidney cleaves angiotensinogen to angiotensin I. Renin-mediated cleavage is tightly regulated and considered the rate-limiting step in the production of biologically active angiotensin peptides []. Angiotensin I is subsequently converted to angiotensin II by the angiotensin-converting-enzyme (ACE) located predominantly on the endothelial cells of the pulmonary vasculature. Angiotensin II plays a pivotal role in controlling blood pressure and sodium homeostasis through its effect on blood vessels, zona glomerulosa of the adrenal cortex, and the kidney []. Additionally, the disequilibrium of the renin-angiotensin-system (RAS) impacts the inflammatory pathways in the lungs and is linked to the development of acute respiratory distress syndrome (ARDS), including the coronavirus disease 2019 (COVID-19) induced lung injury [,].
2.3. Thrombopoietin
The liver is the source of hematopoietic growth factors and iron transport proteins such as hepcidin. Thrombopoietin, a key hematopoietic cytokine synthesized in the liver, induces megakaryocyte progenitor expansion and differentiation. It additionally assists in the maintenance and expansion of hematopoietic stem cells []. Thrombopoietin also determines the lineage of primitive progenitor stem cells and is unique among the hematopoietic cytokines by its effect on both primitive, as well terminally differentiated, cells [].
2.4. Betatrophin and Proprotein Convertase Subtilsin-Kexin Type 9 (PCSK9)
The liver plays a critical role in maintaining lipid balance. Betatrophin, now referred to as angiopoietin-like protein 8 (ANGPTL8), and PCSK9 are critical regulators of lipid metabolism. Though initial reports suggested that betatrophin can stimulate the growth of beta cells of the pancreas, subsequent studies have disproved this []. ANGPTL8 modulates the activity of lipoprotein lipase (LPL) through its interaction with ANGPTL3 and stabilizes triglyceride levels []. Though the exact mechanism by which this hepatocyte-derived factor regulates metabolism is not clearly understood, it has been linked to obesity, diabetes, hypothyroidism, and polycystic ovary syndrome (PCOS). It has the potential to emerge as a critical therapeutic target in the management of metabolic disorders []. PCSK9, synthesized in the liver and several other organs, is a regulatory protein for low-density-lipoprotein (LDL) receptors []. The development of PSCK9 inhibitors as lipid-lowering tools is a significant breakthrough in the management of dyslipidemia and atherosclerotic cardiovascular disease [].
2.5. Hormone-Transport Proteins
The liver produces several important proteins that act as carriers for various hormones and thus indirectly modulate critical endocrine functions. Thyroid-binding globulin, transthyretin, and albumin produced in the liver are all involved in the transportation of thyroxine and tri-iodothyronine []. Cortisol is mainly bound to corticosteroid-binding globulin, again produced from the liver []. Sex hormone-binding globulin not only carries estradiol and testosterone, but can also serve as an early biomarker and a therapeutic target for PCOS []. The levels of these proteins are altered in different physiological and pathological states [,].
3. Endocrine Manifestations of Hepatic Disease
The liver modulates the functioning of the endocrine system directly or indirectly in multiple ways. Liver dysfunction is thus predictably associated with various endocrine disorders. The significant anomalies have been detailed below and depicted in Figure 1.
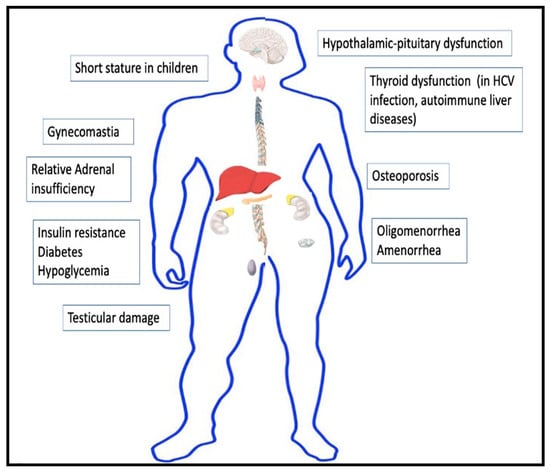
Figure 1.
Endocrine manifestations of cirrhosis.
3.1. Insulin Resistance and Diabetes
Diabetes is a leading cause of the development of NAFLD and cirrhosis. On the other hand, cirrhosis causes insulin resistance and increases the probability of developing diabetes, with a reported prevalence ranging from 30–70% in different studies [,,]. The hyperglycemia arising from liver dysfunction is referred to as hepatogenous diabetes and is pathophysiologically distinct from type 2 diabetes mellitus (T2DM) []. The fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) are often normal in hepatogenous diabetes, and an abnormal oral glucose tolerance test (OGTT) is usually required to establish the diagnosis []. The mechanism of the development of diabetes. in cirrhosis is complex and only partially understood. Insulin resistance from altered secretion of adipokines, inflammatory cytokines, incretins, and free fatty acids play a significant contributory role [,].
Additionally, hypoxia-inducible factors and advanced glycosylation end-products (AGEs) can result in impaired insulin secretion [,]. Hepatitis C virus itself decreases insulin sensitivity by altering insulin signaling and increasing endoplasmic reticulum stress [,,]. Hepatic and systemic insulin resistance often precedes the onset of cirrhosis and is present in individuals with NAFLD [,].
3.2. Hypoglycemia
Hypoglycemia is commonly encountered in patients with advanced cirrhosis, especially if a concurrent infection is present [,]. Hypoglycemia occurs in up to 40% of cases of acute liver failure and is associated with increased mortality [,]. The mechanism behind hypoglycemia is depletion of glycogen stores, decreased gluconeogenesis, and impaired insulin clearance by the liver [,]. Non-islet cell tumor hypoglycemia (NICTH) is a rare paraneoplastic manifestation of hepatocellular carcinoma (HCC) []. Low serum insulin, C-peptide, and beta-hydroxybutyrate in combination with high IGF-2 characterize NICTH [].
3.3. Gonadal Dysfunction
Hypogonadism and gynecomastia are well-recognized manifestations of cirrhosis of the liver. The possible mechanisms include decreased production of sex hormone-binding globulin, decreased hepatic clearance of estrogen, primary testicular defect, hypothalamic-pituitary dysfunction, and direct toxic effects of alcohol on gonads [,]. Women with cirrhosis can manifest menstrual irregularities such as oligomenorrhea or amenorrhea, primarily resulting from hypothalamic-pituitary dysfunction []. Undernutrition and elevated serum prolactin can also produce irregularities in the menstrual cycle [].
Cirrhosis in men can manifest with features of hypogonadism such as loss of secondary sexual characters and decreased libido []. Gynecomastia is reported in up to 44% of men with cirrhosis, and ascribed to the elevated estrogen:testosterone ratio [,]. Testosterone levels are low in patients with cirrhosis, and progressively decrease while the severity of the liver disease increases []. Low testosterone is responsible for body hair loss, sarcopenia, osteoporosis, anemia, and fatigue, and is a marker of increased mortality in cirrhosis [,]. Primary hypogonadism, indicated by an elevation in serum levels of luteinizing hormone (LH), can occur in alcohol-induced cirrhosis. It can be attributed to the direct toxic effect of alcohol on the testis [,]. Hypogonadotropic hypogonadism that partially reverses after liver transplantation is described in most other forms of cirrhosis []. Low testosterone levels stimulate the synthesis of sex-hormone-binding globulin (SHBG) in cirrhosis. SHBG levels are elevated in liver disease, except in advanced stages where the synthetic capacity of the liver is diminished [].
3.4. Skeletal Manifestations
Alteration in bone metabolism generally occurs in cirrhosis of the liver. Hepatic osteodystrophy refers to the skeletal manifestations of cirrhosis and encompasses osteoporosis and, in rare cases, osteomalacia and rickets []. The metabolic bone disease in cirrhosis is multifactorial and results from nutritional factors, proinflammatory state, synthetic defects, and hypogonadism []. Primary biliary cirrhosis (PBC) has been mainly linked to a low bone-turnover state resulting from decreased production of growth factors such as IGF-1, elevated levels of lithocholic acid (known to prevent osteoblast formation), and vitamin K deficiency []. Osteoporosis and fragility fractures are recognized but under-diagnosed complications of cirrhosis, and can be prevented by early diagnosis and treatment [].
3.5. Thyroid Disorders
The autoimmune disorders often tend to coexist, and thyroid dysfunction and high prevalence of thyroid autoantibodies have been observed in autoimmune hepatitis, PBC, and primary sclerosing cholangitis []. Hepatitis C infection is also associated with the development of thyroid disorders []. A meta-analysis of five studies after adjusting for heterogeneity suggested that hepatitis C infection increased the chance of the development of thyroid cancer []. The serum concentration of thyroid-binding globulin (TBG) is elevated in HCC, and normalizes after resection of the tumor [,].
3.6. Adrenal Insufficiency
Adrenal insufficiency is reported in patients with cirrhosis during septic shock and decompensated liver disease []. The term hepato-adrenal syndrome has been used to define relative adrenal insufficiency occurring in patients with cirrhosis. Though the exact mechanism is not clearly understood, diminished hepatic synthesis of cholesterol resulting in the deficiency of substrate for steroid synthesis in the adrenal cortex is a proposed hypothesis [].
3.7. Growth Disorders in Children
Children with cirrhosis commonly exhibit restricted linear growth []. Even though GH levels are high in cirrhosis, decreased IGF-1 and IGFBP3 synthesis by the liver induce growth hormone resistance. Thus, administration of exogenous growth hormone has minimal benefit in children with cirrhosis and short stature []. Liver transplantation partially restores linear growth rate, but delayed puberty and reduced final adult height are still common [].
4. Hepatic Manifestations of Endocrine Disease
Endocrine and metabolic diseases are a common cause of hepatic dysfunction. The common endocrine causes of liver dysfunction have been depicted in Table 2. NAFLD resulting from metabolic disorders such as diabetes, obesity, and dyslipidemia has emerged as one of the leading causes of chronic liver disease over the past two decades. Several other hormonal disturbances affect the functioning of the liver directly or indirectly.

Table 2.
Hepatic manifestations of endocrine disorders.
4.1. Non-Alcoholic Fatty Liver Disease
NAFLD has a bidirectional and complex relationship with metabolic syndrome and insulin resistance. NAFLD refers to a group of disorders characterized by fat accumulation in the liver in the absence of other secondary causes. The spectrum of NAFLD encompasses steatosis or steatohepatitis with associated fibrosis, and can progress to cirrhosis. The risk for HCC is also elevated in patients with NAFLD []. Insulin resistance, a key component of metabolic syndrome, plays an essential role in the pathogenesis of NAFLD []. Obesity, T2DM, and dyslipidemia are strongly associated with the development of NAFLD, though the exact pathophysiologic link is a subject of research [].
Several factors such as genetic and epigenetic factors, nutrition, adipose tissue dysfunction, gut microbiota, inflammation, oxidative stress, adipocytokines, and hepatic iron have been implicated, however the influence of insulin resistance in the pathogenesis of NAFLD remains central []. Uninhibited adipose tissue lipolysis resulting from systemic insulin resistance, coupled with increased lipogenesis leads to increased delivery and deposition of free fatty acids in the liver []. The toxicity of accumulated lipids in hepatic cells triggers further inflammation and damage. Free fatty acids stimulate endoplasmic reticulum stress and mitochondrial pathways of apoptosis. Lipoapoptosis induces hepatic fibrosis and further progression to cirrhosis [].
In recent years, NAFLD and non-alcoholic steatohepatitis has emerged as an important risk factor for development of HCC even in the absence of cirrhosis []. The carcinogenesis results from alteration in complex signaling pathways mediated by genetic, immunologic, metabolic, and endocrine interactions []. Insulin resistance and hyperinsulinemia associated with NAFLD augment IGF-1 synthesis in the liver []. Stimulation of insulin receptor and IGF-1 receptor initiates insulin receptor substrate-1 pathway activation and subsequent downstream induction of PI3K and MAPK pathways []. The activation of these pathways induce cell proliferation, prevent apoptosis, and act as the link between insulin resistance and carcinogenesis of HCC [].
NAFLD is the leading cause of chronic liver disease in many parts of the world and metabolic syndrome, diabetes, and obesity remain its primary drivers []. The strong connection between insulin resistance and NAFLD, NASH, and HCC reinforces the importance of the intricate relationship between endocrine pathways and liver.
4.2. Secondary NAFLD from Other Endocrine Disorders
Steatosis or steatohepatitis has been observed in multiple other endocrine anomalies such as hypothyroidism, Graves’ disease and other causes of thyrotoxicosis, PCOS, Cushing’s syndrome, acromegaly, and pheochromocytoma []. Hypothyroidism is a risk factor for NAFLD. A recent meta-analysis of 26 studies demonstrated that thyroid stimulating hormone (TSH) levels can correlate with development and progression of NAFLD []. However, other reports did not establish the link []. Such an association is mechanistically plausible given the effect of thyroid hormone on fat deposition in the liver and other body parts []. The prevalence of NAFLD is reported to be only 20% in Cushing’s syndrome, in spite of the presence of several features of metabolic syndrome such as central obesity and insulin resistance []. The low prevalence of NAFLD could result from the immunosuppressive effect of cortisol, especially the low grade chronic inflammation mediated by interleukin-6 []. PCOS is also associated with NASH, and the two conditions share common genetic and metabolic factors []. GH deficiency also increases the risk of NAFLD as already discussed in the previous section.
4.3. Other Hepatic Manifestations of Endocrine Disorders
The liver can be the site of metastases for many endocrine cancers such as adrenal carcinoma, pancreatic carcinoma, and testicular and ovarian tumors []. The unique constellation of clinical symptoms observed in carcinoid syndrome usually occurs after extensive hepatic metastases from gastrointestinal carcinoids. The liver otherwise metabolizes the bioactive products secreted into the portal circulation by the tumors []. Cholestasis can be a hepatic manifestation of thyroid disorders []. Neonatal cholestasis can be an indicator of the presence of congenital combined pituitary hormone deficiency or congenital hypothyroidism [,].
5. Sexual Dimorphism in Liver Disorders
Many liver diseases show differential gender distribution. NAFLD is more common in men during the reproductive age group, but is more frequent in women after menopause, indicating a possible protective role of estrogen []. HCC occurs more commonly in men, while the risk of autoimmune liver diseases such as primary biliary cirrhosis and autoimmune hepatitis is more common in women []. Women also show higher vulnerability to alcohol-related liver diseases []. Apart from sex hormones, differences in xenobiotics, immune function, genetic alterations, and receptor expression are presumed to drive the dichotomy [].
6. Liver Function Biochemical Markers as Predictors of Endocrine Dysfunction
In several studies, liver enzymes have correlated with the development of incident diabetes []. γ-glutamyltransferase (GGT) has been proposed as a marker of oxidative stress and is associated with the future risk of diabetes. GGT levels have also been considered an indicator of hepatic fat deposition, which is related to insulin resistance []. In several reports, GGT and alanine aminotransferase in early pregnancy predicted the future occurrence of gestational diabetes mellitus [,]. Table 3 summarizes the liver enzymes which have been linked to the future development of metabolic disorders.

Table 3.
Liver function biochemical markers as predictors of endocrine dysfunction.
7. Hepato-Endocrine Syndromes
We have used the term “hepato-endocrine syndromes” to describe disorders with a common etiology that manifest as combined hepatic and endocrine dysfunction. The various hepato-endocrine syndromes are enumerated in Table 4. Disorders of iron and copper metabolism such as hemochromatosis and Wilson’s disease are notable examples of this syndrome [,]. Polyglandular autoimmune syndromes type 1 and type 2 can develop autoimmune hepatitis and primary biliary cirrhosis, respectively, as their hepatic manifestations []. Hepatitis C virus infection can be associated with thyroiditis and hypothyroidism [].

Table 4.
Hepato-endocrine syndromes.
8. Hepatic Effect of Endocrine Drugs
The endocrine drugs can have harmful as well as beneficial effects on the liver. Both anabolic steroids and estrogens can cause cholestasis, hepatic adenoma, focal nodular hyperplasia, and other hepatic disorders [,]. Acute liver failure has been reported with diverse agents such as propylthiouracil (used for hyperthyroidism) and high doses of methylprednisolone [,]. Orlistat, a commonly used therapy for weight loss, has also been described to cause subacute and acute liver failure [].
On the other hand, the anti-diabetic agents such as pioglitazone and possibly sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-such as peptide-1 receptor agonist (GLP1RA) might possibly have a beneficial effect on NAFLD []. Glucocorticoid is indicated for the treatment of autoimmune hepatitis []. Somatostatin and vasopressin analogs decrease portal blood flow and help control esophageal variceal bleeding [].
9. Endocrine Effects of Drugs Used in Hepatology
Spironolactone, commonly used for the management of ascites in patients with cirrhosis, is an anti-androgen which has beneficial effects in PCOS in women, but causes painful gynecomastia in males [,]. Interferon-alpha used for management of hepatitis C infection can result in thyroid dysfunction []. Beta-blockers have often been associated with erectile dysfunction []. Table 5 depicts the common drug interactions in hepatocrinology.

Table 5.
Pharmacological interactions in hepatocrinology.
10. Conclusions
The spectrum of hepatocrinology envelops diverse interactions between hepatic and endocrine systems in health and disease. We have coined this portmanteau term to increase awareness among clinicians about the complex, multifaceted relationships between these two disciplines. Both diabetes and NAFLD are emerging epidemics, and early recognition of the interconnection between these commonly prevalent disorders might assist in preventing advanced complications such as cirrhosis and HCC. Chronic liver disease results in multiple endocrine dysfunctions in all stages of life. Children with cirrhosis have stunted linear growth; in reproductive age groups hypogonadism remains a concern; and the elderly are affected by osteoporosis. Many of these relations remain unappreciated, and the complications undiagnosed in clinical practice. We hope that the study of hepatocrine interplay under a well-structured rubric will make clinicians aware of these often-missed interactions and improve patient outcome.
Author Contributions
Conceptualization, S.K. and P.R.; methodology, S.B.; software, S.B.; validation, S.K., S.B. and P.R.; formal analysis, S.K.; investigation, P.R.; resources, S.K.; data curation, S.B.; writing—original draft preparation, S.K.; writing—review and editing, S.B.; visualization, P.R.; supervision, S.K.; project administration, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. APC was sponsored by MDPI.
Institutional Review Board Statement
Ethical review and approval were waived for this study was waived as this is a review article.
Informed Consent Statement
Patient consent was waived as this is a review article and data related to individual patient information was not reported.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the contribution of Puja Dutta in preparing the diagram.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wewer Albrechtsen, N.J.; Pedersen, J.; Galsgaard, K.D.; Winther-Sørensen, M.; Suppli, M.P.; Janah, L.; Gromada, J.; Vilstrup, H.; Knop, F.K.; Holst, J.J. The Liver-α-Cell Axis and Type 2 Diabetes. Endocr. Rev. 2019, 40, 1353–1366. [Google Scholar] [CrossRef]
- Bach, L.A. IGF-binding proteins. J. Mol. Endocrinol. 2018, 61, T11–T28. [Google Scholar] [CrossRef] [PubMed]
- Raghow, R. Betatrophin: A liver-derived hormone for the pancreatic β-cell proliferation. World J. Diabetes 2013, 4, 234–237. [Google Scholar] [CrossRef]
- Matsusaka, T.; Niimura, F.; Shimizu, A.; Pastan, I.; Saito, A.; Kobori, H.; Nishiyama, A.; Ichikawa, I. Liver Angiotensinogen Is the Primary Source of Renal Angiotensin II. J. Am. Soc. Nephrol. 2012, 23, 1181–1189. [Google Scholar] [CrossRef]
- Ruchala, P.; Nemeth, E. The pathophysiology and pharmacology of hepcidin. Trends Pharmacol. Sci. 2014, 35, 155–161. [Google Scholar] [CrossRef]
- Hitchcock, I.S.; Kaushansky, K. Thrombopoietin from beginning to end. Br. J. Haematol. 2014, 165, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Sharma, M.; Ferdinand, K. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors: Present perspectives and future horizons. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Selby, C. Sex Hormone Binding Globulin: Origin, Function and Clinical Significance. Ann. Clin. Biochem. Int. J. Lab. Med. 1990, 27, 532–541. [Google Scholar] [CrossRef]
- Schussler, G.C. The Thyroxine-Binding Proteins. Thyroid 2000, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Palha, J.A. Transthyretin as a Thyroid Hormone Carrier: Function Revisited. Clin. Chem. Lab. Med. 2002, 40, 1292–1300. [Google Scholar] [CrossRef]
- Breuner, C.W.; Beyl, H.E.; Malisch, J.L. Corticosteroid-binding globulins: Lessons from biomedical research. Mol. Cell. Endocrinol. 2020, 514, 110857. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhou, X.-X.; Hu, Y.; Li, G.; Wang, Y. The Roles of Insulin-Like Growth Factor Binding Protein Family in Development and Diseases. Adv. Ther. 2021, 38, 885–903. [Google Scholar] [CrossRef] [PubMed]
- Scharf, J.; Ramadori, G.; Braulke, T.; Hartmann, H. Synthesis of insulinlike growth factor binding proteins and of the acid-labile subunit in primary cultures of rat hepatocytes, of Kupffer cells, and in cocultures: Regulation by insulin, insulinlike growth factor, and growth hormone. Hepatology 1996, 23, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Leví, A.M.; Marazuela, M. Treatment of adult growth hormone deficiency with human recombinant growth hormone: An update on current evidence and critical review of advantages and pitfalls. Endocrine 2018, 60, 203–218. [Google Scholar] [CrossRef]
- Engström, W.; Shokrai, A.; Otte, K.; Granerus, M.; Gessbo, A.; Bierke, P.; Madej, A.; Sjolund, M.; Ward, A. Transcriptional regulation and biological significance of the insulin like growth factor II gene. Cell Prolif. 1998, 31, 173–189. [Google Scholar] [CrossRef]
- Adamek, A.; Kasprzak, A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef] [PubMed]
- Colakoğlu, O.; Taşkiran, B.; Colakoğlu, G.; Kizildağ, S.; Ari Ozcan, F.; Unsal, B. Serum insulin like growth factor-1 (IGF-1) and insulin like growth factor binding protein-3 (IGFBP-3) levels in liver cirrhosis. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2007, 18, 245–249. [Google Scholar]
- Stanley, T.L.; Fourman, L.T.; Zheng, I.; McClure, C.M.; Feldpausch, M.N.; Torriani, M.; E Corey, K.; Chung, R.T.; Lee, H.; E Kleiner, D.; et al. Relationship of IGF-1 and IGF-Binding Proteins to Disease Severity and Glycemia in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2021, 106, e520–e533. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Miyake, T.; Miyazaki, M.; Eguchi, T.; Niiya, T.; Yamamoto, S.; Senba, H.; Furukawa, S.; Matsuura, B.; Hiasa, Y. Insulin-like growth factor-1 is inversely associated with liver fibrotic markers in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2018, 10, 1083–1091. [Google Scholar] [CrossRef]
- Takahashi, Y. The Role of Growth Hormone and Insulin-Like Growth Factor-I in the Liver. Int. J. Mol. Sci. 2017, 18, 1447. [Google Scholar] [CrossRef]
- Christopoulos, P.F.; Msaouel, P.; Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer 2015, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Bleach, R.; Sherlock, M.; O’Reilly, M.W.; McIlroy, M. Growth Hormone/Insulin Growth Factor Axis in Sex Steroid Associated Disorders and Related Cancers. Front. Cell Dev. Biol. 2021, 9, 630503. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.; Pipkin, F.B.; Kalsheker, N. Angiotensinogen: Molecular biology, biochemistry and physiology. Int. J. Biochem. Cell Biol. 1996, 28, 1211–1222. [Google Scholar] [CrossRef]
- Leenen, F.H.H.; Blaustein, M.P.; Hamlyn, J.M. Update on angiotensin II: New endocrine connections between the brain, adrenal glands and the cardiovascular system. Endocr. Connect. 2017, 6, R131–R145. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Giulietti, F.; Di Pentima, C.; Giordano, P.; Spannella, F. Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L325–L336. [Google Scholar] [CrossRef]
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef] [PubMed]
- Drachman, J.G. Role of thrombopoietin in hematopoietic stem cell and progenitor regulation. Curr. Opin. Hematol. 2000, 7, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Al Madhoun, A.; Abubaker, J. The Rise and the Fall of Betatrophin/ANGPTL8 as an Inducer of β-Cell Proliferation. J. Diabetes Res. 2016, 2016, 4860595. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Teng, C. Angiopoietin-like proteins 3, 4 and 8: Regulating lipid metabolism and providing new hope for metabolic syndrome. J. Drug Target. 2014, 22, 679–687. [Google Scholar] [CrossRef]
- Navaeian, M.; Asadian, S.; Yazdi, H.A.; Gheibi, N. ANGPTL8 roles in proliferation, metabolic diseases, hypothyroidism, polycystic ovary syndrome, and signaling pathways. Mol. Biol. Rep. 2021, 1–13. [Google Scholar] [CrossRef]
- Reiss, A.B.; Shah, N.; Muhieddine, D.; Zhen, J.; Yudkevich, J.; Kasselman, L.J.; DeLeon, J. PCSK9 in cholesterol metabolism: From bench to bedside. Clin. Sci. 2018, 132, 1135–1153. [Google Scholar] [CrossRef]
- Wong, N.D.; Rosenblit, P.D.; Greenfield, R.S. Advances in dyslipidemia management for prevention of atherosclerosis: PCSK9 monoclonal antibody therapy and beyond. Cardiovasc. Diagn. Ther. 2017, 67, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Henley, D.; Lightman, S. New insights into corticosteroid-binding globulin and glucocorticoid delivery. Neuroscience 2011, 180, 1–8. [Google Scholar] [CrossRef]
- Qu, X.; Donnelly, R. Sex Hormone-Binding Globulin (SHBG) as an Early Biomarker and Therapeutic Target in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2020, 21, 8191. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Kratzsch, J. Corticosteroid-binding globulin: Modulating mechanisms of bioavailability of cortisol and its clinical implications. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 761–772. [Google Scholar] [CrossRef]
- Tahboub, R.; Arafah, B.M. Sex steroids and the thyroid. Best. Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Bragança, A.C.C.; Alvares-Da-Silva, M.R. Prevalence of diabetes mellitus and impaired glucose tolerance in patients with decompensated cirrhosis being evaluated for liver transplantation: The utility of oral glucose tolerance test. Arq. Gastroenterol. 2010, 47, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Marselli, L.; De Simone, P.; Morganti, R.; Coletti, L.; Carrai, P.; Catalano, G.; Tincani, G.; Ghinolfi, D.; Occhipinti, M.; Filipponi, F.; et al. Frequency and characteristics of diabetes in 300 pre-liver transplant patients. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Holstein, A.; Hinze, S.; Thiessen, E.; Plaschke, A.; Egberts, E.-H. Clinical implications of hepatogenous diabetes in liver cirrhosis. J. Gastroenterol. Hepatol. 2002, 17, 677–681. [Google Scholar] [CrossRef]
- Orsi, E.; Grancini, V.; Menini, S.; Aghemo, A.; Pugliese, G. Hepatogenous diabetes: Is it time to separate it from type 2 diabetes? Liver Int. 2016, 37, 950–962. [Google Scholar] [CrossRef]
- Kumar, R. Hepatogenous diabetes: An underestimated problem of liver cirrhosis. Indian J. Endocrinol. Metab. 2018, 22, 552–559. [Google Scholar] [CrossRef]
- Petrides, A.S.; Stanley, T.; Matthews, D.E.; Vogt, C.; Bush, A.J.; Lambeth, H. Insulin resistance in cirrhosis: Prolonged reduction of hyperinsulinemia normalizes insulin sensitivity. Hepatology 1998, 28, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.H.; Forbes, J.M.; Angus, P.W.; Herath, C.B. Development and Progression of Non-Alcoholic Fatty Liver Disease: The Role of Advanced Glycation End Products. Int. J. Mol. Sci. 2019, 20, 5037. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.-T.; Qin, Z.-L.; Ren, H.; Zhao, P.; Qi, Z.-T. Inhibition of IRS-1 by hepatitis C virus infection leads to insulin resistance in a PTEN-dependent manner. Virol. J. 2015, 12, 12. [Google Scholar] [CrossRef]
- Bose, S.K.; Shrivastava, S.; Meyer, K.; Ray, R.B.; Ray, R.B.; Ray, R. Hepatitis C Virus Activates the mTOR/S6K1 Signaling Pathway in Inhibiting IRS-1 Function for Insulin Resistance. J. Virol. 2012, 86, 6315–6322. [Google Scholar] [CrossRef] [PubMed]
- Lerat, H.; Imache, M.R.; Polyte, J.; Gaudin, A.; Mercey, M.; Donati, F.; Baudesson, C.; Higgs, M.R.; Picard, A.; Magnan, C.; et al. Hepatitis C virus induces a prediabetic state by directly impairing hepatic glucose metabolism in mice. J. Biol. Chem. 2017, 292, 12860–12873. [Google Scholar] [CrossRef]
- Manco, M. Insulin Resistance and NAFLD: A Dangerous Liaison beyond the Genetics. Children 2017, 4, 74. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Caviglia, G.; Bugianesi, E. Insulin Resistance across the Spectrum of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 155. [Google Scholar] [CrossRef]
- Nouel, O.; Bernuau, J.; Rueff, B.; Benhamou, J.P. Hypoglycemia. A common complication of septicemia in cirrhosis. Arch. Intern. Med. 1981, 141, 1477–1478. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-J.; Liao, C.-J. Predisposing factors for hypoglycaemia in the emergency department. J. Int. Med. Res. 2019, 47, 2404–2412. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, P.; Kumar, V.; Sarin, S.K.; Kumar, A. Etiology and prognostic factors of acute liver failure in children. Indian Pediatr. 2013, 50, 677–679. [Google Scholar] [CrossRef]
- Pfortmueller, C.A.; Wiemann, C.; Funk, G.-C.; Leichtle, A.B.; Fiedler, G.M.; Exadaktylos, A.K.; Lindner, G. Hypoglycemia is associated with increased mortality in patients with acute decompensated liver cirrhosis. J. Crit. Care 2014, 29, 316.e7–316.e12. [Google Scholar] [CrossRef] [PubMed]
- Anno, T.; Kaneto, H.; Shigemoto, R.; Kawasaki, F.; Kawai, Y.; Urata, N.; Kawamoto, H.; Kaku, K.; Okimoto, N. Hypoinsulinemic hypoglycemia triggered by liver injury in elderly subjects with low body weight: Case reports. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Oldenbeuving, G.; McDonald, J.R.; Goodwin, M.L.; Sayilir, R.; Reijngoud, D.J.; Gladden, L.B.; Nijsten, M.W.N. A patient with acute liver failure and extreme hypoglycaemia with lactic acidosis who was not in a coma: Causes and consequences of lactate-protected hypoglycaemia. Anaesth. Intensive Care 2014, 42, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liang, X.; Chen, Y.; Huang, F.; Fan, W.; Xue, J.; Li, C. Hepatocellular carcinoma with worsened hypoglycemia after transarterial chemoembolization: A case report and systematic review. Int. J. Clin. Exp. Pathol. 2020, 13, 3167–3173. [Google Scholar]
- Yu, B.; Douli, R.; Suarez, J.A.; Gutierrez, V.P.; Aldiabat, M.; Khan, M. Non-islet cell tumor hypoglycemia as an initial presentation of hepatocellular carcinoma coupled with end-stage liver cirrhosis: A case report and review of literature. World J. Hepatol. 2020, 12, 519–524. [Google Scholar] [CrossRef]
- Garg, M.K.; Puri, P.; Brar, K.S.; Pandit, A.; Srivastava, S.; Kharb, S. Assessment of thyroid and gonadal function in liver diseases. Indian J. Endocrinol. Metab. 2015, 19, 89–94. [Google Scholar] [CrossRef]
- Kumar, K.V.S.H.; Pawah, A.K.; Manrai, M. Occult endocrine dysfunction in patients with cirrhosis of liver. J. Fam. Med. Prim. Care 2016, 5, 576–580. [Google Scholar] [CrossRef]
- Valimäki, M.; Pelkonen, R.; Salaspuro, M.; Härkönen, M.; Hirvonen, E.; Ylikahri, R. Sex Hormones in Amenorrheic Women with Alcoholic Liver Disease. J. Clin. Endocrinol. Metab. 1984, 59, 133–138. [Google Scholar] [CrossRef]
- Cundy, T.F.; Butler, J.; Pope, R.M.; Saggar-Malik, A.K.; Wheeler, M.J.; Williams, R. Amenorrhoea in women with non-alcoholic chronic liver disease. Gut 1991, 32, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Schipilliti, M.; Ciarleglio, F.A.; Lenzi, A.; D’Amico, D. Male hypogonadism in cirrhosis and after liver transplantation. J. Endocrinol. Investig. 2008, 31, 470–478. [Google Scholar] [CrossRef]
- Cavanaugh, J.; Niewoehner, C.B.; Nuttall, F.Q. Gynecomastia and cirrhosis of the liver. Arch. Intern. Med. 1990, 150, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Neong, S.F.; Billington, E.O.; Congly, S.E. Sexual Dysfunction and Sex Hormone Abnormalities in Patients with Cirrhosis: Review of Pathogenesis and Management. Hepatology 2018, 69, 2683–2695. [Google Scholar] [CrossRef]
- Sinclair, M.; Grossmann, M.; Gow, P.J.; Angus, P.W. Testosterone in men with advanced liver disease: Abnormalities and implications. J. Gastroenterol. Hepatol. 2015, 30, 244–251. [Google Scholar] [CrossRef]
- Zifroni, A.; Schiavi, R.C.; Schaffner, F. Sexual function and testosterone levels in men with nonalcoholic liver disease. Hepatology 1991, 14, 479–482. [Google Scholar] [CrossRef]
- Grossmann, M.; Hoermann, R.; Gani, L.; Chan, I.; Cheung, A.; Gow, P.J.; Li, A.; Zajac, J.D.; Angus, P. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clin. Endocrinol. 2012, 77, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zietz, B.; Lock, G.; Plach, B.; Drobnik, W.; Grossmann, J.; Schölmerich, J.; Straub, R.H. Dysfunction of the hypothalamic-pituitary-glandular axes and relation to Child-Pugh classification in male patients with alcoholic and virus-related cirrhosis. Eur. J. Gastroenterol. Hepatol. 2003, 15, 495–501. [Google Scholar]
- Gluud, C.; Bahnsen, M.; Bennett, P.; Brodthagen, U.A.; Dietrichson, O.; Johnsen, S.G.; Nielsen, J.; Micic, S.; Svendsen, L.B.; Svenstrup, B. Hypothalamic-pituitary-gonadal function in relation to liver function in men with alcoholic cirrhosis. Scand. J. Gastroenterol. 1983, 18, 939–944. [Google Scholar] [CrossRef]
- Ehnert, S.; Aspera-Werz, R.H.; Ruoß, M.; Dooley, S.; Hengstler, J.G.; Nadalin, S.; Relja, B.; Badke, A.; Nussler, A.K. Hepatic Osteodystrophy-Molecular Mechanisms Proposed to Favor Its Development. Int. J. Mol. Sci. 2019, 20, 2555. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Loperto, I.; Camera, S.; Cossiga, V.; Di Somma, C.; Colao, A.; Caporaso, N.; Morisco, F. Osteoporosis across chronic liver disease. Osteoporos. Int. 2016, 27, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Glass, L.M.; Su, G.L.-C. Metabolic Bone Disease in Primary Biliary Cirrhosis. Gastroenterol. Clin. N. Am. 2016, 45, 333–343. [Google Scholar] [CrossRef]
- Barbu, E.-C.; Chiţu-Tișu, C.-E.; Lazar, M.; Olariu, C.; Bojincă, M.; Ionescu, R.A.; Ion, D.A.; Badarau, I.A. Hepatic Osteodystrophy: A Global (Re)View of the Problem. Acta Clin. Croat. 2017, 56, 512–525. [Google Scholar] [CrossRef]
- Silveira, M.G.; Mendes, F.D.; Diehl, N.N.; Enders, F.T.; Lindor, K.D. Thyroid dysfunction in primary biliary cirrhosis, primary sclerosing cholangitis and non-alcoholic fatty liver disease. Liver Int. 2009, 29, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferri, C.; Fallahi, P.; Ferrari, S.M.; Ghinoi, A.; Rotondi, M.; Ferrannini, E. Thyroid Disorders in Chronic Hepatitis C Virus Infection. Thyroid. Off. J. Am. Thyroid. Assoc. 2006, 16, 563–572. [Google Scholar] [CrossRef]
- Wang, P.; Jing, Z.; Liu, C.; Xu, M.; Wang, P.; Wang, X.; Yin, Y.; Cui, Y.; Ren, D.; Rao, X. Hepatitis C virus infection and risk of thyroid cancer: A systematic review and meta-analysis. Arab. J. Gastroenterol. 2017, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kalk, W.J.; Kew, M.C.; Danilewitz, M.D.; Jacks, F.; Van Der Walt, L.A.; Levin, J. Thyroxine Binding Globulin and Thyroid Function Tests in Patients with Hepatocellular Carcinoma. Hepatology 2007, 2, 72S–76S. [Google Scholar] [CrossRef] [PubMed]
- Nagasue, N.; Ohmori, H.; Hashimoto, N.; Tachibana, M.; Kubota, H.; Uchida, M.; Yu, L. Thyroxine-binding globulin and thyroid hormones after resection of hepatocellular carcinoma. Am. J. Gastroenterol. 1997, 92, 1187–1189. [Google Scholar]
- Fede, G.; Spadaro, L.; Tomaselli, T.; Privitera, G.; Germani, G.; Tsochatzis, E.; Thomas, M.; Bouloux, P.-M.; Burroughs, A.K.; Purrello, F. Adrenocortical dysfunction in liver disease: A systematic review. Hepatology 2012, 55, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Chiriac, S.; Stanciu, C. Update on adrenal insufficiency in patients with liver cirrhosis. World J. Gastroenterol. 2013, 19, 445–456. [Google Scholar] [CrossRef]
- Alonso, E.M. Growth and developmental considerations in pediatric liver transplantation. Liver Transplant. 2008, 14, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Bucuvalas, J.C.; Cutfield, W.; Horn, J.; Sperling, M.A.; Heubi, J.E.; Campaigne, B.; Chernausek, S.D. Resistance to the growth-promoting and metabolic effects of growth hormone in children with chronic liver disease. J. Pediatr. 1990, 117, 397–402. [Google Scholar] [CrossRef]
- Mohammad, S.; Grimberg, A.; Rand, E.; Anand, R.; Yin, W.; Alonso, E.M. Long-Term Linear Growth and Puberty in Pediatric Liver Transplant Recipients. J. Pediatr. 2013, 163, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M. The Liver as an Endocrine Organ—Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Pafundi, P.; Galiero, R.; Caturano, A.; Morone, M.; Silvestri, C.; Giordano, M.; Salvatore, T.; Sasso, F. Mechanisms of Non-Alcoholic Fatty Liver Disease in the Metabolic Syndrome. A Narrative Review. Antioxidants 2021, 10, 270. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef]
- Seppälä-Lindroos, A.; Vehkavaara, S.; Häkkinen, A.-M.; Goto, T.; Westerbacka, J.; Sovijärvi, A.; Halavaara, J.; Yki-Järvinen, H. Fat Accumulation in the Liver Is Associated with Defects in Insulin Suppression of Glucose Production and Serum Free Fatty Acids Independent of Obesity in Normal Men. J. Clin. Endocrinol. Metab. 2002, 87, 3023–3028. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Kohli, R.; Gores, G.J. Mechanisms of Lipotoxicity in NAFLD and Clinical Implications. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 131–140. [Google Scholar] [CrossRef]
- Baffy, G.; Brunt, E.M.; Caldwell, S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J. Hepatol. 2012, 56, 1384–1391. [Google Scholar] [CrossRef]
- Kutlu, O.; Kaleli, H.N.; Ozer, E. Molecular Pathogenesis of Nonalcoholic Steatohepatitis-(NASH-) Related Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- De Minicis, S.; Agostinelli, L.; Rychlicki, C.; Sorice, G.P.; Saccomanno, S.; Candelaresi, C.; Giaccari, A.; Trozzi, L.; Pierantonelli, I.; Mingarelli, E.; et al. HCC development is associated to peripheral insulin resistance in a mouse model of NASH. PLoS ONE 2014, 9, e97136. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Kaseb, A.O.; Tsimberidou, A.M.; Wolff, R.A.; Kurzrock, R. Identification of novel therapeutic targets in the PI3K/AKT/mTOR pathway in hepatocellular carcinoma using targeted next generation sequencing. Oncotarget 2014, 5, 3012–3022. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. Off. J. Int. Assoc. Study Liver 2017, 37 (Suppl. S1), 81–84. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. NAFLD in Some Common Endocrine Diseases: Prevalence, Pathophysiology, and Principles of Diagnosis and Management. Int. J. Mol. Sci. 2019, 20, 2841. [Google Scholar] [CrossRef]
- Guo, Z.; Li, M.; Han, B.; Qi, X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig. Liver Dis. 2018, 50, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Jaruvongvanich, V.; Sanguankeo, A.; Upala, S. Nonalcoholic Fatty Liver Disease Is Not Associated with Thyroid Hormone Levels and Hypothyroidism: A Systematic Review and Meta-Analysis. Eur. Thyroid. J. 2017, 6, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Lugari, S.; Mantovani, A.; Nascimbeni, F.; Lonardo, A. Hypothyroidism and nonalcoholic fatty liver disease—A chance association? Horm. Mol. Biol. Clin. Investig. 2018, 41, 347–353. [Google Scholar] [CrossRef]
- Ahmed, A.; Rabbitt, E.; Brady, T.; Brown, C.; Guest, P.; Bujalska, I.J.; Doig, C.; Newsome, P.N.; Hubscher, S.; Elias, E.; et al. A Switch in Hepatic Cortisol Metabolism across the Spectrum of Non Alcoholic Fatty Liver Disease. PLoS ONE 2012, 7, e29531. [Google Scholar]
- Tarantino, G.; Finelli, C. Pathogenesis of hepatic steatosis: The link between hypercortisolism and non-alcoholic fatty liver disease. World J. Gastroenterol. 2013, 19, 6735–6743. [Google Scholar] [CrossRef] [PubMed]
- Macut, D.; Bjekić-Macut, J.; Livadas, S.; Stanojlović, O.; Hrnčić, D.; Rašić-Marković, A.; Milutinović, D.V.; Mladenović, V.; Andrić, Z. Nonalcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome. Curr. Pharm. Des. 2019, 24, 4593–4597. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, J.; de Wilt, J.H.W.; Simmer, F.; Overbeek, L.; Lemmens, V.; Nagtegaal, I. Incidence and origin of histologically confirmed liver metastases: An explorative case-study of 23,154 patients. Oncotarget 2016, 7, 55368–55376. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Lee, L.; Jensen, R.T. Carcinoid-syndrome: Recent advances, current status and controversies. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 22–35. [Google Scholar] [CrossRef]
- Piantanida, E.; Ippolito, S.; Gallo, D.; Masiello, E.; Premoli, P.; Cusini, C.; Rosetti, S.; Sabatino, J.; Segato, S.; Trimarchi, F.; et al. The interplay between thyroid and liver: Implications for clinical practice. J. Endocrinol. Investig. 2020, 43, 885–899. [Google Scholar] [CrossRef]
- Chan, U.; Chan, W.-T.; Ting, W.-H.; Ho, C.-S.; Liu, H.-C.; Lee, H.-C. Cholestasis caused by panhypopituitarism and acquired cytomegalovirus infection in a 2-month-old male infant: A case report. Medicine 2017, 96, e6757. [Google Scholar] [CrossRef]
- Korkmaz, L.; Akın, M.A.; Güneş, T.; Daar, G.; Baştuğ, O.; Yıkılmaz, A.; Kurtoğlu, S. Unusual Course of Congenital Hypothyroidism and Route of the L-Thyroxine Treatment in a Preterm Newborn. J. Clin. Res. Pediatric Endocrinol. 2014, 6, 177–179. [Google Scholar] [CrossRef]
- Villalba, N.L.; Zulfiqar, A.-A.; Saint-Mezard, V.; Alonso, M.B.; Kechida, M.; Zamorano, N.F.; Ortega, S.S. Myxedema coma: Four patients diagnosed at the Internal Medicine Department of the Dr. Negrin University Hospital in Spain. Pan Afr. Med. J. 2019, 34, 7. [Google Scholar] [CrossRef]
- Abebe, A.; Eck, L.M.; Holyoak, M. Severe cholestatic jaundice associated with Graves’ disease. Clin. Case Rep. 2018, 6, 2240–2245. [Google Scholar] [CrossRef]
- Subedi, A.; Kumar, V.C.S.; Sharma, A.; Hoilat, G.; John, S. Persistent lactic acidosis in the Mauriac syndrome in type 1 diabetes mellitus. Bayl. Univ. Med Cent. Proc. 2021, 34, 382–383. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Kur, P.; Kolasa-Wołosiuk, A.; Misiakiewicz-Has, K.; Wiszniewska, B. Sex Hormone-Dependent Physiology and Diseases of Liver. Int. J. Environ. Res. Public Health 2020, 17, 2620. [Google Scholar] [CrossRef] [PubMed]
- Agabio, R.; Pisanu, C.; Gessa, G.L.; Franconi, F. Sex Differences in Alcohol Use Disorder. Curr. Med. Chem. 2017, 24, 2661–2670. [Google Scholar] [CrossRef]
- Biswas, S.; Ghose, S. Divergent impact of gender in advancement of liver injuries, diseases, and carcinogenesis. Front. Biosci. Sch. Ed. 2018, 10, 65–100. [Google Scholar] [CrossRef][Green Version]
- Kaneko, K.; Yatsuya, H.; Li, Y.; Uemura, M.; Chiang, C.; Hirakawa, Y.; Ota, A.; Tamakoshi, K.; Aoyama, A. Association of gamma-glutamyl transferase and alanine aminotransferase with type 2 diabetes mellitus incidence in middle-aged Japanese men: 12-year follow up. J. Diabetes Investig. 2018, 10, 837–845. [Google Scholar] [CrossRef]
- Koenig, G.; Seneff, S. Gamma-Glutamyltransferase: A Predictive Biomarker of Cellular Antioxidant Inadequacy and Disease Risk. Dis. Markers 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, J.S.; Han, Y.J.; Kim, W.; Bang, S.H.; Kim, B.J.; Park, C.-W.; Kim, M.Y. Elevated Alanine Aminotransferase in Early Pregnancy and Subsequent Development of Gestational Diabetes and Preeclampsia. J. Korean Med Sci. 2020, 35, e198. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, L.; Zhang, G.; Varkaneh, H.K.; Rahmani, J.; Clark, C.; Ryan, P.M.; Abdulazeem, H.; Salehisahlabadi, A. The association of plasma levels of liver enzymes and risk of gestational diabetes mellitus: A systematic review and dose–response meta-analysis of observational studies. Acta Diabetol. 2019, 57, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Avagnina, S.; Barantani, E.G.; Ciccarone, A.M.; Corica, F.; Dall’Aglio, E.; Grave, R.D.; Morpurgo, P.S.; Tomasi, F.; Vitacolonna, E. Aminotransferase and gamma-glutamyltranspeptidase levels in obesity are associated with insulin resistance and the metabolic syndrome. J. Endocrinol. Investig. 2005, 28, 333–339. [Google Scholar] [CrossRef]
- Crownover, B.K.; Covey, C.J. Hereditary hemochromatosis. Am. Fam. Physician 2013, 87, 183–190. [Google Scholar]
- Mulligan, C.; Bronstein, J.M. Wilson Disease: An Overview and Approach to Management. Neurol. Clin. 2020, 38, 417–432. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Frommer, L. Polyglandular autoimmune syndromes. J. Endocrinol. Investig. 2018, 41, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.A.; Jones, T.L.; Ianna, E.A.; Foy, A.; Reeves, G.E.M. Thyroid disease in chronic hepatitis C infection treated with combination interferon-α and ribavirin: Management strategies and future perspective. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2013, 19, 292–300. [Google Scholar] [CrossRef]
- Ellingwood, S.S.; Cheng, A. Biochemical and clinical aspects of glycogen storage diseases. J. Endocrinol. 2018, 238, R131–R141. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Chang, Y.; Ryu, S.; Cainzos-Achirica, M.; Kwon, M.-J.; Zhang, Y.; Choi, Y.; Ahn, J.; Rampal, S.; Zhao, D.; et al. Hepatitis B and C virus infection and diabetes mellitus: A cohort study. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Qu, Q.; Wang, S.; Chen, S.; Zhou, L.; Rui, J.-A. Prognostic role and significance of paraneoplastic syndromes in hepatocellular carcinoma. Am. Surg. 2014, 80, 191–196. [Google Scholar] [CrossRef]
- Niedfeldt, M.W. Anabolic Steroid Effect on the Liver. Curr. Sports Med. Rep. 2018, 17, 97–102. [Google Scholar] [CrossRef]
- Ponnatapura, J.; Kielar, A.; Burke, L.M.; Lockhart, M.E.; Abualruz, A.-R.; Tappouni, R.; Lalwani, N. Hepatic complications of oral contraceptive pills and estrogen on MRI: Controversies and update-Adenoma and beyond. Magn. Reson. Imaging 2019, 60, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Akmal, A.; Kung, J. Propylthiouracil, and methimazole, and carbimazole-related hepatotoxicity. Expert Opin. Drug Saf. 2014, 13, 1397–1406. [Google Scholar] [CrossRef]
- Zoubek, M.E.; Pinazo-Bandera, J.; Ortega-Alonso, A.; Hernández, N.; Crespo, J.; Contreras, F.; Medina-Cáliz, I.; Sanabria-Cabrera, J.; Sanjuan-Jiménez, R.; González-Jiménez, A.; et al. Liver injury after methylprednisolone pulses: A disputable cause of hepatotoxicity. A case series and literature review. United Eur. Gastroenterol. J. 2019, 7, 825–837. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Terziroli Beretta-Piccoli, B.; Mieli-Vergani, G.; Vergani, D. Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J. Gastroenterol. 2017, 23, 6030–6048. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Pharmacologic Management of Portal Hypertension. Clin. Liver Dis. 2019, 23, 713–736. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef]
- Lainscak, M.; Pelliccia, F.; Rosano, G.; Vitale, C.; Schiariti, M.S.M.; Greco, C.; Speziale, G.; Gaudio, C. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int. J. Cardiol. 2015, 200, 25–29. [Google Scholar] [CrossRef]
- Jadali, Z. Autoimmune thyroid disorders in hepatitis C virus infection: Effect of interferon therapy. Indian J. Endocrinol. Metab. 2013, 17, 69–75. [Google Scholar] [CrossRef]
- Sharp, R.P.; Gales, B.J. Nebivolol versus other beta blockers in patients with hypertension and erectile dysfunction. Ther. Adv. Urol. 2017, 9, 59–63. [Google Scholar] [CrossRef]
- Chang, H.-T.; Pan, H.-J.; Lee, C.-H. Prevention of Tamoxifen-related Nonalcoholic Fatty Liver Disease in Breast Cancer Patients. Clin. Breast Cancer 2018, 18, e677–e685. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Beland, F.; Chen, S.; Liu, F.; Guo, L.; Fang, J.-L. Mechanisms of tolvaptan-induced toxicity in HepG2 cells. Biochem. Pharmacol. 2015, 95, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Wang, D.Q.-H.; Portincasa, P. Cholesterol cholelithiasis: Part of a systemic metabolic disease, prone to primary prevention. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 157–171. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).