Efficacy of Platelet-Rich Plasma Therapy in Oral Lichen Planus: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Inclusion Criteria
2.3. Exclusion Criteria
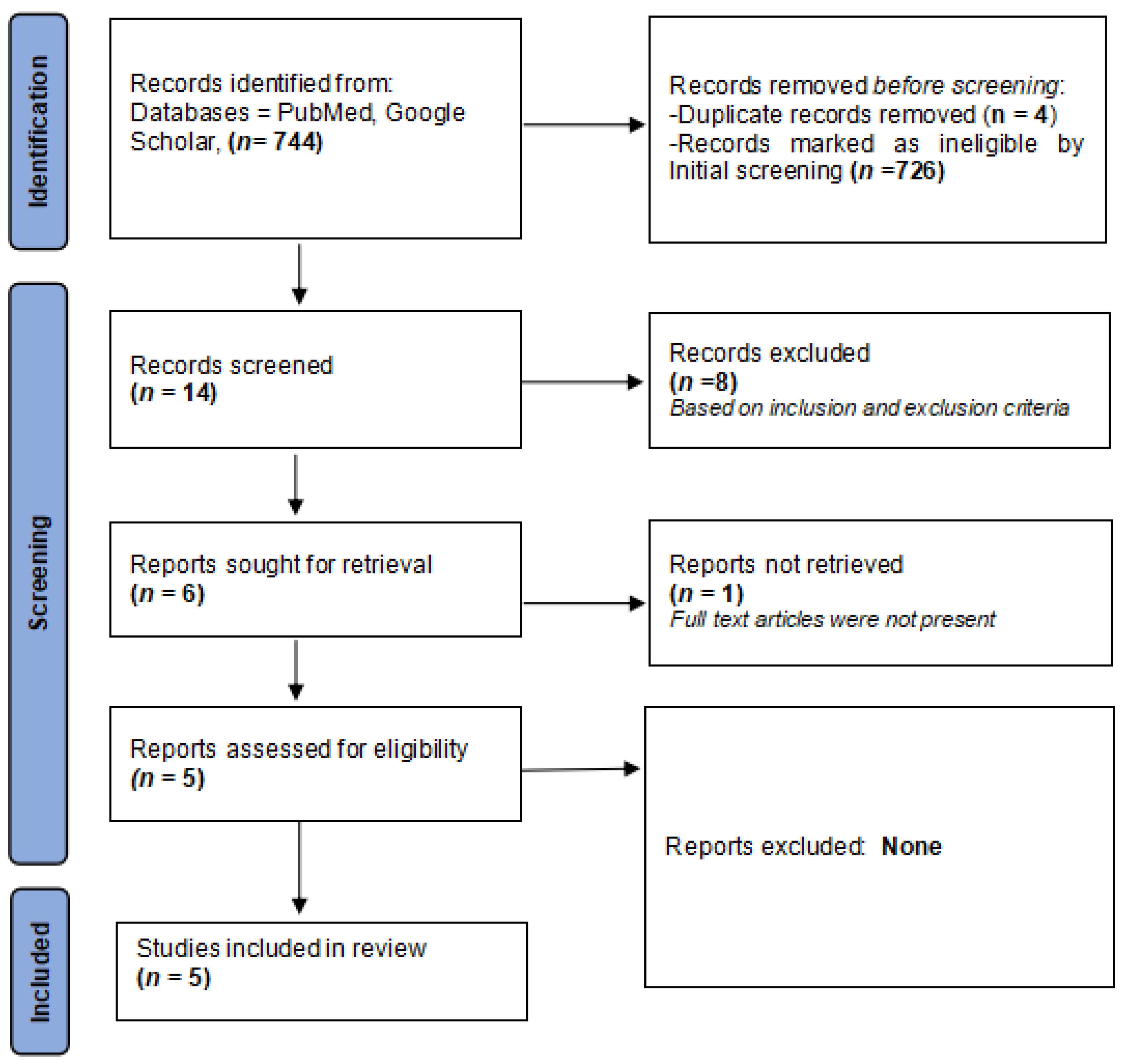
2.4. Literature Search and Identification of Studies
2.5. Study Selection
2.6. Outcome Parameters
2.7. Data Extraction
2.8. Risk of Bias Assessment
3. Results
3.1. Study Characteristics
| S. No. | Author(s)/Year/Country | Type of Study | Age/Sex/ Follow-Up | Sample Size | OLP Diagnosis | Treatment Plan | Test of Significance | Outcome | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Loré B et al., 2016 [73] Italy | Pilot study | 8 males and 12 females, mean age of 56 years (range 40–74) completed the study with follow-up at 2, 4, 8, and 12 weeks. | 20 | Clinical and histopathological Diagnosis | OLP patients were divided into three groups. Reticular OLP patients were treated with cyclosporin mouth rinses OD for 8 weeks, Plaque-like OLP patients were treated with 0.05% retinoic acid lotion BID for 8 weeks, and erosive OLP patients were treated with PRP gel once a week for 8 weeks respectively. Evaluation for clinical improvement (complete response, partial response, and no response) was noted at 2, 4, 8, and 12 weeks. | Not Applicable | (I) Reticular (n = 10) Treatment-Cyclosporin Complete: 2 Partial: 5 No response: 3 (II) Plaque (n = 5) Treatment-Retinoic acid Complete: 3 Partial: 1 No response: 1 (III) Erosive (n = 5) Treatment-PRP Complete: 2 Partial: 2 No response: 1 | OLP is associated with periods of remissions and exacerbations, hence, clinical management should be based on the clinical phenotype of OLP. Periodic follow-up with a detailed clinical examination is imperative. |
| 2. | Ahuja US et al., 2020 [74] India | Prospective, case control, randomized clinical trial. | 18 females & 2 males in the age range of 28–60 years (mean age 44.5 years); 4 months follow up | 20 | Clinical and histopathological Diagnosis | 20 OLP patients were divided into 2 groups. 10 patients in each group were given weekly intralesional injections of corticosteroid and PRP respectively for 2 months. The patients were followed up for 4 months to evaluate pain/burning, erythema, and size of the lesion. | Unpaired t-test | Pain Scores: At the 4-month follow-up: NOT significant Lesion Size: At the 4-month follow-up: NOT significant Erythema scores: At the 4-month follow-up: NOT significant | The efficacy of intralesional PRP therapy was found to be similar to that of intralesional triamcinolone acetonide in the treatment of erosive OLP. Furthermore, PRP therapy exhibited less recurrence and no adverse effects. |
| 3. | Shinnawi UE et al., 2021 [75] Egypt | Cohort Study | 7 females & 3 males in the age range of 50–65 years | 10 | Clinical and histopathological Diagnosis | 10 erosive OLP were given weekly intralesional PRP injections for 4 weeks. The patients were evaluated for pain (VAS) and the size of the lesion. | Friedman test and Wilcoxon test | Pain Reduction: At 4 weeks follow-up: Significant Clinical Scores: At 4 weeks follow-up: Significant | PRP injections exhibited significant efficacy in ameliorating the signs and symptoms in steroid-resistant erosive OLP cases. |
| 4. | Hijazi AH et al., 2022 [76] Egypt | Pilot randomized controlled clinical trial | 18 females & 2 males in the age range of 24–65 years | 20 | Clinical and histopathological Diagnosis | 20 OLP patients were divided into 2 groups. 10 patients in each group were given weekly intralesional injections of PRP and corticosteroid respectively for a month. | Wilcoxon test | Pain Reduction: (I) At 4 weeks follow-up: Significant (II) At 3-month follow-up: Significant (III) At the end of the treatment: NOT significant Clinical Scores: (I) At 4 weeks follow-up: Significant (II) At 17 weeks follow-up: NOT significant (III) At end of treatment: NOT significant | Injectable PRP therapy may be regarded as an efficacious therapeutic regimen for erosive OLP cases. |
| 5. | ElGhareeb MI et al., 2023 [77] Egypt | Case-control study | 14 females & 10 males in the age range of 30–72 years; 3-month follow-up. | 24 | Clinical and histopathological Diagnosis | 24 OLP patients were divided into 2 groups. 12 patients in each group were given intralesional injections of PRP and corticosteroid respectively every 2 weeks for 2 months. | Mann–Whitney test, Paired Wilcoxon Test and Chi-square test. | REU: (I) PRP (before) vs. Steroids (before): NOT significant (II) PRP (after) vs. Steroids (after): NOT significant (III) PRP (before) vs. PRP (after): Significant (IV) Steroids (before) vs. Steroids (after): Significant NRS: (I) NRS (before) vs. Steroids (before): NOT significant (II) NRS (after) vs. Steroids (after): NOT significant (III) NRS (before) vs. NRS (after): Significant Steroids (before) vs. Steroids (after): Significant | Injectable PRP therapy exhibited a safe therapeutic profile in OLP patients. However, intralesional PRP therapy was associated with more adverse effects (especially pain) and a higher relapse of OLP lesions after a 3-month follow-up. |
3.2. Outcome Parameters
3.3. Assessment of Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Didona, D.; Caro, R.D.C.; Santos, A.M.S.; Solimani, F.; Hertl, M. Therapeutic strategies for oral lichen planus: State of the art and new insights. Front. Med. 2022, 9, 997190. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jawanda, M.K. Oral Lichen Planus: An Update on Etiology, Pathogenesis, Clinical Presentation, Diagnosis and Management. Indian J. Dermatol. 2015, 60, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Boch, K.; Langan, E.A.; Kridin, K.; Zillikens, D.; Ludwig, R.J.; Bieber, K. Lichen Planus. Front. Med. 2021, 8, 737813. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, N.; Jayanthi, P.; Rao, U.K.; Ranganathan, K. Oral lichen planus: An update on pathogenesis and treatment. J. Oral Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef]
- Alrashdan, M.S.; Cirillo, N.; McCullough, M. Oral lichen planus: A literature review and update. Arch. Dermatol. Res. 2016, 308, 539–551. [Google Scholar] [CrossRef]
- Carbone, M.; Arduino, P.G.; Carrozzo, M.; Gandolfo, S.; Argiolas, M.R.; Bertolusso, G.; Conrotto, D.; Pentenero, M.; Broccoletti, R. Course of oral lichen planus: A retrospective study of 808 northern Italian patients. Oral Dis. 2009, 15, 235–243. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Gould, A.; Kurago, Z.; Fantasia, J.; Muller, S. Diagnosis of oral lichen planus: A position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 332–354. [Google Scholar] [CrossRef]
- Li, C.; He, H.; Wang, J.; Xia, X.; Zhang, M.; Wu, Q. Possible roles of exosomal mi-RNAs in the pathogenesis of oral lichen planus. Am. J. Transl. Res. 2019, 11, 5313–5323. [Google Scholar]
- Li, Y.; Wang, K.; Zhang, B.; Tu, Q.; Yao, Y.; Cui, B.; Ren, B.; He, J.; Shen, X.; Van-Nostrand, J.D.; et al. Salivary mycobiome dysbiosis and its potential impact on bacteriome shifts and host immunity in oral lichen planus. Int. J. Oral. Sci. 2019, 11, 13. [Google Scholar] [CrossRef]
- Hasan, S.; Ahmed, S.; Kiran, R.; Panigrahi, R.; Thachil, J.M.; Saeed, S. Oral lichen planus and associated comorbidities: An approach to holistic health. J. Family Med. Prim. Care 2019, 8, 3504–3517. [Google Scholar] [CrossRef]
- Santonocito, S.; Polizzi, A.; De Pasquale, R.; Ronsivalle, V.; Lo Giudice, A.; Isola, G. Analysis of the Efficacy of Two Treatment Protocols for Patients with Symptomatic Oral Lichen Planus: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2020, 18, 56. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef]
- Saeed, S.; Choudhury, P.; Ahmad, S.A.; Alam, T.; Panigrahi, R.; Aziz, S.; Kaleem, S.M.; Priyadarshini, S.R.; Sahoo, P.K.; Hasan, S. Vitamin D in the Treatment of Oral Lichen Planus: A Systematic Review. Biomedicines 2022, 10, 2964. [Google Scholar] [CrossRef]
- Waingade, M.; Medikeri, R.S.; Gaikwad, S. Effectiveness of hyaluronic acid in the management of oral lichen planus: A systematic review and meta-analysis. J. Dent. Anesth. Pain Med. 2022, 22, 405–417. [Google Scholar] [CrossRef]
- Elenbaas, A.; Enciso, R.; Al-Eryani, K. Oral lichen planus: A review of clinical features, etiologies, and treatments. Dent. Rev. 2022, 2, 100007. [Google Scholar] [CrossRef]
- Gupta, S.; Ghosh, S.; Gupta, S. Interventions for the management of oral lichen planus: A review of the conventional and novel therapies. Oral Dis. 2017, 23, 1029–1042. [Google Scholar] [CrossRef]
- Kramer, I.R.; Lucas, R.B.; Pindborg, J.J.; Sobin, L.H. Definition of Leukoplakia and related lesions: An aid to studies on oral precancer. Oral Surg. Oral Med. Oral Pathol. 1978, 46, 518–539. [Google Scholar]
- Van der Meij, E.H.; Van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef]
- Idrees, M.; Kujan, O.; Shearston, K.; Farah, C.S. Oral lichen planus has a very low malignant transformation rate: A systemic review and meta-analysis using strict diagnostic and inclusion criteria. J. Oral Pathol. Med. 2021, 50, 287–298. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Scully, C.; Gil-Montoya, J.A. Oral lichen planus: Controversies surrounding malignant transformation. Oral Dis. 2008, 14, 229–243. [Google Scholar] [CrossRef]
- Tsushima, F.; Sakurai, J.; Uesugi, A.; Oikawa, Y.; Ohsako, T.; Mochizuki, Y.; Hirai, H.; Kayamori, K.; Harada, H. Malignant transformation of oral lichen planus: A retrospective study of 565 Japanese patients. BMC Oral Health 2021, 21, 298. [Google Scholar] [CrossRef] [PubMed]
- Radochová, V.; Ivančaková, R.K.; Heneberk, O.; Slezák, R. The Characteristics of Patients with Oral Lichen Planus and Malignant Transformation-A Retrospective Study of 271 Patients. Int. J. Environ. Res. Public Health 2021, 18, 6525. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.A.; Ruiz-Ávila, I.; González-Ruizd, L.; Ayéne, A.; Gil-Montoya, J.A.; Ramos-Garcíaa, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Troiano, G.; Cordaro, M.; Corsalini, M.; Gioco, G.; Muzio, L.L.; Pignatelli, P.; Lajolo, C. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2019, 25, 693–709. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P.; Warnakulasuriya, S. An appraisal of highest quality studies reporting malignant transformation of oral lichen planus based on a systematic review. Oral Dis. 2021, 27, 1908–1918. [Google Scholar] [CrossRef]
- Arduino, P.G.; Magliano, A.; Gambino, A.; Macciotta, A.; Carbone, M.; Conrotto, D.; Karimi, D.; Carrozzo, M.; Broccoletti, R. Risk of Malignant Transformation in 3173 Subjects with histopathologically Confirmed Oral Lichen Planus: A 33-Year Cohort Study in Northern Italy. Cancers 2021, 13, 5740. [Google Scholar] [CrossRef]
- Rotaru, D.; Chisnoiu, R.; Picos, A.M.; Picos, A.; Chisnoiu, A. Treatment trends in oral lichen planus and oral lichenoid lesions. Exp. Ther. Med. 2020, 20, 198. [Google Scholar] [CrossRef]
- Andabak-Rogulj, A.; Vindiš, E.; Aleksijević, L.H.; Škrinjar, I.; Juras, D.V.; Ascic, A.; Brzak, B.L. Different Treatment Modalities of Oral Lichen Planus-A Narrative Review. Dent. J. 2023, 11, 26. [Google Scholar] [CrossRef]
- Sridharan, K.; Sivarama Krishnan, G. Interventions for oral lichen planus: A systematic review and network meta-analysis of randomized clinical trials. Aust. Dent. J. 2021, 66, 295–303. [Google Scholar] [CrossRef]
- Sun, S.L.; Liu, J.J.; Zhong, B.; Wang, J.K.; Jin, X.; Xu, H.; Yin, F.Y.; Liu, T.N.; Chen, Q.M.; Zeng, X. Topical calcineurin inhibitors in the treatment of oral lichen planus: A systematic review and meta-analysis. Br. J. Dermatol. 2019, 181, 1166–1176. [Google Scholar] [CrossRef]
- Su, Z.; Hu, J.; Cheng, B.; Tao, X. Efficacy and safety of topical administration of tacrolimus in oral lichen planus: An updated systematic review and meta-analysis of randomized controlled trials. J. Oral Pathol. Med. 2022, 51, 63–73. [Google Scholar] [CrossRef]
- Mattsson, U.; Magnusson, B.; Jontell, M. Squamous cell carcinoma in a patient with oral lichen planus treated with topical application of tacrolimus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 10, e19–e25. [Google Scholar] [CrossRef]
- Polizzi, A.; Santonocito, S.; Lo Giudice, A.; Alibrandi, A.; De Pasquale, R.; Isola, G. Analysis of the response to two pharmacological protocols in patients with oral lichen planus: A randomized clinical trial. Oral Dis. 2023, 29, 755–763. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Gieler, U.; Steinhoff, M. Lichen planus: A comprehensive evidence-based analysis of medical treatment. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1847–1862. [Google Scholar] [CrossRef]
- Chauhan, P.; De, D.; Handa, S.; Narang, T.; Saikia, U.N. A prospective observational study to compare efficacy of topical triamcinolone acetonide 0.1% oral paste, oral methotrexate, and a combination of topical triamcinolone acetonide 0.1% and oral methotrexate in moderate to severe oral lichen planus. Dermatol. Ther. 2018, 31, e12563. [Google Scholar] [CrossRef]
- Beck, H.I.; Brandrup, F. Treatment of erosive lichen planus with dapsone. Acta Derm. Venereol. 1986, 66, 366–367. [Google Scholar]
- Falk, D.K.; Latour, D.L.; King, L.E. Dapsone in the treatment of erosive lichen planus. J. Am. Acad. Dermatol. 1985, 12, 567–570. [Google Scholar] [CrossRef]
- Petruzzi, M.; Lucchese, A.; Lajolo, C.; Campus, G.; Lauriteno, D.; Serpico, R. Topical retinoids in oral lichen planus treatment: An overview. Dermatology 2013, 226, 61–67. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, G.; Zeng, H.; Xiong, C.; Lin, M.; Zhou, H.M. A randomized double-blind, positive-control trial of topical thalidomide in erosive oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 188–195. [Google Scholar] [CrossRef]
- Hasan, S.; Saeed, S.; Rai, A.; Kumar, A.; Choudhury, P.; Panigrahi, R.; Sahu, P.K. Thalidomide: Clinical Implications in Oral Mucosal Lesions—An Update. Ann. Med. Health Sci. Res. 2018, 8, 21–28. [Google Scholar]
- Lu, S.Y.; Chen, W.J.; Eng, H.L. Dramatic response to levamisole and low-dose prednisolone in 23 patients with oral lichen planus: A 6-year prospective follow-up study. Oral Pathol. Oral Radiol. Endod. 1995, 80, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Perween, N.; Saeed, S.; Kaur, M.; Gombra, V.; Rai, A. Evaluation of 5% Amlexenox Oral Paste and Rebamipide Tablets in Treatment of Recurrent Aphthous Stomatitis and Comparison with Dologel CT. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 5228–5234. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhu, X.; Dan, H.; Zhou, Y.; Liu, C.; Wang, F.; Li, Y.; Liu, N.; Chen, Q.; Xu, Y.; et al. Amlexanox is as effective as dexamethasone in topical treatment of erosive oral lichen planus: A short-term pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.I.; EI-Sayed, N.M.; Darwis, Z.E.; Fahmy, R.A. The effect of topically applied hyaluronic acid gel versus topical corticosteroid in the treatment of erosive oral lichen planus. Alex. Dent. J. 2019, 44, 57–63. [Google Scholar] [CrossRef]
- Dillenburg, C.S.; Martins, M.A.; Munerato, M.C.; Marques, M.M.; Carrard, V.C.; Sant’Ana Filho, M.; Castilho, R.M.; Martins, M.D. Efficacy of laser phototherapy in comparison to topical clobetasol for the treatment of oral lichen planus: A randomized controlled trial. J. Biomed. Opt. 2014, 19, 068002. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Kalakonda, B.; Al-Soneidar, W.A.; Al-Shamiri, H.M.; Alakhali, M.S.; Alaizari, N. Efficacy of low-level laser therapy in management of symptomatic oral lichen planus: A systematic review. Lasers Med. Sci. 2017, 32, 1429–1437. [Google Scholar] [CrossRef]
- He, Y.; Deng, J.; Zhao, Y.; Tao, H.; Dan, H.; Xu, H.; Chen, Q. Efficacy evaluation of photodynamic therapy for oral lichen planus: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 302. [Google Scholar] [CrossRef]
- Sandhu, S.; Klein, B.A.; Al-Hadlaq, M.; Chirravur, P.; Bajonaid, A.; Xu, Y.; Intini, R.; Hussein, M.; Vacharotayangul, P.; Sroussi, H.; et al. Oral lichen planus: Comparative efficacy and treatment costs—A systematic review. BMC Oral Health 2022, 22, 161. [Google Scholar] [CrossRef]
- Mutafchieva, M.Z.; Draganova-Filipova, M.N.; Zagorchev, P.I.; Tomov, G.T. Oral lichen planus—Known and unknown: A review. Folia Med. 2018, 60, 528–535. [Google Scholar] [CrossRef]
- Anitua, E.; Eguia, A.; Alkhraisat, M.H.; Pinas, L. Oral Lichen Planus Treated with Plasma Rich in Growth Factors. Cutis 2022, 109, 163–166. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Kramer, M.E.; Keaney, T.C. Systematic review of platelet-rich plasma (PRP) preparation and composition for the treatment of androgenetic alopecia. J. Cosmet. Dermatol. 2018, 17, 666–671. [Google Scholar] [CrossRef]
- Huber, S.C.; de Lima Montalvao, S.A.; Sachetto, Z.; Santos Duarte Lana, J.F.; Annichino-Bizzacchi, J.M. Characterization of autologous platelet rich plasma (PRP) and its biological effects in patients with Behcet’s Disease. Regen. Ther. 2021, 18, 339–346. [Google Scholar] [CrossRef]
- Saif, D.S.; Hegazy, N.N.; Zahran, E.S. Evaluating the Efficacy of Intra-articular Injections of Platelet Rich Plasma (PRP) in Rheumatoid Arthritis Patients and its Impact on Inflammatory Cytokines, Disease Activity and Quality of Life. Curr. Rheumatol. Rev. 2021, 17, 232–241. [Google Scholar] [CrossRef]
- Badsha, H.; Harifi, G.; Murrell, W.D. Platelet-rich plasma for treatment of rheumatoid arthritis: Case series and review of literature. Case Rep. Rheumatol. 2020, 2020, 8761485. [Google Scholar] [CrossRef]
- Shively, D.; Amin, N. Platelet-rich plasma for rheumatoid arthritis: A case series. Cureus 2021, 13, e19629. [Google Scholar] [CrossRef]
- Afify, A.A.; Zuelfakkar, N.M.; Eshafi, M.A. Fractional CO2 laser, platelet rich plasma and narrow band ultraviolet B in the treatment of Vitiligo (A randomized clinical trial). Lasers Med. Sci. 2021, 36, 1479–1486. [Google Scholar] [CrossRef]
- Chakravdhanula, U.; Anbarasu, K.; Verma, V.K.; Beevi, S.S. Clinical efficacy of platelet rich plasma in combination with methotrexate in chronic plaque psoriatic patients. Dermatol. Ther. 2016, 29, 446–450. [Google Scholar] [CrossRef]
- Trink, A.; Sorbellini, E.; Bezzola, P.; Rodella, L.; Rezzani, R.; Ramot, Y.; Rinaldi, F. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br. J. Dermatol. 2013, 169, 690–694. [Google Scholar] [CrossRef]
- Villalpando, B.K.; Wyles, S.P.; Schaefer, L.A.; Bodiford, K.J.; Bruce, A.J. Platelet-rich plasma for the treatment of lichen sclerosus. Plast. Aesthet. Res. 2021, 8, 63. [Google Scholar] [CrossRef]
- Bolanca, Z.; Goren, A.; Getaldic-Svarc, B.; Vucic, M.; Situm, M. Platelet-rich plasma as a novel treatment for lichen planopilaris. Dermatol. Ther. 2016, 29, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Gambino, A. Possible use of platelet gel in ulcerative-erosive oral lesions. In Proceedings of the 5th National and 1st International Symposium of Italian Society of Oral Pathology and Medicine, Ancona, Italy, 19–20 October 2018. [Google Scholar] [CrossRef]
- El-Komy, M.H.M.; Hassan, A.S.; AbdelRaheem, H.M.; Doss, S.S.; EL-Kaliouby, M.; Saleh, N.; Saleh, M.A. Platelet-rich plasma for resistant oral erosions of pemphigus vulgaris: A pilot study. Wound Repair Regen. 2015, 23, 953–955. [Google Scholar] [CrossRef] [PubMed]
- El-Komy, M.H.M.; Saleh, N.; Saleh, M.A. Autologous platelet-rich plasma and triamcinolone acetonide intralesional injection in the treatment of oral erosions of pemphigus vulgaris: A pilot study. Arch. Dermatol. Res. 2018, 310, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Pinas, L.; Alkhraisat, M.H.; Suarez-Fernandez, R.; Anitua, E. Biomolecules in the treatment of lichen planus refractory to corticosteroid therapy: Clinical and histopathological assessment. Ann. Anat. 2018, 216, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Pixley, J.N.; Cook, M.K.; Singh, R.; Larrondo, J.; McMichael, A.J. A comprehensive review of platelet-rich plasma for the treatment of dermatologic disorders. J. Dermatol. Treat. 2023, 34, 2142035. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Desai, R.; Kaye, A.D. Is Platelet-Rich Plasma a Future Therapy in Pain Management? Med. Clin. N. Am. 2016, 100, 199–217. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.S.; Van Eps, J.L.; Cabrera, F.J.; Barbosa, Z.; Del Rosal, G.M.; Weiner, B.K.; Ellsworth, W.A.; Tasciotti, E. Platelet-rich plasma: A bio mimetic approach to enhancement of surgical wound healing. J. Surg. Res. 2017, 207, 33–44. [Google Scholar] [CrossRef]
- Merigo, E.; Oppici, A.; Parlatore, A.; Cella, L.; Clini, F.; Fontana, M.; Fornaini, C. Platelet-Rich Plasma (PRP) Rinses for the Treatment of Non-Responding Oral Lichen Planus: A Case Report. Biomedicines 2018, 6, 15. [Google Scholar] [CrossRef]
- Shaik, S.; Jyothi, P.N.; Kumar, B.V.; Suman, S.V.; Praveen, K.S.; Sravanthi, M. Platelet rich plasma a new prospective in treatment of recalcitrant erosive lichen planus–a case report. Int. J. Res. Rep. Dent. 2020, 3, 1–5. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.E.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias Visualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Meth. 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Loré, B.; Saraceno, R.; Poladas, G.; Fida, M.; Khoury, C.; Arcuri, C.; Magnato, R. Oral lichen planus: Therapy and phenotype. G. Ital. Dermatol. Venereol. 2018, 153, 459–463. [Google Scholar] [CrossRef]
- Ahuja, U.S.; Puri, N.; More, C.B.; Gupta, R.; Gupta, D. Comparative evaluation of effectiveness of autologous platelet rich plasma and intralesional corticosteroids in the management of erosive oral Lichen planus—A clinical study. J. Oral Biol. Craniofac. Res. 2020, 10, 714–718. [Google Scholar] [CrossRef]
- EL-Shinnawi, U.; Mowafey, B. Clinical Evaluation of The Efficiency of Intralesional Injection of Autologous Platelet Rich Plasma In Treatment Of Erosive Oral Lichen planus. Egypt. Dental. J. 2021, 67, 457–463. [Google Scholar] [CrossRef]
- Hijazi, A.H.; Ahmed, W.; Gaafar, S. Efficacy of intralesional injections of platelet-rich plasma in patients with oral lichen planus: A pilot randomized clinical trial. Clin. Exp. Dent. Res. 2022, 8, 707–714. [Google Scholar] [CrossRef]
- ElGhareeb, M.I.; Ghoneimy, S.; Elsayed, A. Intralesional injection of platelet-rich plasma versus steroid in the treatment of oral lichen planus. J. Cosmet. Dermatol. 2023. [Google Scholar] [CrossRef]
- Deng, X.; Wang, Y.; Jiang, L.; Li, J.; Chen, Q. Updates on immunological mechanistic insights and targeting of the oral lichen planus microenvironment. Front. Immunol. 2023, 13, 1023213. [Google Scholar] [CrossRef]
- Ashkar, S.; Weber, G.F.; Panoutsakopoulou, V.; Sanchirico, M.E.; Jansson, M.; Zawaideh, S.; Rittling, S.R.; Denhardt, D.T.; Glimcher, M.J.; Cantor, H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 2000, 287, 860–864. [Google Scholar] [CrossRef]
- Stoop, R.; Gál, I.; Glant, T.; McNeish, J.D.; Mikecz, K. Trafficking of CD44-deficient murine lymphocytes under normal and inflammatory conditions. Eur. J. Immunol. 2002, 32, 2532–2542. [Google Scholar] [CrossRef]
- Chaiyarit, P.; Jintakanon, D.; Klanrit, P.; Siritapetawee, M.; Thongprasom, K. Immunohistochemical analyses of survivin and heat shock protein 90 expression in patients with oral lichen planus. J. Oral Pathol. Med. 2009, 38, 55–62. [Google Scholar] [CrossRef]
- Okada, H.; Mak, T.W. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat. Rev. Cancer 2004, 4, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, A.; Mascitti, M.; Rubini, C.; Bambini, F.; Zizzi, A.; Offidani, A.; Ganzetti, G.; Laino, L.; Cicciù, M.; Muzio, L.L. Active inflammatory biomarkers in oral lichen planus. Int. J. Immunopathol. Pharmacol. 2015, 28, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Toader, M.P.; Taranu, T.; Constantin, M.M.; Olinici, D.; Mocanu, M.; Costan, V.V.; Toader, S. High serum level of interleukin-6 is linked with dyslipidemia in oral lichen planus. Exp. Ther. Med. 2021, 22, 987. [Google Scholar] [CrossRef] [PubMed]
- Bagan, J.; Compilato, D.; Paderni, C.; Campisi, G.; Panzarella, V.; Picciotti, M.; Lorenzini, G.; Di Fede, O. Topical therapies for oral lichen planus management and their efficacy: A narrative review. Curr. Pharm. Des. 2012, 18, 5470–5480. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Alanazi, R.; Alhajj, M.N.; Daer, A.; Hunaish, A.A.; Nabhan, A.B.; Al-Sosowa, A.A. Efficacy of topical hyaluronic acid for symptomatic oral lichen planus: A Systematic Review. J. Oral Res. 2021, 10, 9946. [Google Scholar] [CrossRef]
- Andia, I.; Abate, M. Platelet rich plasma: Underlying biology and clinical correlates. Regen. Med. 2013, 8, 645–658. [Google Scholar] [CrossRef]
- Andia, I. Platelet-rich plasma biology. In Clinical Indications and Treatment Protocols with Platelet-Rich Plasma in Dermatology; Alves, R., Grimalt, R., Eds.; Ediciones Mayo: Barcelona, Spain, 2016; pp. 3–15. [Google Scholar]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Current clinical recommendations for use of platelet-rich plasma. Curr. Rev. Musculoskelet. Med. 2018, 11, 624–634. [Google Scholar] [CrossRef]
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.; Dellinger, E.P.; Ko, C.Y.; Duane, T.M.; et al. American College of Surgeons and Surgical Infection Society: Surgical site infection guidelines, 2016 update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef]
- Hamman, B.L.; Stout, L.Y.; Theologes, T.T.; Sass, D.M.; da Graca, B.; Filardo, G. Relation between topical application of platelet-rich plasma and vancomycin and severe deep sternal wound infections after a first median sternotomy. Am. J. Cardiol. 2014, 113, 1415–1419. [Google Scholar] [CrossRef]
- Patel, A.N.; Selzman, C.H.; Kumpati, G.S.; McKellar, S.H.; Bull, D.A. Evaluation of autologous platelet rich plasma for cardiac surgery: Outcome analysis of 2000 patients. J. Cardiothorac. Surg. 2016, 11, 62. [Google Scholar] [CrossRef]
- Dorge, H.; Sellin, C.; Bury, M.C.; Drescher, A.; Seipelt, R.; Grossmann, M.; Danner, B.C.; Schoendube, F.A. Incidence of deep sternal wound infection is not reduced with autologous platelet rich plasma in high-risk cardiac surgery patients. Thorac. Cardiovasc. Surg. 2013, 61, 180–184. [Google Scholar] [CrossRef]
- Albanese, A.; Licata, M.E.; Polizzi, B.; Campisi, G. Platelet-rich plasma (PRP) in dental and oral surgery: From the wound healing to bone regeneration. Immun. Ageing 2013, 10, 23. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. Platelet-Rich Plasma and its Use for Cicatricial and Non-Cicatricial Alopecias: A Narrative Review. Dermatol. Ther. 2020, 10, 623–633. [Google Scholar] [CrossRef]
- Hrycay, E.G.; Bandiera, S.M. Involvement of cytochrome P450 in reactive oxygen species formation and cancer. Adv. Pharmacol. 2015, 74, 35–84. [Google Scholar] [CrossRef]
- Kesarwala, A.H.; Krishna, M.C.; Mitchell, J.B. Oxidative stress in oral diseases. Oral Dis. 2016, 22, 9–18. [Google Scholar] [CrossRef]
- Panchal, F.H.; Ray, S.; Munshi, R.P.; Bhalerao, S.S.; Nayak, C.S. Alterations in lipid metabolism and antioxidant status in lichen planus. Indian J. Dermatol. 2015, 60, 439–444. [Google Scholar] [CrossRef]
- Tohidnezhad, M.; Wruck, C.J.; Slowik, A.; Kweider, N.; Beckmann, R.; Bayer, A.; Houben, A.; Brandenburg, L.O.; Varoga, D.; Sönmez, T.T.; et al. Role of platelet-released growth factors in detoxification of reactive oxygen species in osteoblasts. Bone 2014, 65, 9–17. [Google Scholar] [CrossRef]
- Rhodus, N.L.; Cheng, B.; Myers, S.; Miller, L.; Ho, V.; Ondrey, F. The feasibility of monitoring NF-kappa B associated cytokines: TNF-alpha, IL-1alpha, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol. Carcinog. 2005, 44, 77–82. [Google Scholar] [CrossRef]
- Giannopoulou, M.; Dai, C.; Tan, X.; Wen, X.; Michalopoulos, G.K.; Liu, Y. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-κB signaling. Am. J. Pathol. 2008, 173, 30–41. [Google Scholar] [CrossRef]
- Saxena, A.; Khosraviani, S.; Noel, S.; Mohan, D.; Donner, T.; Hamad, A.R. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 2015, 74, 27–34. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef]
- Cognasse, F.; Boussoulade, F.; Chavarin, P.; Acquart, S.; Fabrigli, P.; Lamy, B.; Garraud, O. Release of potential immunomodulatory factors during platelet storage. Transfusion 2006, 46, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef]
- Srivastava, K.; Cockburn, I.A.; Swaim, A.; Thompson, L.E.; Tripathi, A.; Fletcher, C.A.; Shirk, E.M.; Sun, H.; Kowalska, M.A.; Fox-Talbot, K.; et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe 2008, 4, 179–187. [Google Scholar] [CrossRef]
- Liu, C.Y.; Battaglia, M.; Lee, S.H.; Sun, Q.H.; Aster, R.H.; Visentin, G.P. Platelet factor 4 differentially modulates CD4+CD25+ (regulatory) versus CD4+CD25- (nonregulatory) T cells. J. Immunol. 2005, 174, 2680–2686. [Google Scholar] [CrossRef]
- Shi, G.; Field, D.J.; Ko, K.A.; Ture, S.; Srivastava, K.; Levy, S.; Kowalska, M.A.; Poncz, M.; Fowell, D.J.; Morrell, C.N. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. J. Clin. Investig. 2014, 124, 543–552. [Google Scholar] [CrossRef]
- Chainani-Wu, N.; Silverman, S., Jr.; Reingold, A.; Bostrom, A.; Lozada-Nur, F.; Weintraub, J. Validation of instruments to measure the symptoms and signs of oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 51–58. [Google Scholar] [CrossRef]
- Wiriyakijja, P.; Porter, S.; Fedele, S.; Hodgson, T.; McMillan, R.; Shephard, M.; Riordain, R.N. The patient acceptable symptom state in oral lichen planus: Identification of cut-off threshold scores in measures of pain and quality of life. Clin. Oral Investig. 2021, 25, 3699–3709. [Google Scholar] [CrossRef]
- Thongprasom, K.; Luangjarmekorn, L.; Sererat, T.; Taweesap, W. Relative efficacy of fluocinolone acetonide compared with triamcinolone acetonide in treatment of oral lichen planus. J. Oral Pathol. Med. 1992, 21, 456–458. [Google Scholar] [CrossRef]
- Escudier, M.; Ahmed, N.; Shirlaw, P.; Setterfield, J.; Tappuni, A.; Black, M.M.; Challacombe, S.J. A scoring system for mucosal disease severity with special reference to oral lichen planus. Br. J. Dermatol. 2007, 157, 765–770. [Google Scholar] [CrossRef]
- Piboonniyom, S.-O.; Treister, N.; Pitiphat, W.; Woo, S.-B. Scoring system for monitoring oral lichenoid lesions: A preliminary study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 696–703. [Google Scholar] [CrossRef]
- Baek, K.; Choi, Y. The microbiology of oral lichen planus: Is microbial infection the cause of oral lichen planus? Mol. Oral Microbiol. 2018, 33, 22–28. [Google Scholar] [CrossRef]
- Du, G.H.; Wang, Y.F.; Chen, J.J.; Deng, Y.W.; Han, X.Z.; Tang, G.Y. Potential association between Fusobacterium nucleatum enrichment on oral mucosal surface and oral lichen planus. Oral Dis. 2020, 26, 122–130. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home oral care of periodontal patients using antimicrobial gel with postbiotics, lactoferrin, and aloe barbadensis leaf juice powder vs. conventional chlorhexidine gel: A split-mouth randomized clinical trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]

| S. No. | Salient Features | Mechanism |
|---|---|---|
| 1. | Anti-oxidant and Anti-inflammatory | PRP treatment can prevent oxidative damage by activating nuclear factor (derived-erythrocyte) type-2 (Nrf2), which in turn increases the signaling of antioxidant response elements. Hepatocyte growth factor (HGF), a potent anti-inflammatory cytokine inhibits the NF-κB signaling mechanism, thereby reducing inflammation. Platelets in PRP may serve as a potential source of inflammatory mediators and regulators, and release a host of anti-inflammatory cytokines. e.g., IL-1 receptor antagonist (IL-1ra), soluble tumor necrosis factor (TNF) receptor (sTNF-R) I and II, IL-4, IL-10, IL-13, and interferon γ. IL-1ra suppresses IL-1’s bioactivity by blocking its receptors. sTNF-RI and RII can attach themselves to free TNFα, which curbs signal transduction. IL-4, IL-10, and IL-13 can promote the generation of IL-1ra and decrease the production of TNFα-induced prostaglandin E2. Interferon γ stimulates the production of IL-18-binding protein, which inhibits IL-18 production. |
| 2. | Immunomodulatory | Platelets in PRP release various substances capable of modulating the inflammatory reaction by interacting with leukocytes and endothelial cells. Among the most prominent immunomodulators are transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), soluble ligand (sCD40L), and platelet factor 4 (PF4). TGF-β serves as the primary immunosuppressant and differentiation of T regulator cells (Treg) depend on TGF-β. CD40L, present mainly on activated platelets and T cells, acts as a transmembrane protein with a significant function in both innate and adaptive immune systems. Platelet factor 4 (PF4), a protein released from α-granules of activated platelets, assists in T cell trafficking, and may also play a role in Treg development. PRP may have a significant immunological role in sustaining Th cell homeostasis and limiting the Th17 cell development and response. |
| 3. | Wound healing and tissue regeneration | Growth factors in PRP (PDGF, VEGF, EGF, IGF, TGF-β, and fibronectin) promotes cell migration and proliferation, angiogenesis, and tissue regeneration. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriram, S.; Hasan, S.; Alqarni, A.; Alam, T.; Kaleem, S.M.; Aziz, S.; Durrani, H.K.; Ajmal, M.; Dawasaz, A.A.; Saeed, S. Efficacy of Platelet-Rich Plasma Therapy in Oral Lichen Planus: A Systematic Review. Medicina 2023, 59, 746. https://doi.org/10.3390/medicina59040746
Sriram S, Hasan S, Alqarni A, Alam T, Kaleem SM, Aziz S, Durrani HK, Ajmal M, Dawasaz AA, Saeed S. Efficacy of Platelet-Rich Plasma Therapy in Oral Lichen Planus: A Systematic Review. Medicina. 2023; 59(4):746. https://doi.org/10.3390/medicina59040746
Chicago/Turabian StyleSriram, Shyamkumar, Shamimul Hasan, Abdullah Alqarni, Tanveer Alam, Sultan Mohammed Kaleem, Shahid Aziz, Humayoun Khan Durrani, Muhammed Ajmal, Ali Azhar Dawasaz, and Shazina Saeed. 2023. "Efficacy of Platelet-Rich Plasma Therapy in Oral Lichen Planus: A Systematic Review" Medicina 59, no. 4: 746. https://doi.org/10.3390/medicina59040746
APA StyleSriram, S., Hasan, S., Alqarni, A., Alam, T., Kaleem, S. M., Aziz, S., Durrani, H. K., Ajmal, M., Dawasaz, A. A., & Saeed, S. (2023). Efficacy of Platelet-Rich Plasma Therapy in Oral Lichen Planus: A Systematic Review. Medicina, 59(4), 746. https://doi.org/10.3390/medicina59040746








