Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Venous Combined Micro-Macrothrombosis
Abstract
:1. Introduction
2. Endotheliopathy
3. Thrombocytopenia
4. Thrombosis
- Macrothrombosis includes DVT, VTE, CVST, PTE, splanchnic vein thrombosis (SVT) and Budd-Chiari syndrome, portal vein thrombosis, acute ischemic stroke, acute myocardial infarction, aortic thrombus and renal vein thrombosis, etc.
- Microthrombosis (i.e., VMTD) occurs in TTP, TTP-like syndrome, ARDS, diffuse encephalopathic stroke, microvascular myocardial infarction, hemolytic-uremic syndrome, transient ischemic attack, microaneurysm and thrombosis of retinal artery, MODS, and hepatic veno-occlusive disease.
- Heparin-induced thrombocytopenia with thrombosis syndrome, especially white clot syndrome, is a unique aberrant hemostatic disease without vascular injury.
- Gangrene syndrome associated with arterial combined micro-macrothrombosis includes symmetrical peripheral gangrene (SPG), purpura fulminans, Fournier’s gangrene in acute promyelocytic leukemia (APL), Burger’s disease, diabetic gangrene, Raynaud’s phenomenon, and acute necrotizing fasciitis.
- Fibrin clot disease occurs in APL as aberrant hemostatic disease without vascular injury.
- Acute “disseminated intravascular coagulation (DIC)” is a form of VMTD with hemorrhagic complication.
- Concurrent microthrombosis and macrothrombosis in both arterial and venous systems in paroxysmal nocturnal hemoglobinuria (PNH) is an unresolved disease yet.
5. Cerebral Venous Sinus Thrombosis
6. Pathogenesis of Venous Combined Micro-macrothrombotic Syndromes
7. Molecular Evidence of Venous Endotheliopathy
8. Diagnostic and Therapeutic Consideration
8.1. Diagnostic Perspective
8.2. Therapeutic Approach
8.3. “Special Note” on TTP-Like Syndrome and ADAMTS13 after COVID-19 Vaccines
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franchini, M.; Liumbruno, G.M.; Pezzo, M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. Eur. J. Haematol. 2021, 107, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.A.; Parsons, G.T.; Gering, S.K.; Meier, A.R.; Hutchinson, I.V.; Robicsek, A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA 2021, e2113443. [Google Scholar]
- Chang, J.C. Acute Respiratory Distress Syndrome as an Organ Phenotype of Vascular Microthrombotic Disease: Based on Hemostatic Theory and Endothelial Molecular Pathogenesis. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619887437. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.C. COVID-19 Sepsis: Pathogenesis and Endothelial Molecular Mechanisms Based on “Two-Path Unifying Theory” of Hemostasis and Endotheliopathy-Associated Vascular Microthrombotic Disease, and Proposed Therapeutic Approach with Antimicrothrombotic Therapy. Vasc. Health Risk Manag. 2021, 17, 273–298. [Google Scholar] [CrossRef]
- Chang, J.C. Sepsis and septic shock: Endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb. J. 2019, 17, 10. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.C. TTP-like syndrome: Novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb. J. 2018, 16, 20. [Google Scholar] [CrossRef] [Green Version]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef]
- Huisman, W.; Martina, B.E.; Rimmelzwaan, G.F.; Gruters, R.A.; Osterhaus, A.D. Vaccine-induced enhancement of viral infections. Vaccine 2009, 27, 505–512. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef]
- Kuter, D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br. J. Haematol. 2021. [Google Scholar] [CrossRef]
- Abdalkader, M.; Shaikh, S.P.; Siegler, J.E.; Cervantes-Arslanian, A.M.; Tiu, C.; Radu, R.A.; Tiu, V.E.; Jillella, D.V.; Mansour, O.Y.; Vera, V.; et al. Cerebral Venous Sinus Thrombosis in COVID-19 Patients: A Multicenter Study and Review of Literature. J. Stroke Cerebrovasc. Dis. 2021, 30, 105733. [Google Scholar] [CrossRef]
- Pishko, A.M.; Bussel, J.B.; Cines, D.B. COVID-19 vaccination and immune thrombocytopenia. Nat. Med. 2021, 27, 1145–1146. [Google Scholar] [CrossRef] [PubMed]
- Saudagar, V.; Patil, S.; Goh, S.; Pothiawala, S. Vigilance regarding immune thrombocytopenic purpura after COVID-19 vaccine. Ir. J. Med. Sci. 2021, 1–2. [Google Scholar]
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination: A systematic review. J. Neurol. Sci. 2021, 428, 117607. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J.G.; Gupta, A.; Sardar, P.; Henry, B.M.; Lewin, J.C.; Lippi, G.; Lavie, C.J. Clinical Characteristics and Pharmacological Management of COVID-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia With Cerebral Venous Sinus Thrombosis: A Review. JAMA Cardiol. 2021. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.Y. Myocarditis after Covid-19 mRNA Vaccination. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- The Chief Medical Office of Health Alberta. Myocarditis and/or Pericarditis following COVID-19 Vaccines. Available online: https://www.alberta.ca/assets/documents/health-myocarditis-and-pericarditis-following-covid.pdf (accessed on 22 July 2021).
- Chang, J.C. Thrombocytopenia in critically ill patients due to vascular microthrombotic disease: Pathogenesis based on “two-activation theory of the endothelium”. Vascul. Dis. Ther. 2017, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zufferey, A.; Kapur, R.; Semple, J.W. Pathogenesis and Therapeutic Mechanisms in Immune Immune Thrombocytopenia (ITP). J. Clin. Med. 2017, 6, 16. [Google Scholar] [CrossRef]
- Audia, S.; Mahévas, M.; Samson, M.; Godeau, B.; Bonnotte, B. Pathogenesis of immune thrombocytopenia. Autoimmun. Rev. 2017, 16, 620–632. [Google Scholar] [CrossRef]
- LeVine, D.N.; Brooks, M.B. Immune thrombocytopenia (ITP): Pathophysiology update and diagnostic dilemmas. Vet. Clin. Pathol. 2019, 48 (Suppl. 1), 17–28. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, J. Pathogenesis in immune thrombocytopenia: New insights. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 306–312. [Google Scholar] [CrossRef]
- McGonagle, D.; De Marco, G.; Bridgewood, C. Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection. J. Autoimmun. 2021, 121, 102662. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. Laboratory testing for suspected COVID-19 vaccine-induced (immune) thrombotic thrombocytopenia. Int. J. Lab. Hematol. 2021, 43, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Terpos, E.; Politou, M.; Ntanasis-Stathopoulos, I.; Karalis, V.; Merkouri, E.; Fotiou, D.; Gavriatopoulou, M.; Malandrakis, P.; Kastritis, E.; Trougakos, I.P.; et al. High Prevalence of Anti-PF4 Antibodies Following ChAdOx1 nCov-19 (AZD1222) Vaccination Even in the Absence of Thrombotic Events. Vaccines 2021, 9, 712. [Google Scholar] [CrossRef]
- Chang, J.C. Hemostasis based on a novel ‘two-path unifying theory’ and classification of hemostatic disorders. Blood Coagul. Fibrinolysis. 2018, 29, 573–584. [Google Scholar] [CrossRef] [PubMed]
- İremli, B.G.; Şendur, S.N.; Ünlütürk, U. Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine: Post-vaccination ASIA Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 2066–2605. [Google Scholar] [CrossRef]
- Domiguez, M.P.; Medina, G.; Sánchez Valadez, T.I.; Jara, L.J. Two Cases of Graves’ Disease Following SARS-CoV-2 Vaccination: An Autoimmune/Inflammatory Syndrome Induced by Adjuvants. Thyroid 2021, 31. [Google Scholar]
- Keshavarz, P.; Yazdanpanah, F.; Rafiee, F.; Mizandari, M. Lymphadenopathy Following COVID-19 Vaccination: Imaging Findings Review. Acad. Radiol. 2021, 28, 1058–1071. [Google Scholar] [CrossRef]
- Hiller, N.; Goldberg, S.N.; Cohen-Cymberknoh, M.; Vainstein, V.; Simanovsky, N. Lymphadenopathy Associated with the COVID-19 Vaccine. Cureus 2021, 13, e13524. [Google Scholar]
- Talotta, R.; Robertson, E.S. Antiphospholipid antibodies and risk of post-COVID-19 vaccination thrombophilia: The straw that breaks the camel’s back? Cytokine Growth Factor Rev. 2021, 60, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Uzun, G.; Althaus, K.; Bakchoul, T. No Correlation between Anti-PF4 and Anti-SARS-CoV-2 Antibodies after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 385, 1334–1336. [Google Scholar] [CrossRef]
- Castillo-Martínez, D.; Torres, Z.; Amezcua-Guerra, L.M.; Pineda, C. Are antiphospholipid antibodies just a common epiphenomenon or are they causative of immune-mediated coagulopathy in COVID-19? Clin. Rheumatol. 2021, 40, 3015–3019. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.T.; Engebjerg, M.C.; Farkas, D.K.; Jensen, A.Ø.; Nørgaard, M.; Zhao, S.; Sørensen, H.T. Risk of venous thromboembolism in patients with primary chronic immune thrombocytopenia: A Danish population-based cohort study. Br. J. Haematol. 2011, 152, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F. ITP and thrombosis: An intriguing association. Blood Adv. 2017, 1, 2280. [Google Scholar] [CrossRef]
- Moulis, G.; Audemard-Verger, A.; Arnaud, L.; Luxembourger, C.; Montastruc, F.; Gaman, A.M.; Svenungsson, E.; Ruggeri, M.; Mahevas, M.; Gerfaud-Valentin, M.; et al. Risk of thrombosis in patients with primary immune thrombocytopenia and antiphospholipid antibodies: A systematic review and meta-analysis. Autoimmun. Rev. 2016, 15, 203–209. [Google Scholar] [CrossRef]
- Chang, J.C. Thrombogenesis and thrombotic disorders based on ‘two-path unifying theory of hemostasis’: Philosophical, physiological, and phenotypical interpretation. Blood Coagul Fibrinolysis 2018, 29, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Stroke Classification: Critical Role of Unusually Large von Willebrand Factor Multimers and Tissue Factor on Clinical Phenotypes Based on Novel “Two-Path Unifying Theory” of Hemostasis. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620913634. [Google Scholar] [CrossRef]
- Chang, J.C. Disseminated intravascular coagulation: New identity as endotheliopathy-associated vascular microthrombotic disease based on in vivo hemostasis and endothelial molecular pathogenesis. Thromb. J. 2020, 18, 25. [Google Scholar] [CrossRef]
- Giladi, O.; Steinberg, D.M.; Peleg, K.; Tanne, D.; Givon, A.; Grossman, E.; Klein, Y.; Avigdori, S.; Greenberg, G.; Katz, R.; et al. Head trauma is the major risk factor for cerebral sinus-vein thrombosis. Thromb. Res. 2016, 137, 26–29. [Google Scholar] [CrossRef]
- Salih, F.; Schönborn, L.; Kohler, S.; Franke, C.; Möckel, M.; Dörner, T.; Bauknecht, H.C.; Pille, C.; Graw, J.A.; Alonso, A.; et al. Vaccine-Induced Thrombocytopenia with Severe Headache. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shahsavarzadeh, T.; Javanmard, S.H.; Saadatnia, M. Impact of factor VIII and von Willebrand factor plasma levels on cerebral venous and sinus thrombosis: Are they independent risk factors? Eur. Neurol. 2011, 66, 243–246. [Google Scholar] [CrossRef]
- Bugnicourt, J.M.; Roussel, B.; Tramier, B.; Lamy, C.; Godefroy, O. Cerebral venous thrombosis and plasma concentrations of factor VIII and von Willebrand factor: A case control study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 699–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, A.; Wilson, E.; Harber, M.; Brunton, C.; Sweny, P. Cerebral venous sinus thrombosis in minimal change nephrotic syndrome. Nephrol. Dial. Transplant. 1995, 10, 30–34. [Google Scholar] [CrossRef]
- Bolayır, A.; Çiğdem, B. The relationship between ABO blood types and development of cerebral venous sinus thrombosis. Cum. Med. J. 2017, 39, 602–607. [Google Scholar] [CrossRef]
- Rawala, M.S.; Noorani, M.M.; Gulati, R.; Waqas, S.; Dave, D. Elevated Factor VIII Level Associated with Transverse Cerebral Venous Sinus Thrombosis. Am. J. Case Rep. 2019, 20, 274–277. [Google Scholar] [CrossRef]
- De Michele, M.; Iacobucci, M.; Chistolini, A.; Nicolini, E.; Pulcinelli, F.; Cerbelli, B.; Merenda, E.; Schiavo, O.G.; Sbardella, E.; Berto, I.; et al. Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: A catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 2021, 12, 4663. [Google Scholar] [CrossRef]
- Anadure, R.K.; Nagaraja, D.; Christopher, R. Plasma factor VIII in non-puerperal cerebral venous thrombosis: A prospective case-control study. J. Neurol. Sci. 2014, 339, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Vecht, L.; Zuurbier, S.M.; Meijers, J.C.M.; Coutinho, J.M. Elevated factor VIII increases the risk of cerebral venous thrombosis: A case-control study. J. Neurol. 2018, 265, 1612–1617. [Google Scholar] [CrossRef]
- Yokota, H.; Ida, Y.; Sugiura, S.; Sasaki, K.; Itoh, H. Cerebral venous sinus thrombosis with increased factor VIII activity in an adult with iron deficiency anemia. Neurol. India 2014, 62, 674–675. [Google Scholar] [CrossRef]
- Thachil, J. Lessons learnt from COVID-19 coagulopathy. EJHaem 2021. [Google Scholar] [CrossRef] [PubMed]
- Afshari, F.T.; Yakoub, K.M.; Zisakis, A.; Thomas, A.; Ughratdar, I.; Sturman, S.; Belli, A. Traumatic dural venous sinus thrombosis; a challenge in management of head injury patients. J. Clin. Neurosci. 2018, 57, 169–173. [Google Scholar] [CrossRef]
- Mastellos, D.C.; Skendros, P.; Lambris, J.D. Is complement the culprit behind COVID-19 vaccine-related adverse reactions? J. Clin. Investig. 2021, 131, e151092. [Google Scholar] [CrossRef]
- Behet, M.C.; Kurtovic, L.; van Gemert, G.J.; Haukes, C.M.; Siebelink-Stoter, R.; Graumans, W.; van de Vegte-Bolmer, M.G.; Scholzen, A.; Langereis, J.D.; Diavatopoulos, D.A.; et al. The Complement System Contributes to Functional Antibody-Mediated Responses Induced by Immunization with Plasmodium falciparum Malaria Sporozoites. Infect. Immun. 2018, 86, e00920-17. [Google Scholar] [CrossRef] [Green Version]
- Lind, L.; Hulthe, J.; Johansson, A.; Hedner, E. Endotoxin-induced and vaccine-induced systemic inflammation both impair endothelium-dependent vasodilation, but not pulse wave reflection. Vasc. Health Risk Manag. 2012, 8, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapp, B.R.; Hingorani, A.D.; Kharbanda, R.K.; Mohamed-Ali, V.; Stephens, J.W.; Vallance, P.; MacAllister, R.J. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc. Res. 2004, 64, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yuan, X.; Chen, H.; Chaturvedi, S.; Braunstein, E.M.; Brodsky, R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood 2020, 136, 2080–2089. [Google Scholar] [CrossRef]
- Hattori, R.; Hamilton, K.K.; McEver, R.P.; Sims, P.J. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J. Biol. Chem. 1989, 264, 9053–9060. [Google Scholar] [CrossRef]
- Hamad, I.; Hunter, A.C.; Szebeni, J.; Moghimi, S.M. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol. Immunol. 2008, 46, 225–232. [Google Scholar] [CrossRef]
- Pannuzzo, M.; Esposito, S.; Wu, L.P.; Key, J.; Aryal, S.; Celia, C.; Di Marzio, L.; Moghimi, S.M.; Decuzzi, P. Overcoming Nanoparticle-Mediated Complement Activation by Surface PEG Pairing. Nano Lett. 2020, 20, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Wibroe, P.P.; Petersen, S.V.; Bovet, N.; Laursen, B.W.; Moghimi, S.M. Soluble and immobilized graphene oxide activates complement system differently dependent on surface oxidation state. Biomaterials 2016, 78, 20–26. [Google Scholar] [CrossRef]
- Szebeni, J.; Muggia, F.; Gabizon, A.; Barenholz, Y. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: Prediction and prevention. Adv. Drug Deliv. Rev. 2011, 63, 1020–1030. [Google Scholar] [CrossRef]
- Lin, J.J.; Hsiao, H.J.; Chan, O.W.; Wang, Y.; Hsia, S.H.; Chiu, C.H. Increased serum thrombomodulin level is associated with disease severity and mortality in pediatric sepsis. PLoS ONE 2017, 12, e0182324. [Google Scholar] [CrossRef] [Green Version]
- Lutz, H.U.; Stammler, P.; Bianchi, V.; Trüeb, R.M.; Hunziker, T.; Burger, R.; Jelezarova, E.; Späth, P.J. Intravenously applied IgG stimulates complement attenuation in a complement-dependent autoimmune disease at the amplifying C3 convertase level. Blood 2004, 103, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.M.; Basta, M.; Fries, L.F. The effects of intravenous immune globulin on complement-dependent immune damage of cells and tissues. Clin. Immunol. Immunopathol. 1992, 62 Pt 2, S82–S86. [Google Scholar] [CrossRef]
- McCrae, K. Immune Thrombocytopenia with Pulmonary Embolism and Deep-Vein Thrombosis: Recommendations for Bone Marrow Aspirate and Biopsy. Hematologist 2011, 8, 6. [Google Scholar] [CrossRef]
- Sarpatwari, A.; Bennett, D.; Logie, J.W.; Shukla, A.; Beach, K.J.; Newland, A.C.; Sanderson, S.; Provan, D. Thromboembolic events among adult patients with primary immune thrombocytopenia in the United Kingdom General Practice Research Database. Haematologica 2010, 95, 1167–1175. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Yavaşoğlu, İ. Vaccination and Thrombotic Thrombocytopenic Purpura. Turk. J. Haematol. 2020, 37, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, J.; Schnetzke, U.; Kentouche, K.; Prims, F.; Baier, M.; Herfurth, K.; Schlosser, M.; Busch, M.; Hochhaus, A.; Wolf, G. Acquired thrombotic thrombocytopenic purpura after first vaccination dose of BNT162b2 mRNA COVID-19 vaccine. Ann. Hematol. 2021, 1–3. [Google Scholar]
- Sissa, C.; Al-Khaffaf, A.; Frattini, F.; Gaiardoni, R.; Mimiola, E.; Montorsi, P.; Melara, B.; Amato, M.; Peyvandi, F.; Franchini, M. Relapse of thrombotic thrombocytopenic purpura after COVID-19 vaccine. Transfus. Apher. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.H.B.; Khan, A.A.; WMemon, S. Thrombotic thrombocytopenic purpura: A new menace after COVID bnt162b2 vaccine. Int. J. Hematol. 2021, 114, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Selvaratnam, V.; Rajasuriar, J.S. Thrombotic thrombocytopenic purpura after ChAdOx1 nCoV-19 vaccine. BMJ Case Rep. 2021, 14, e246049. [Google Scholar] [CrossRef] [PubMed]
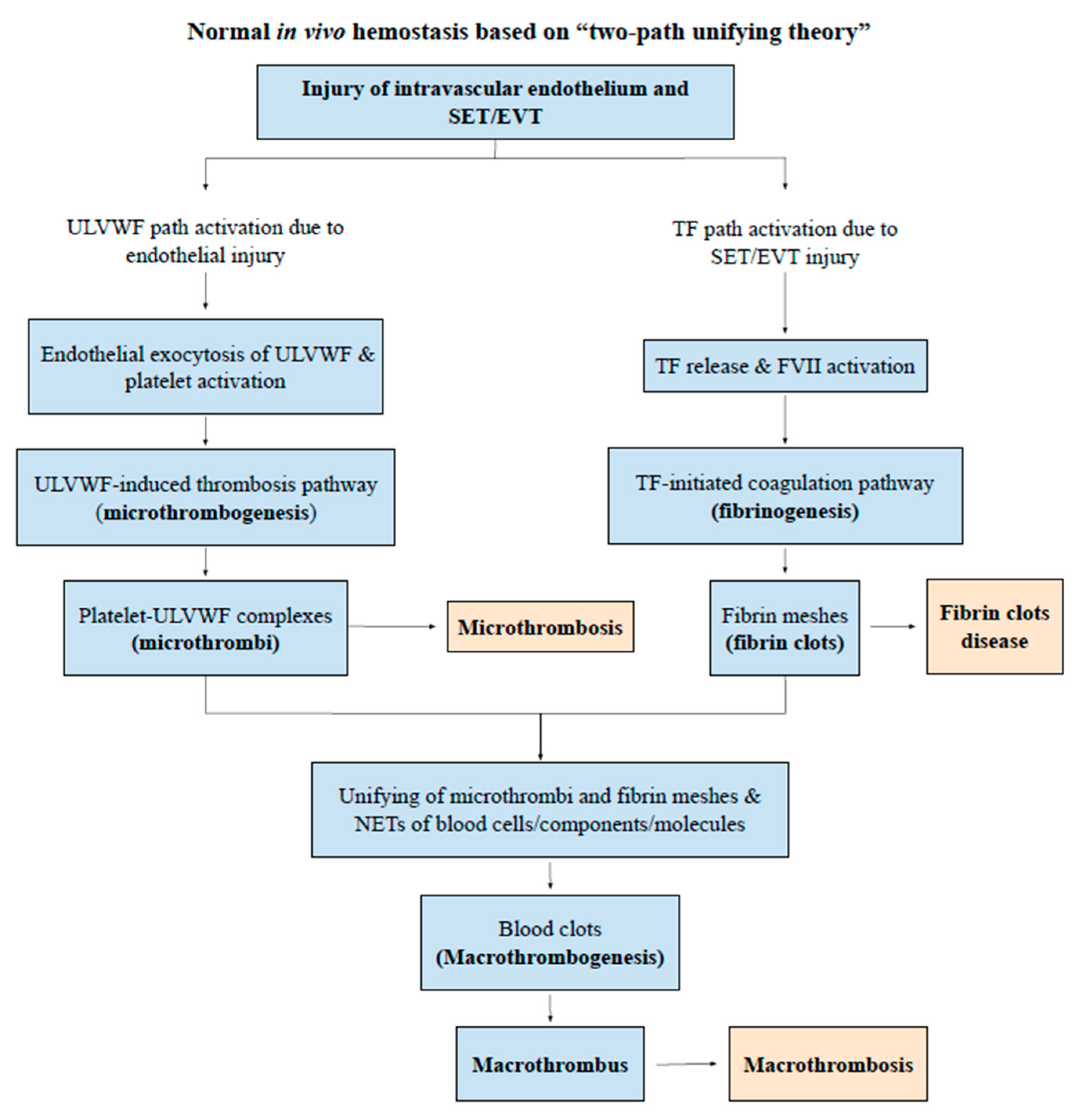
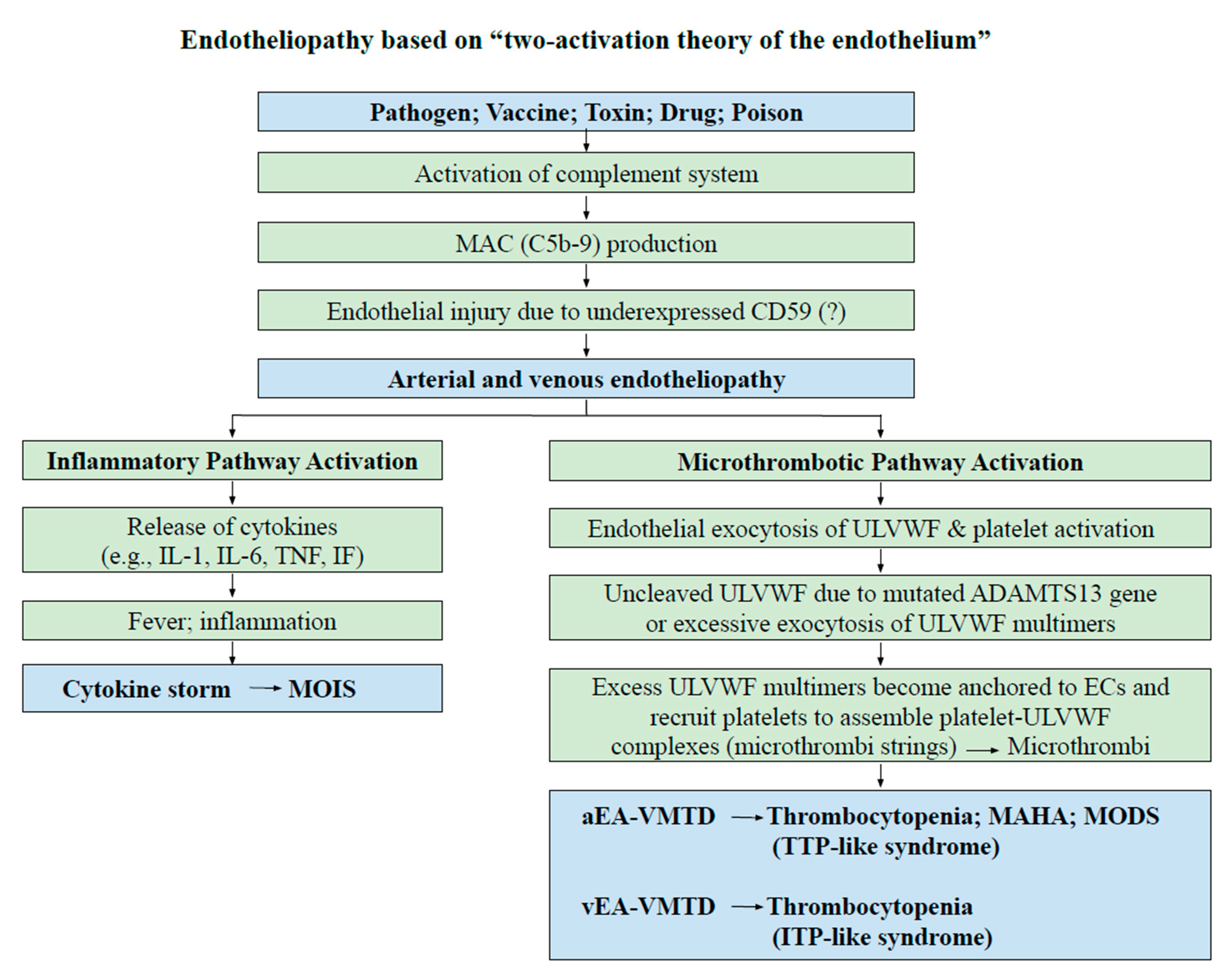
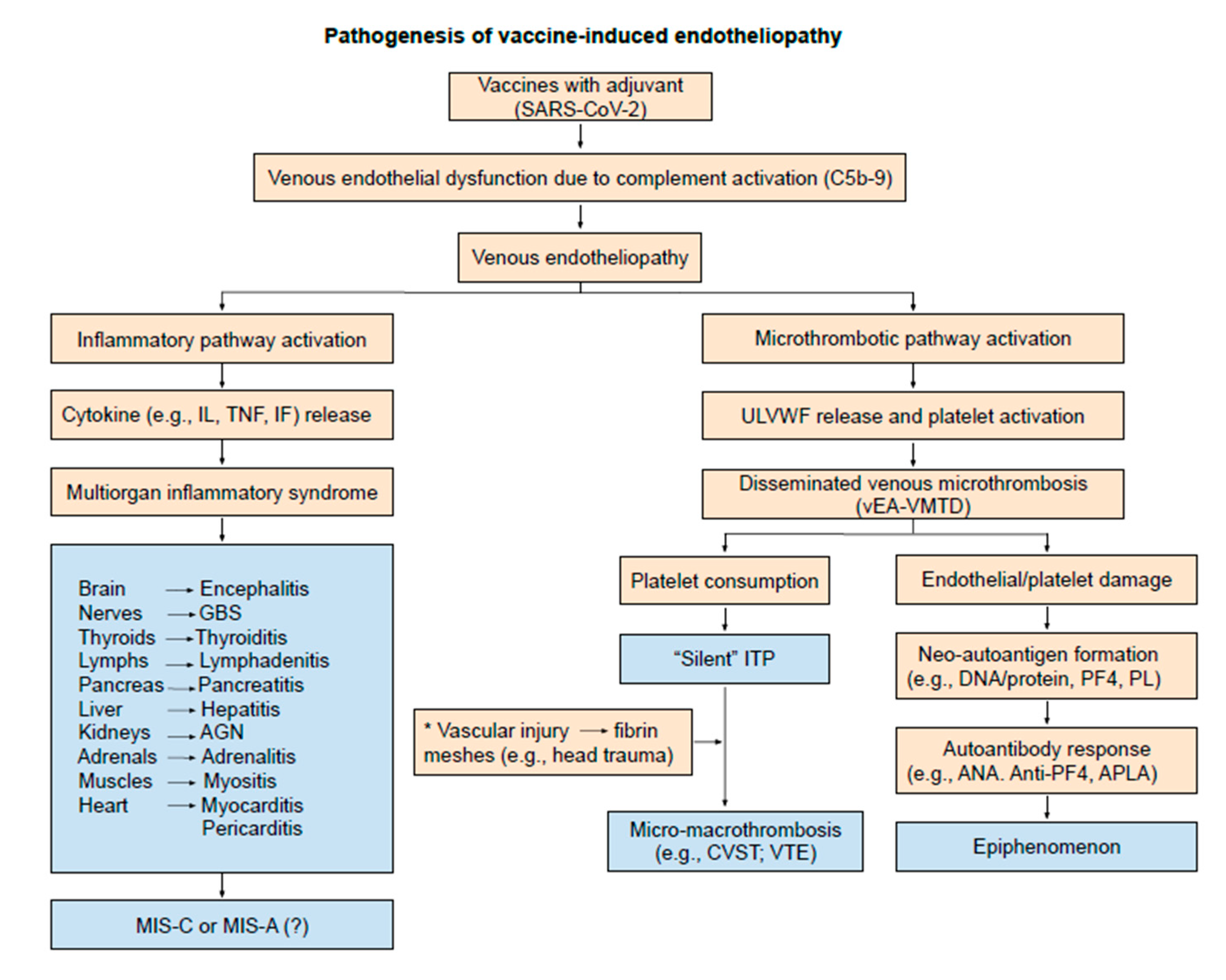
| Clinical Phenotype | Arterial Endotheliopathy | Venous Endothelipathy |
|---|---|---|
| Underlying pathology | aEA-VMTD | vEA-VMTD |
| Physiological/hemodynamic differences in the vascular network | Efferent circulation from the heart (oxygenated blood delivery to the organs) | Afferent circulation into the heart (deoxygenated blood delivery to the lungs) |
| High pressure flow | Low pressure flow | |
| High shear stress | Low shear stress | |
| Capillary and arteriolar microvascular event | Venous and pulmonary microvascular event | |
| Primary cause and result | ||
| Vascular injury (ECs) | Sepsis-induced microvascular endotheliopathy | Sepsis-induced venous endotheliopathy |
| Vaccine-induced venous endotheliopathy | ||
| Vascular pathology site | Disseminated aEA-VMTD at the microvasculature | Transient or “silent” vEA-VMTD at venous system |
| Activated hemostatic path | ULVWF path | ULVWF path |
| Thrombosis component | Microthrombi strings | Microthrombi strings |
| Clinical phenotypes | TTP-like syndrome
| ITP-like syndrome
|
| Phenotypes | Distal DVT (de novo Venous Macrothrombosis) | Proximal/Central DVT (Combined Venous Micro-Macro thrombosis) |
|---|---|---|
| Disease examples | ||
| Venous thrombosis | ||
| DVT | Distal DVT | Proximal/central(catheter) DVT |
| VTE | VTE; multiple PTE | |
| Other complex venous thrombosis | IVCT; SVT; PVT; BCS; SVCT; CVST | |
| Mechanisms of vascular injury | ||
| Event | Local trauma (rarely with surgery/vascular access) | Underlying disease (vEA-VMTD (e.g., sepsis)) + local trauma (commonly with surgery/vascular access) |
| Extent of vascular damage | Local ECs/SET injury | Disseminated ECs injury + local/regional ECs/SET injury |
| Pathogenesis | ||
| Activated thrombosis path | ULVWF and TF paths from local trauma | ULVWF path from systemic vEA-VMTD and TF path from regional trauma |
| Thrombi character | Macrothrombus | Combined micro-macrothrombi composed of “microthrombi strings–fibrin meshes” |
| Severity | Typically, solitary, benign, and self-limited | Serious and often with multiple/large thrombi |
| Severe inflammation | Absent | May be present and can be severe |
| Venous EA-VMTD | Absent | Commonly present (e.g., ITP-like syndrome) |
| MOIS | Absent | May be present |
| Diagnostic findings/markers | ||
| Consumptive thrombocytopenia | Does not occur | Sometimes occurs |
| ULVWF/VWF antigen | Normal | Overexpressed |
| FVIII activity | Normal | Increased |
| ADAMTS13 activity | Normal | Mild to moderately decreased |
| D-dimer | Normal | Markedly increased |
| Immune: ANA; APLA; Anti-DNA antibodies | Negative | May be positive |
| Therapeutic approach per theory | No treatment or short-term anticoagulant | Anticoagulant and antimicrothrombotic/anticomplement agent (?) |
| Pathology/Phenotypes | Arterial Micro-Macrothrombotic Syndrome (aEA-VMTD) | Venous Micro-Macrothrombotic Syndrome (vEA-VMTD) |
|---|---|---|
| Primary pathogenesis | ||
| Cause example | Pathogen-associated endotheliopathy (SARS-CoV-2) | Pathogen-induced endotheliopathy (SARS-CoV-2) |
| Vaccine-associated endotheliopathy (SARS-CoV-2 vaccines) | ||
| Activated hemostatic path | ULVWF path via damaged ECs | ULVWF path via damaged ECs |
| Involved vessels | Capillaries/arterioles | Veins/venules |
| Underlying thrombophlia | ADAMTS13 insufficiency | ADAMTS13 insufficiency(?) |
| Endothelial pathogenesis | Complement activation | Complement activation |
| Phenotype and marker | ||
| Thrombosis character | Arterial microvascular microthrombi | Venous microthrombi |
| Clinical phenotype | TTP-like syndrome | ITP-like syndrome (“Silent” ITP) |
| Consumptive thrombocytopenia | Consumptive thrombocytopenia | |
| MAHA | MOIS | |
| MODS/MOIS | ||
| Endothelial markers | ULVWF ( ↑ VWF antigen ↑ FVIII activity) | ULVWF (↑ VWF antigen ↑ FVIII activity) |
| Secondary pathogenesis | ||
| Cause of additional vascular injury | From arterial vascular access in hospital | From venous vascular access in hospital |
| From arterial vascular injury outside hospital | From incidental head injury (?) after vaccine (e.g., CVST) | |
| Activated hemostatic path | TF path via damaged SET | TF path via damaged SET |
| Affected vessels | Terminal arterial trees | Localized veins at injury site |
| Molecular phenotype | Microthrombi and fibrin meshes | Microthrombi and fibrin meshes |
| Pathologic phenotype | Numerous, minute macrothrombi shower composed of microthrombi and fibrin meshes in arterioles and capillaries in the digits, producing well-demarcated peripheral gangrene | Large, multiple, irregular, connected macrothrombi made of microthrombi and fibrin meshes in local or regional veins, producing DVT complex and venous stasis |
| Clinical syndrome | Arterial micro-macrothrombosis (Gangrene syndrome) | Venous micro-macrothrombosis (complex DVT syndrome) |
| Examples of syndromes | SPG, PF, ANF, diabetic gangrene, limb gangrene, Fournier’s disease, Burger’s disease, acrocyanosis) | VTE, CVST, IVCT, PTE, BCS, PVT, SVCT, SVT |
| Combined Micro-Macrothrombosis due to aEA-VMTD (Gangrene Syndromes) (e.g., Sepsis) | Combined Micro-Macrothrombosis due to vEA-VMTD (Proximal/Central DVT) (e.g., Sepsis or after Vaccination) |
|---|---|
| Clinical features | Clinical features |
| Fever/fatigue/myalgia | Fever/fatigue/myalgia |
| TTP-like syndrome with thrombocytopenia and MAHA | ITP-like syndrome (“Silent” ITP) |
| MODS (e.g., ARDS; encephalopathy) | MOIS (e.g., myocarditis; pericarditis) |
| Gangrene syndromes (e.g., SPG; limb gangrene) | VTE (e.g., VTE; CVST; PTE; SVT) |
| “Gangrene” | “Venous congestion syndrome” |
| Laboratory changes (demonstrated) | Laboratory changes (demonstrated or expected) |
| Endothelial (ULVWF path) markers | Endothelial (ULVWF path) markers |
| Consumptive thrombocytopenia | Consumptive thrombocytopenia |
| Overexpressed ULVWF/VWF antigen | Overexpressed ULVWF/VWF antigen |
| Increased FVIII activity | Increased FVIII activity |
| Increased thrombomodulin | Increased thrombomodulin |
| Endothelial epiphenomenon | Endothelial epiphenomenon |
| Positive ANA | Positive ANA |
| Positive anti-dsDNA | Positive anti-dsDNA |
| Positive anti-PL antibodies | Positive anti-PL antibodies |
| Positive PF4 antibodies | Positive PF4 antibodies |
| Tissue factor path markers | Tissue factor path markers |
| Positive D-dimer | Positive D-dimer |
| TF-bearing microvesicles in circulation | TF-bearing microvesicles in circulation (expected) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.C.; Hawley, H.B. Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Venous Combined Micro-Macrothrombosis. Medicina 2021, 57, 1163. https://doi.org/10.3390/medicina57111163
Chang JC, Hawley HB. Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Venous Combined Micro-Macrothrombosis. Medicina. 2021; 57(11):1163. https://doi.org/10.3390/medicina57111163
Chicago/Turabian StyleChang, Jae C., and H. Bradford Hawley. 2021. "Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Venous Combined Micro-Macrothrombosis" Medicina 57, no. 11: 1163. https://doi.org/10.3390/medicina57111163







