Assessment of Immature Granulocytes Percentage to Predict Severe Bacterial Infection in Latvian Children: An Analysis of Secondary Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Protocol and Definitions
2.3. Data Collection and Laboratory Assays
2.4. Ethical Considerations
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van den Bruel, A.; Haj-Hassan, T.; Thompson, M.; Buntinx, F.; Mant, D. European Research Network on Recognising Serious Infection. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: A systematic review. Lancet 2010, 375, 834–845. [Google Scholar] [CrossRef]
- Craig, J.C.; Williams, G.J.; Jones, M.; Codarini, M.; Macaskill, P.; Hayen, A. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: Prospective cohort study of 15 781 febrile illnesses. BMJ 2010, 340, c1594. [Google Scholar] [CrossRef] [PubMed]
- Nijman, R.; Vergouwe, Y.; Thompson, M.; van Veen, M.; van Meurs, A.; van der Lei, J. Clinical prediction model to aid emergency doctors managing febrile children at risk of serious bacterial infections: Diagnostic study. BMJ 2013, 346, f1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galetto-Lacour, A.; Zamora, S.A.; Andreola, B.; Bressan, S.; Lacroix, L.; Da Dalt, L. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch. Dis. Child. 2010, 95, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Nijman, R.G.; Moll, H.A.; Smit, F.J.; Gervaix, A.; Weerkamp, F.; Vergouwe, Y.; de Rijke, Y.B.; Oostenbrink, R. C-reactive protein, procalcitonin and the labscore for detecting serious bacterial infections in febrile children at the emergency department: A prospective observational study. Pediatr. Infect. Dis. J. 2014, 33, e273–e279. [Google Scholar] [CrossRef] [PubMed]
- Yo, C.H.; Hsieh, P.S.; Lee, S.H.; Wu, J.Y.; Chang, S.S.; Tasi, K.C.; Lee, C.C. Comparison of the test characteristics of procalcitonin to C-reactive protein and leukocytosis for the detection of serious bacterial infections in children presenting with fever without source: A systematic review and meta-analysis. Ann. Emerg. Med. 2012, 60, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Kim, N.D.; Luster, A.D. Neutrophils cascading their way to inflammation. Trends Immunol. 2011, 32, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, B.; Hamaguchi, Y. Autometed enumeration of Immature granulocytes. Am. J. Clin. Pathol. 2007, 128, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Senthilnayagam, B.; Kumar, T.; Sukumaran, J.; Jeya, M. Automated Measurement of Immature Granulocytes. Performance Characteristics and Utility in Routine Clinical Practice. Pathol. Res. Int. 2012. [Google Scholar] [CrossRef] [PubMed]
- Ansari-Lari, M.A.; Kickler, T.S.; Borowitz, M.J. Immature Granulocyte Measurement Using the Sysmex XE-2100: Relationship to Infection and Sepsis. Am. J. Clin. Pathol. 2003, 120, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.O.; Park, S.H.; Park, S.H.; Park, J.S.; Huh, J.W.; Lim, C.M.; Koh, Y.; Hong, S.B.; Jang, S. Fraction of immature granulocytes reflects severity but not mortality in sepsis. Scand. J. Clin. Lab. Investig. 2015, 75, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Geest, P.J.; Mohseni, M.; Brouwer, R.; van der Hoven, B.; Steyerberg, E.W.; Groeneveld, A.B. Immature granulocytes predict microbial infection and its adverse sequelae in the intensive care unit. J. Crit. Care 2014, 29, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Nierhaus, A.; Klatte, S.; Linssen, J.; Eismann, N.M.; Wichmann, D.; Hedke, J.; Braune, S.A.; Kluge, S. Revisiting the white blood cell count: Immature granulocytes count as a diagnostic marker to discriminate between SIRS and sepsis—A prospective, observational study. BMC Immunol. 2013, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Mare, T.A.; Treacher, D.F.; Shankar-Hari, M.; Beale, R.; Lewis, S.M.; Chambers, D.J.; Brown, K.A. The diagnostic and prognostic significance of monitoring blood levels of immature neutrophils in patients with systemic inflammation. Crit. Care 2015, 19, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roehrl, M.H.; Lantz, D.; Sylvester, C.; Wang, J.Y. Age-dependent reference ranges for automated assessment of immature granulocytes and clinical significance in an outpatient setting. Arch. Pathol. Lab. Med. 2011, 135, 471–477. [Google Scholar] [PubMed]
- Craig, J.C.; Williams, G.J.; Jones, M. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: Prospective cohort of 15 781 febrile illness. BMJ 2010, 340, c1594. [Google Scholar] [CrossRef] [PubMed]
- Selig, C.; Nothdurft, W. Cytokines and progenitore cells of granulocytopoiesis in peripheral blood of patients with bacterial infections. Infect. Immun. 1995, 63, 104–109. [Google Scholar] [PubMed]
- Nigro, K.G.; O’Riordan, M.; Mollloy, E.J.; Walsh, M.C.; Sandhaus, L.M. Performance of an automated immature granulocyte count as a predictor of neonatal sepsis. Am. J. Clin. Pathol. 2005, 123, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Simson, E.; Groner, W. The state of the art for the automated WBC differentials. Lab. Hematol. 1995, 1, 13–22. [Google Scholar]
- Park, D.H.; Park, K.; Park, J.; Park, H.H.; Chae, H.; Lim, J.; Oh, E.J.; Kim, Y.; Park, Y.K.; Han, K. Screening of sepsis using leukocyte cell population data from the Coulter automatic blood cell analyzer DxH800. Int. J. Lab. Hematol. 2011, 33, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.; Kunka, S.; Fujimo, H.; Hamaguchi, Y.; Davis, B.H.; Machin, S.J. Evaluation of immature granulocyte counts by the XE_IG master: Upgrated sofware for the XE-2100 automated hematology analyzer. Lab. Hematol. 2003, 9, 117–124. [Google Scholar] [PubMed]
- Nahm, C.H.; Choi, J.W.; Lee, J. Delta neutrophil index in automated immature granulocyte counts for assesing diseases severity of patients with sepsis. Ann. Clin. Lab. Sci. 2008, 38, 241–246. [Google Scholar] [PubMed]
- Pavare, J.; Grope, I.; Kalnins, I.; Gardovska, D. High-mobility group box-1 protein, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in children with community acquired infections and bacteraemia—A prospective study. BMC Infect. Dis. 2010, 16, 10–28. [Google Scholar] [CrossRef] [PubMed]
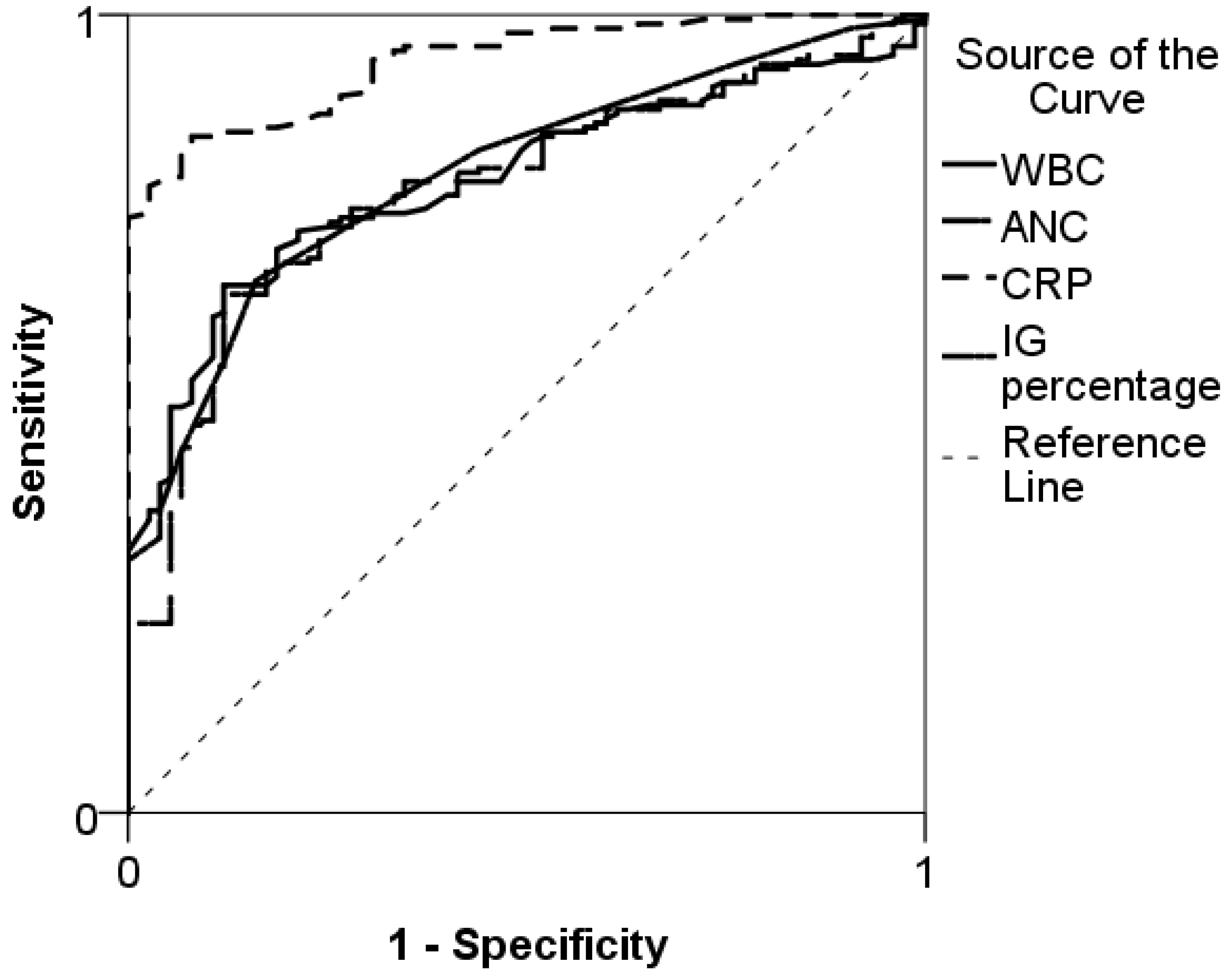
| Characteristics of the Study Sample | Patients Without SBI (n = 75) | SBI Patients (n = 183) |
|---|---|---|
| Baseline Characteristics | ||
| Boys, n (%) | 36 (48) | 78 (42) |
| Age, median (IQR), months | 37 (16–96) | 63 (13–140) |
| Length of hospitalization, median (IQR), days | 3 (1–5) | 10 (5–14) |
| Infection Focus, n (%) | ||
| Intra-abdominal | 0 (0) | 44 (24) |
| Nephritis/pyelonephritis | 0 (0) | 42 (23) |
| Lower respiratory tract | 12 (16) | 36 (20) |
| Osteomyelitis | 0 (0) | 21 (11) |
| Meningitis | 0 (0) | 11 (7) |
| Occult bacteremia | 0 (0) | 10 (5) |
| Skin/soft tissue infection | 0 (0) | 10(5) |
| Upper respiratory tract | 11 (15) | 5 (3) |
| Toxic shock syndrome | 0 (0) | 2 (1) |
| Pericarditis | 0 (0) | 1 (0.5) |
| Gastroenteritis | 52 (69) | 1 (0.5) |
| Infection Markers | ||
| IG percentage | 0.3 (0.25–0.40) | 0.6 (0.4–1.2) |
| CRP, median (IQR), mg/L | 6 (1–23) | 106 (56–200) |
| ANC, median (IQR), ×109/L | 7.1 (4.5–8.6) | 12.2 (12.2–16.8) |
| WBC, median (IQR), ×109/L | 9.9 (7.5–12.0) | 17 (11.6–22.9) |
| Variable | Cutoff Value | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) |
|---|---|---|---|---|---|
| IG percentage | 0.45 | 66 (59–73) | 84 (73–91) | 90 (84–95) | 51 (42–60) |
| CRP, mg/L | 56.5 | 75 (68–81) | 100 (95–100) | 100 (97–100) | 62 (53–71) |
| ANC, ×109/L | 8.6 | 73 (65–79) | 73 (61–82) | 86 (80–91) | 52 (42–62) |
| WBC, ×109/L | 11.75 | 74 (67–80) | 73 (61–82) | 54 (80–91) | 87 (44–64) |
| WBC and IG percentage | NA | 85 (79–90) | 64 (52–74) | 85 (78–90) | 64 (52–75) |
| WBC and CRP | NA | 92 (88–96) | 85 (74–92) | 92 (86–94) | 83 (73–90) |
| WBC, CRP and IG percentage | NA | 93 (88–96) | 86 (77–93) | 94 (89–97) | 84 (74–91) |
| Variables Included | WBC | CRP | IG % | ||||
|---|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | ||
| Model 1 | WBC | 1.23 | (1.15–1.32) | NA | NA | NA | NA |
| Model 2 | CRP | NA | NA | 1.07 | (1.05–1.1) | NA | NA |
| Model 3 | IG% | NA | NA | NA | NA | 3.40 | (1.65–5.15) |
| Model 4 | WBC, CRP | 1.23 | (1.10–1.37) | 1.07 | (1.04–1.12) | NA | NA |
| Model 5 | WBC, CRP, IG% | 1.31 | (1.14–1.49) | 2.24 | (1.20–3.38) | 1.08 | (1.05–1.11) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavare, J.; Grope, I.; Gardovska, D. Assessment of Immature Granulocytes Percentage to Predict Severe Bacterial Infection in Latvian Children: An Analysis of Secondary Data. Medicina 2018, 54, 56. https://doi.org/10.3390/medicina54040056
Pavare J, Grope I, Gardovska D. Assessment of Immature Granulocytes Percentage to Predict Severe Bacterial Infection in Latvian Children: An Analysis of Secondary Data. Medicina. 2018; 54(4):56. https://doi.org/10.3390/medicina54040056
Chicago/Turabian StylePavare, Jana, Ilze Grope, and Dace Gardovska. 2018. "Assessment of Immature Granulocytes Percentage to Predict Severe Bacterial Infection in Latvian Children: An Analysis of Secondary Data" Medicina 54, no. 4: 56. https://doi.org/10.3390/medicina54040056




