Exploring the Structure–Activity Relationship of Bentonites for Enhanced Refinement of Recycled Vegetable Oil
Highlights
- Solid State NMR is able to distinguish between the different forms of Si and Al
- The Bronsted acidity of bentonites is determined by the substitution of Si by Al in quaternary sites
- Ball milling process affects the chemical environment of Si
- Design of Experiments allowed to improve the pour point in recycled vegetable oils subjected to bentonite processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ball Milling
2.3. Vegetable Oil Processing
2.4. Pour Point Determination
2.5. Headspace Solid-Phase Microextraction (HS-SPME)
2.6. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Retention Indexes
2.7. NMR Analysis
2.8. Karl Fischer Titration
2.9. FT-IR Spectroscopy
2.10. Design of Experiments (DoE)
3. Results and Discussion
3.1. FT-IR Spectroscopy Analysis
3.2. NMR Morphological Analysis
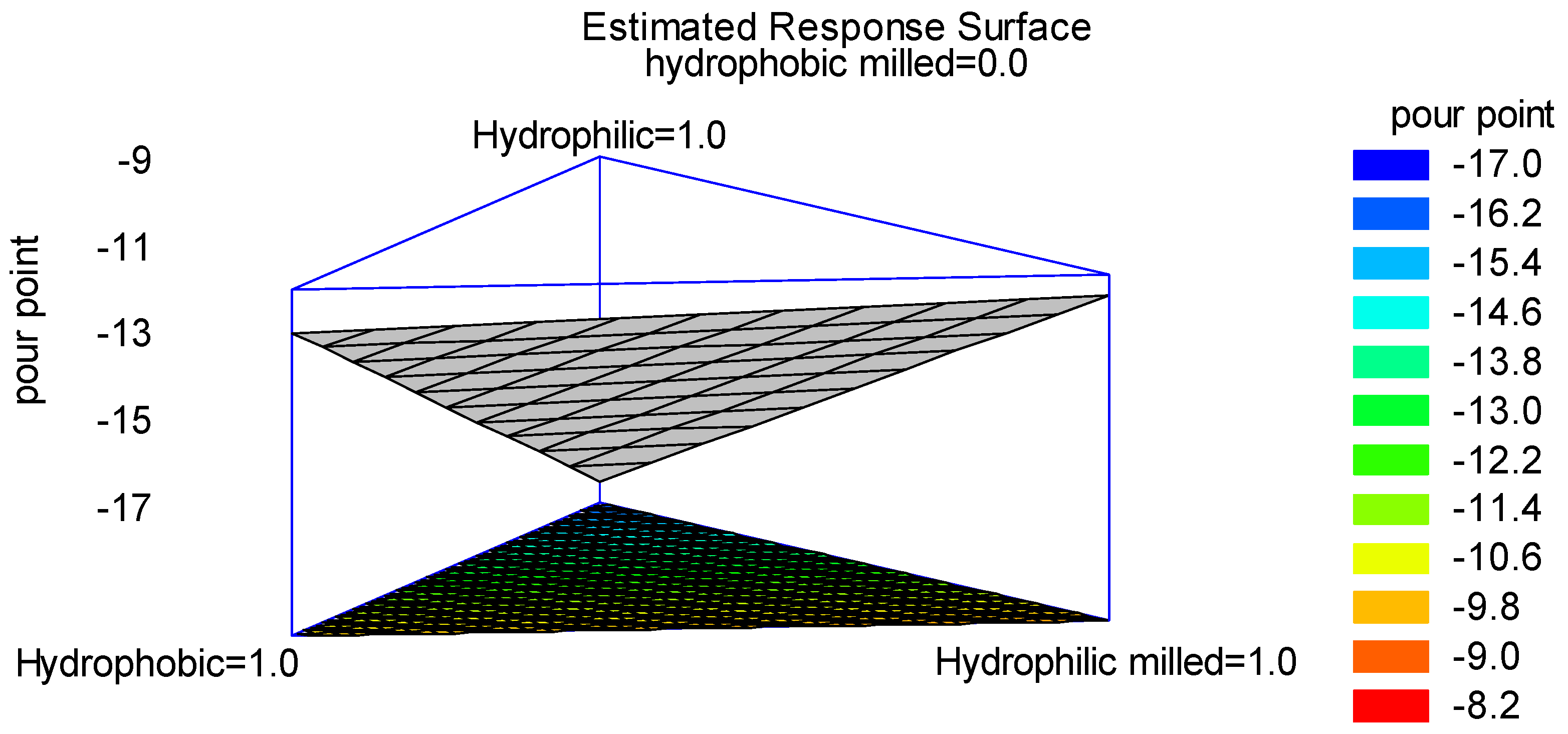
3.3. Design of Experiments (DoE)
3.4. Optimization by Statistical Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cárdenas, J.; Orjuela, A.; Sanchez, D.L.; Narváez, P.C.; Katryniok, B.; Clark, J. Pre-treatment of used cooking oils for the production of green chemicals: A review. J. Clean. Prod. 2021, 289, 125129. [Google Scholar] [CrossRef]
- Foo, W.H.; Koay, S.S.N.; Chia, S.R.; Chia, W.Y.; Tang, D.Y.Y.; Nomanbhay, S.; Chew, K.W. Recent advances in the conversion of waste cooking oil into value-added products: A review. Fuel 2022, 324, 124539. [Google Scholar] [CrossRef]
- Mannu, A.; Garroni, S.; Ibanez Porras, J.; Mele, A. Available technologies and materials for waste cooking oil recycling. Processes 2020, 8, 366. [Google Scholar] [CrossRef]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, 6627013. [Google Scholar] [CrossRef]
- Mannu, A.; Almendras Flores, P.; Briatico, F.; Di Pietro, M.E.; Mele, A. Sustainable Production of Raw Materials from Waste Cooking Oils. RSC Sustain. 2025; Advance article. [Google Scholar]
- Mannu, A.; Poddighe, M.; Garroni, S. Application of IR and UV–VIS spectroscopies and multivariate analysis for the classification of waste vegetable oils. Resour. Conserv. Recycl. 2022, 178, 106088. [Google Scholar] [CrossRef]
- Tu, D.; Li, H.; Wu, Z.; Zhao, B.; Li, Y. Application of Headspace Solid-Phase Microextraction and Multivariate Analysis for the Differentiation Between Edible Oils and Waste Cooking Oil. Food Anal. Methods 2014, 7, 1263–1270. [Google Scholar] [CrossRef]
- Shi, B.; Guo, X.; Liu, H.; Jiang, K.; Liu, L.; Yan, N.; Farag, M.A.; Liu, L. Dissecting Maillard reaction production in fried foods: Formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chem. 2024, 438, 137994. [Google Scholar] [CrossRef]
- Bai, S.; You, L.; Ji, C.; Zhang, T.; Wang, Y.; Geng, D.; Gao, S.; Bi, Y.; Luo, R. Formation of volatile flavor compounds, maillard reaction products and potentially hazard substance in China stir-frying beef sao zi. Food Res. Int. 2022, 159, 111545. [Google Scholar] [CrossRef]
- Lee Kuek, S.; Tarmizi, A.H.A.; Razak, R.A.A.; Jinap, S.; Norliza, S.; Sanny, M. Contribution of lipid towards acrylamide formation during intermittent frying of French fries. Food Control. 2020, 118, 107430. [Google Scholar] [CrossRef]
- Mannu, A.; Di Pietro, M.E.; Petretto, G.L.; Taleb, Z.; Serouri, A.; Taleb, S.; Sacchetti, A.; Mele, A. Recycling of used vegetable oils by powder adsorption. Waste Manag. Res. 2023, 41, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Mistry, N.; Patel, V.; Parekh, P.; Naik, P.; Kumar, N.S.; Vekariya, R.; Khimani, M. Adsorbent Materials, Materials from Natural Sources, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; 26p, ISBN 9781032636368. [Google Scholar]
- Mannu, A.; Poddighe, M.; Mureddu, M.; Castia, S.; Mulas, G.; Murgia, F.; Di Pietro, M.E.; Mele, A.; Garroni, S. Impact of morphology of hydrophilic and hydrophobic bentonites on improving the pour point in the recycling of waste cooking oils. Appl. Clay Sci. 2024, 262, 107607. [Google Scholar] [CrossRef]
- Meirawaty, M.; Palit, C.; Setyorini, D.A.; Jambak, M.A. Bentonite applications in simple purification of bulk cooking oil as alternative solutions for household cost efficiency. J. Community Based Environ. Eng. Manag. 2021, 5, 63–72. [Google Scholar] [CrossRef]
- Aritonang, B.; Ritonga, A.H.; Harefa, K.; Wiratma, D.Y.; Herlina. Purification of used Cooking Oil using a Combination of Activated Carbon and Bentonite Adsorbents. J. Farm. (JFM) 2024, 7, 31–40. [Google Scholar] [CrossRef]
- ASTM D97; Standard Test Method for Pour Point of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2022.
- Van Del Dool, H.; Kartz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chrom. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; de Menezes, S.M.C.; Goodfellow, R.; Granger, P. NMR Nomenclature. Nuclear Spin Properties and Conventions for Chemical Shifts—(IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
- Murray, H.H. Chapter 6 Bentonite Applications. Dev. Clay Sci. 2006, 2, 111–130. [Google Scholar]
- Available online: https://www.sigmaaldrich.com/IT/it/product/sigald/285234 (accessed on 12 December 2024).
- Hayati-Ashtiani, M. Use of FTIR Spectroscopy in the Characterization of Natural and Treated Nanostructured Bentonites (Montmorillonites). Part. Sci. Technol. Int. J. 2012, 30, 553–564. [Google Scholar] [CrossRef]
- Pavón, E.; Alba, M.D. Swelling layered minerals applications: A solid state NMR overview. Prog. Nucl. Magn. Reson. Spectrosc. 2021, 124–125, 99–128. [Google Scholar] [CrossRef]
- Lippmaa, E.; Magi, M.; Samoson, A.; Engelhardt, G.; Grimmer, A.-R. Structural studies of silicates by solid-state high-resolution 29Si NMR. J. Am. Chem. Soc. 1980, 102, 4889–4893. [Google Scholar] [CrossRef]
- Rhee, C.H.; Kim, H.K.; Chang, H.; Lee, J.S. Nafion/sulfonated montmorillonite composite: A new concept electrolyte membrane for direct methanol fuel cells. Chem. Mater. 2005, 17, 1691–1697. [Google Scholar] [CrossRef]
- Okada, K.; Arimitsu, N.; Kameshima, Y.; Nakajima, A.; MacKenzie, K.J.D. Solid acidity of 2:1 type clay minerals activated by selective leaching. Appl. Clay Sci. 2006, 31, 185–193. [Google Scholar] [CrossRef]
- Vajglová, Z.; Kumar, N.; Peurla, M.; Peltonen, J.; Heinmaad, I.; Yu Murzin, D. Synthesis and physicochemical characterization of beta zeolite–bentonite composite materials for shaped catalysts. Catal. Sci. Technol. 2018, 8, 6150–6162. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. Characterisation of calcined raw clays suitable as supplementary cementitious materials. Appl. Clay Sci. 2018, 162, 391–402. [Google Scholar] [CrossRef]
- Oloyede, C.T.; Jekayinfa, S.O.; Alade, A.O.; Ogunkunle, O.; Laseinde, O.T.; Adebayo, A.O.; Abdulkareem, A.I.; Smaisim, G.F.; Fattah, I.M.R. Synthesis of Biobased Composite Heterogeneous Catalyst for Biodiesel Production Using Simplex Lattice Design Mixture: Optimization Process by Taguchi Method. Energies 2023, 16, 2197. [Google Scholar] [CrossRef]
- Monton, C.; Wunnakup, T.; Suksaeree, J.; Charoenchai, L.; Chankana, N. Investigation of the Interaction of Herbal Ingredients Contained in Triphala Recipe Using Simplex Lattice Design: Chemical Analysis Point of View. Int. J. Food Sci. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Purcaro, G.; Szafnauer, R. Tackling Food Fraud: Solid-Phase Microextraction Using Multi-Step Enrichment to Enhance Aroma Profiling in Olive Oil. Column 2021, 17, 20–25. [Google Scholar]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Sun, Z.; Hu, X.; Shen, Q.; Wu, J. Authentication of edible vegetable oils adulterated with used frying oil by Fourier Transform Infrared Spectroscopy. Food Chem. 2012, 132, 1607–1613. [Google Scholar] [CrossRef]
- Wu, C.-M.; Chen, S.-Y. Volatile compounds in oils after deep frying or stir frying and subsequent storage. J. Am. Oil Chem. Soc. 1992, 69, 858–865. [Google Scholar] [CrossRef]
- Duquenne, V. What can lattices do for experimental designs? Math. Soc. Sci. 1986, 11, 243–281. [Google Scholar] [CrossRef]
- Jankovic, A.; Chaudhary, G.; Goia, F. Designing the design of experiments (DOE)—An investigation on the influence of different factorial designs on the characterization of complex systems. Energy Build. 2021, 250, 111298. [Google Scholar] [CrossRef]

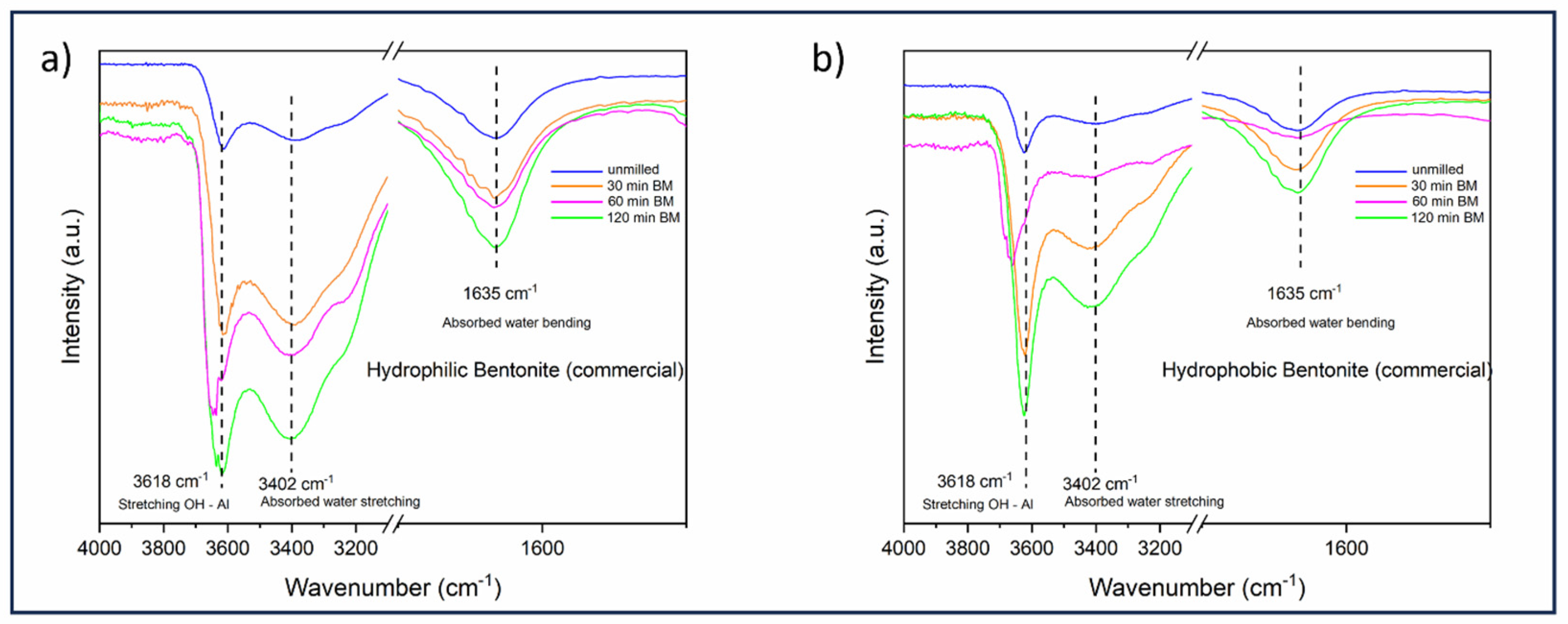
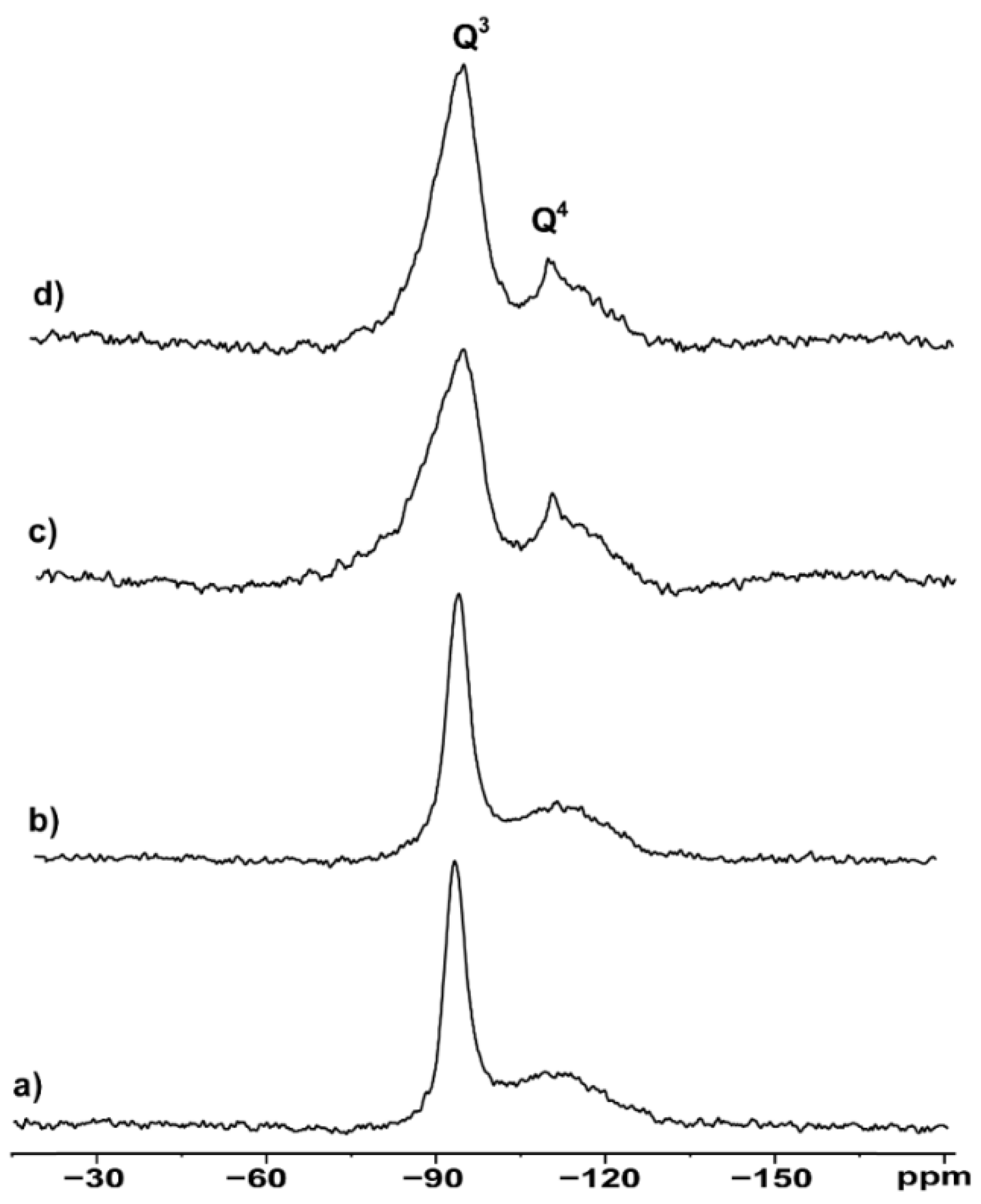
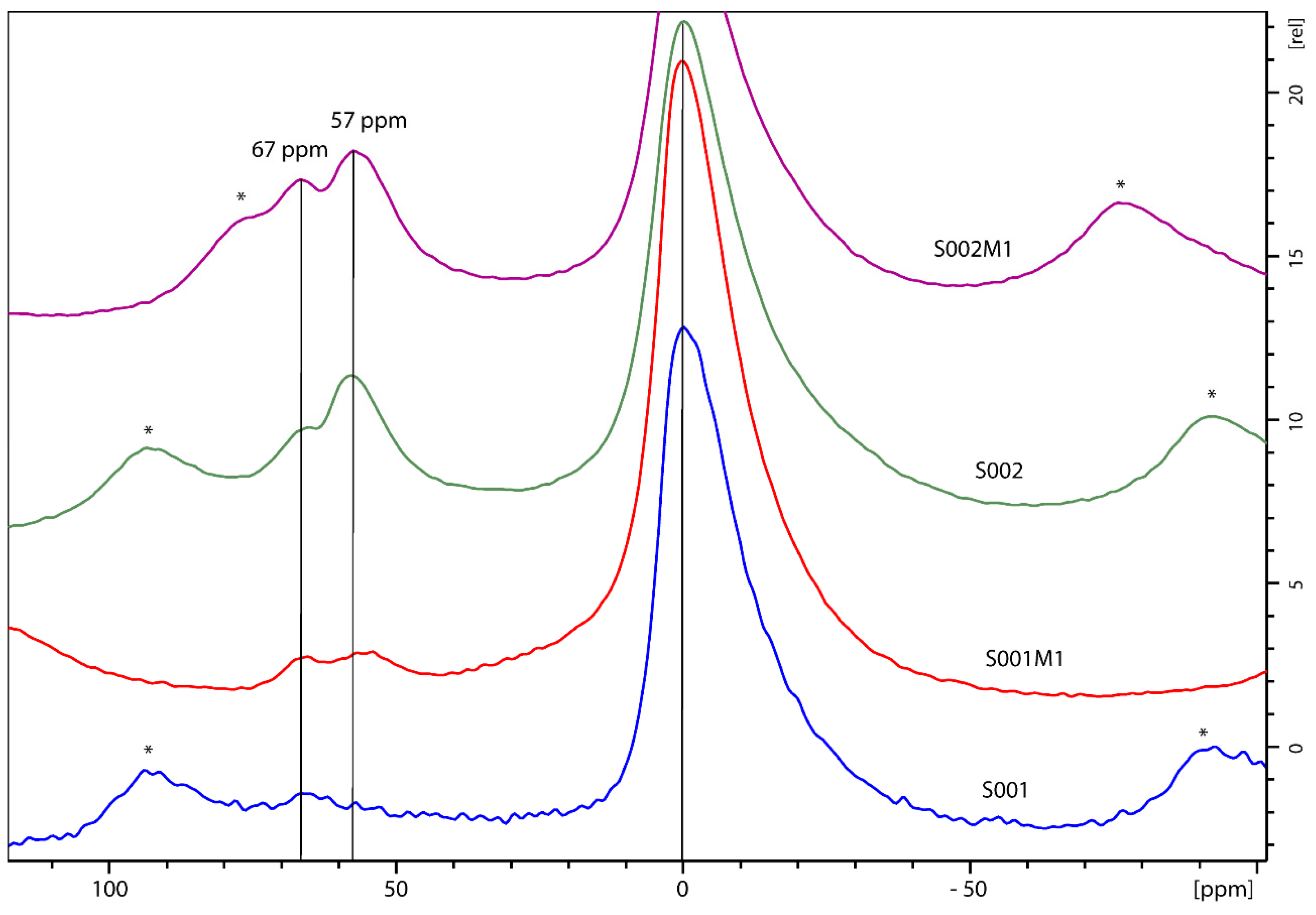
| Spectrum | Sample | LW Q3 (Hz) | LW Q4 (Hz) | Q4/Q3 Ratio |
|---|---|---|---|---|
| A | hydrophilic (S001) | 491 | 345 | 0.137 |
| B | hydrophilic milled (S001M1) | 489 | 354 | 0.150 |
| C | hydrophobic (S002) | 834 | 476 | 0.190 |
| D | hydrophobic milled (S002M2) | 841 | 531 | 0.186 |
| Bentonite | Octahedral | d (ppm) | Al Sites Fully Condensed | d (ppm) | Al Sites in Layered Bentonite | d (ppm) |
|---|---|---|---|---|---|---|
| S001 | 91.63% | 0.25 | 0% | - | 8.36% | 69.32 |
| S001M1 | 91.61% | 0.87 | 4.22% | 55.70 | 4.15% | 55.97 |
| S002 | 74.50% | 0.10 | 13.76% | 58.45 | 11.70% | 68.54 |
| S002M1 | 74.50% | 0.10 | 13.76% | 58.45 | 11.70% | 68.54 |
| Experiment | Bentonite | Response 1 | Response 2 |
|---|---|---|---|
| 1 | S002M1 | PP * | VC ** |
| 2 | S002 | PP * | VC ** |
| 3 | S001M1 | PP * | VC ** |
| 4 | S001 | PP * | VC ** |
| Compound | Retention Times (RTs) * | TQ | S001 | S001M1 | S002 | S002M1 |
|---|---|---|---|---|---|---|
| Pentanal | 3.87 | 1.09 | 0.87 | 1.27 | 0.30 | 0.75 |
| Hexanal | 6.63 | 12.02 | 9.32 | 17.82 | 9.22 | 6.55 |
| 2-Hexenal, (E)- | 10.68 | 0.43 | 0.33 | 0.51 | 0.06 | 0.26 |
| Heptanal | 9.69 | 1.21 | 0.85 | 1.80 | 0.04 | 0.58 |
| Octanal | 12.81 | 3.10 | 2.48 | 3.59 | 1.15 | 1.14 |
| 2-Heptenal, (Z)- | 13.82 | 9.54 | 8.37 | 10.00 | 5.94 | 5.94 |
| 2-Octenal, (E)- | 16.68 | 4.13 | 3.69 | 3.82 | 2.85 | 2.83 |
| Nonanal | 15.77 | 8.37 | 8.05 | 7.17 | 5.60 | 4.30 |
| 2-Nonenal, (E)- | 19.36 | 1.08 | 1.25 | 0.93 | 1.33 | 1.24 |
| 2-Decenal, (Z)- | 22.00 | 5.73 | 9.22 | 3.82 | 8.59 | 8.92 |
| 2,4-Decadienal, (E,E)- | 25.74 | 3.23 | 7.92 | 2.90 | 8.69 | 10.14 |
| 2-Undecenal | 24.44 | 3.23 | 5.42 | 2.05 | 6.41 | 6.97 |
| 2-Heptanone | 9.61 | 1.36 | 0.53 | 2.00 | 0.12 | 0.33 |
| 2-Octanone | 12.68 | 1.10 | 0.65 | 1.35 | 0.37 | 0.25 |
| Total ketones | 2.46 | 1.19 | 3.35 | 0.48 | 0.59 | |
| Acetic acid | 17.83 | 4.00 | 7.31 | 6.34 | 9.67 | 10.59 |
| Butanoic acid | 22.34 | 2.12 | 1.41 | 2.09 | 2.61 | 1.94 |
| Pentanoic acid | 24.79 | 4.38 | 5.19 | 3.58 | 5.37 | 5.42 |
| Hexanoic acid | 27.12 | 15.25 | 11.35 | 11.30 | 15.45 | 16.01 |
| Heptanoic acid | 30.06 | 1.07 | 0.98 | 0.55 | 1.71 | 2.15 |
| Octanoic acid | 32.21 | 0.60 | 0.95 | 0.28 | 1.61 | 1.70 |
| Nonanoic acid | 33.81 | 0.32 | 0.59 | 0.13 | 1.24 | 0.94 |
| 1-Pentanol | 11.86 | 1.17 | 0.83 | 1.32 | 0.84 | 0.61 |
| 1-Hexanol | 14.80 | 4.12 | 2.03 | 5.73 | 1.89 | 1.73 |
| 1-Octen-3-ol | 17.40 | 3.17 | 2.06 | 2.88 | 0.96 | 0.91 |
| 1-Heptanol | 17.52 | 1.28 | 0.85 | 1.58 | 0.60 | 0.72 |
| 2-Hepten-1-ol, (E)- | 18.94 | 1.03 | 0.60 | 1.10 | 0.68 | 0.48 |
| 1-Octanol | 20.06 | 3.27 | 4.92 | 1.17 | 5.72 | 6.28 |
| Dodecane | 10.02 | 0.99 | 0.67 | 0.65 | 0.19 | 0.14 |
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Volatiles | TQ | S001 | S001M1 | S002 | S002M1 | |
| Aldehydes | 53.15 | 57.78 | 55.71 | 50.17 | 49.62 | |
| Ketones | 2.46 | 1.19 | 3.35 | 0.48 | 0.59 | |
| Acids | 27.73 | 27.77 | 24.26 | 37.64 | 38.76 | |
| Alcohols | 14.04 | 11.30 | 13.79 | 10.68 | 10.74 | |
| Alkanes | 0.99 | 0.67 | 0.65 | 0.19 | 0.14 | |
| Pour Point (PP, °C) | −2 °C | −16.5 | −9.5 | −10 | −10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannu, A.; Castia, S.; Petretto, G.L.; Garroni, S.; Castiglione, F.; Mele, A. Exploring the Structure–Activity Relationship of Bentonites for Enhanced Refinement of Recycled Vegetable Oil. Materials 2025, 18, 1059. https://doi.org/10.3390/ma18051059
Mannu A, Castia S, Petretto GL, Garroni S, Castiglione F, Mele A. Exploring the Structure–Activity Relationship of Bentonites for Enhanced Refinement of Recycled Vegetable Oil. Materials. 2025; 18(5):1059. https://doi.org/10.3390/ma18051059
Chicago/Turabian StyleMannu, Alberto, Simona Castia, Giacomo Luigi Petretto, Sebastiano Garroni, Franca Castiglione, and Andrea Mele. 2025. "Exploring the Structure–Activity Relationship of Bentonites for Enhanced Refinement of Recycled Vegetable Oil" Materials 18, no. 5: 1059. https://doi.org/10.3390/ma18051059
APA StyleMannu, A., Castia, S., Petretto, G. L., Garroni, S., Castiglione, F., & Mele, A. (2025). Exploring the Structure–Activity Relationship of Bentonites for Enhanced Refinement of Recycled Vegetable Oil. Materials, 18(5), 1059. https://doi.org/10.3390/ma18051059












