Monitoring of Physiological Parameters to Predict Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review
Abstract
:1. Introduction
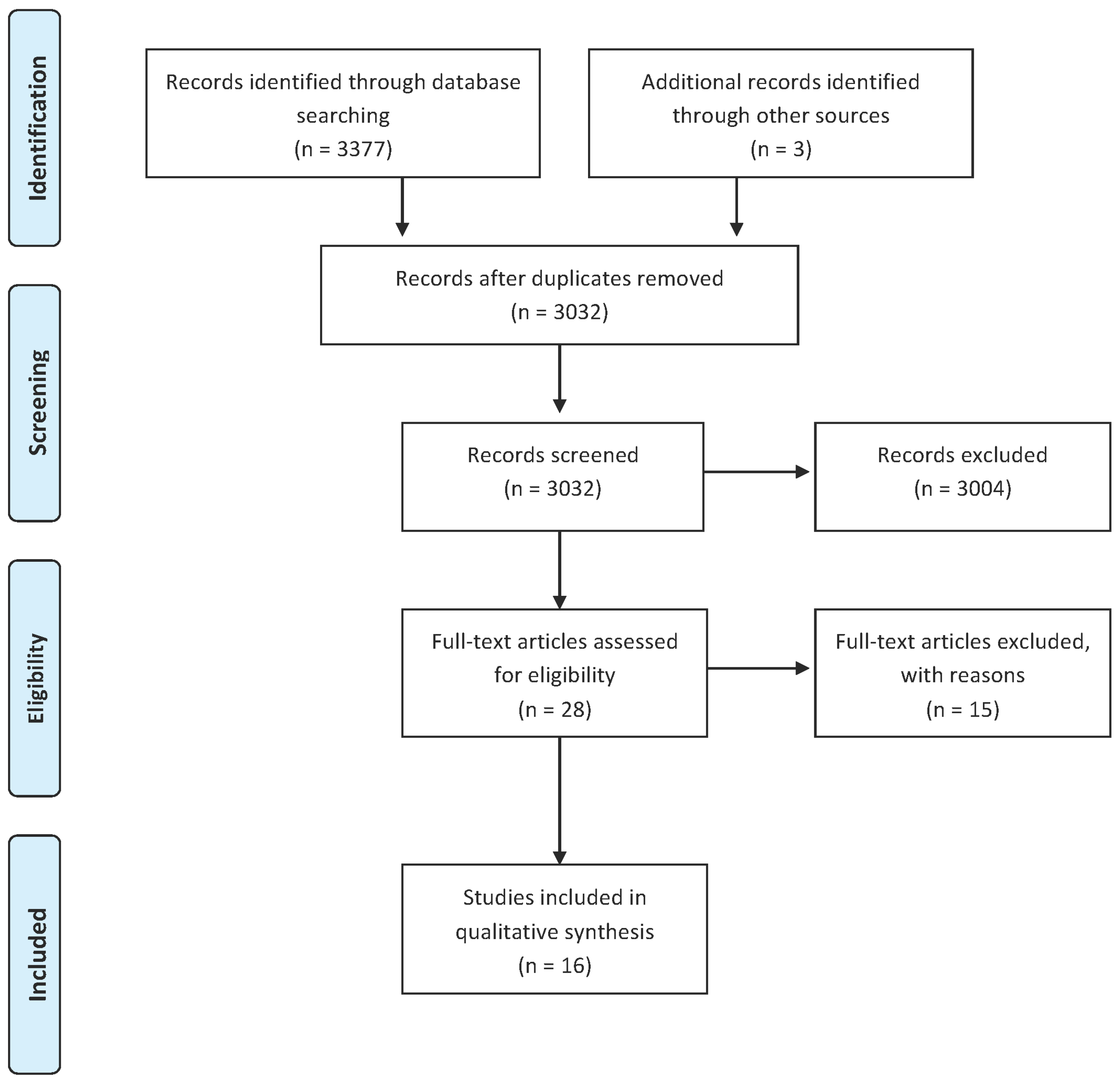
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Collection
2.5. Quality Assessment
2.6. Synthesis of Results
3. Results
Quality Assessment
4. Monitoring Vital Signs to Predict Exacerbation
4.1. Heart Rate and Oxygen Saturation
4.2. Respiratory Rate
4.3. Blood Pressure and Temperature
5. Monitoring Lung Function to Predict Exacerbations
6. Monitoring Respiratory Sounds to Predict Exacerbations
7. Methodological Considerations
7.1. Alarm limits
7.2. Monitoring Characteristics
7.3. Intermittent vs. Continuous Monitoring
8. Discussion
Strength and Limitations
9. Conclusions
Acknowledgments
Author Contributions
Conflict of interest
Appendix A
| 1 | lung diseases, obstructive/ or exp. bronchitis/ or exp. pulmonary disease, chronic obstructive/ | 84,036 | Advanced | Display Results More |
| 2 | emphysema$.mp. | 31,994 | Advanced | Display Results More |
| 3 | bronchiti$.mp. | 30,780 | Advanced | Display Results More |
| 4 | (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp. | 96,121 | Advanced | Display Results More |
| 5 | (copd or coad or cobd or aecb).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 33,479 | Advanced | Display Results More |
| 6 | 1 or 2 or 3 or 4 or 5 | 153,864 | Advanced | Display Results More |
| 7 | telemedicine/ or telerehabilitation/ | 14,118 | Advanced | Display Results More |
| 8 | (telemonitor* or tele-monitor* or tele-health* or telehealth* or telemedicine or tele-medicine or tele-rehabilitat* or telerehabilitat*).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 18,772 | Advanced | Display Results More |
| 9 | (e-health or ehealth or m-health or mhealth or mobile health).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 8219 | Advanced | Display Results More |
| 10 | exp. Telemetry/ | 10,614 | Advanced | Display Results More |
| 11 | (telemetr* or tele-metr*).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 12,888 | Advanced | Display Results More |
| 12 | Monitoring, Ambulatory/ | 6635 | Advanced | Display Results More |
| 13 | (monitoring adj4 (ambulatory or home$)).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 23,156 | Advanced | Display Results More |
| 14 | Domiciliary.mp. | 2364 | Advanced | Display Results More |
| 15 | software/ or mobile applications/ or user-computer interface/ | 114,192 | Advanced | Display Results More |
| 16 | (software* or app? or iphone or ipad or android or smartphone* or smart-phone*).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 205,344 | Advanced | Display Results More |
| 17 | or/7–16 | 284,600 | Advanced | Display Results More |
| 18 | (exacerbat* or deteriorat*).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 176,862 | Advanced | Display Results More |
| 19 | heart rate/ | 149,127 | Advanced | Display Results More |
| 20 | Pulse/ | 16,765 | Advanced | Display Results More |
| 21 | ((heart* or pulse* or cardiac) adj3 rate*).mp. | 229,964 | Advanced | Display Results More |
| 22 | respiratory rate/ or Respiration/ | 75,932 | Advanced | Display Results More |
| 23 | ((respirat* or breath*) adj3 rate*).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 24,774 | Advanced | Display Results More |
| 24 | exp. Oximetry/ | 13,116 | Advanced | Display Results More |
| 25 | oximetr*.mp. | 15,161 | Advanced | Display Results More |
| 26 | Oxygen/ | 150,124 | Advanced | Display Results More |
| 27 | SPO2.mp. | 3207 | Advanced | Display Results More |
| 28 | oxygen.mp. | 519,842 | Advanced | Display Results More |
| 29 | (physiological adj4 (variable* or measure*)).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 10,547 | Advanced | Display Results More |
| 30 | early diagnosis/ | 19,913 | Advanced | Display Results More |
| 31 | (early adj4 (detect* or diagnos*)).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 176,122 | Advanced | Display Results More |
| 32 | predict*.mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 1,238,846 | Advanced | Display Results More |
| 33 | or/18–32 | 2,291,354 | Advanced | Display Results More |
| 34 | 6 and 17 and 33 | 795 | Advanced |
| Database | Subject Heading | Keyword |
|---|---|---|
| Medline | lung diseases, obstructive/ or exp. bronchitis/ or exp. pulmonary disease, chronic obstructive/ telemedicine/ or telerehabilitation/ exp. Telemetry/ Monitoring, Ambulatory/ software/ or mobile applications/ or user-computer interface/ heart rate/ Pulse/ respiratory rate/ or Respiration/ exp. Oximetry/ Oxygen/ early diagnosis/ | emphysema$. bronchiti$. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)). (copd or coad or cobd or aecb) (telemonitor* or tele-monitor* or tele-health* or telehealth* or telemedicine or tele-medicine or tele-rehabilitat* or telerehabilitat*) (telemetr* or tele-metr*) (monitoring adj4 (ambulatory or home$)). Domiciliary. (software* or app? or iphone or ipad or android or smartphone* or smart-phone*). (exacerbat* or deteriorat*). ((heart* or pulse* or cardiac) adj3 rate*). ((respirat* or breath*) adj3 rate*). oximetr*. SPO2. oxygen. (physiological adj4 (variable* or measure*)). (early adj4 (detect* or diagnos*)). predict*. |
| Embase | lung diseases, obstructive/ or exp. bronchitis/ or exp. pulmonary disease, chronic obstructive/ exp. telemonitoring/ or exp. telemedicine/ telerehabilitation/ exp. telephone telemetry/ or exp. telemetry/ exp. ambulatory monitoring/ computer program/ or exp. communication software/ or exp. mobile application/ exp. computer interface/ heart rate variability/ or exp. heart rate/ exp. pulse rate/ or exp. “heart rate and rhythm”/ exp. breathing/ or exp. breathing rate/ exp. oximetry/ or exp. measurement/ or exp. pulse oximetry/ exp. oxygen breathing/ exp. early diagnosis/ | emphysema$. bronchiti$. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)). (copd or coad or cobd or aecb). (telemonitor* or tele-health* or telehealth* or telemedicine or tele-medicine or tele-monitor*). (e-health or ehealth or m-health or mhealth or mobile health). (telemetr* or tele-metr*). ((respirat* or breath*) adj3 rate*). oximetr*. SPO2. Oxygen (physiological adj4 (variable* or measure*)). (early adj4 (detect* or diagnos*)). predict*. (monitoring adj4 (ambulatory or home$)). Domiciliary. (software* or app? or iphone or ipad or android or smartphone* or smart-phone*). (exacerbat* or deteriorat*). ((heart* or pulse* or cardiac) adj3 rate*). |
| AMED | pulmonary disease chronic obstructive/ or bronchitis/ or pulmonary emphysema/ or lung diseases obstructive/ telemedicine/ home care services/ internet/ or exp. computers/ or software/ disease progression/ heart rate/ Pulse/ exp. Respiration/ Oxygen/ monitoring physiologic/ or respiratory function tests/ diagnosis/ | emphysema$. bronchiti$. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)). (copd or coad or cobd or aecb). (telemonitor* or tele-monitor* or tele-health* or telehealth* or telemedicine or tele-medicine or tele-rehabilitat* or telerehabilitat*). (e-health or ehealth or m-health or mhealth or mobile health). (telemetr* or tele-metr*). ((monitoring adj4 (ambulatory or home$)). Domiciliary. (software* or app? or iphone or ipad or android or smartphone* or smart-phone*). (exacerbat* or deteriorat*). ((heart* or pulse* or cardiac) adj3 rate*). ((respirat* or breath*) adj3 rate*). oximetr*. SPO2. oxygen. (physiological adj4 (variable* or measure*)). (early adj4 (detect* or diagnos*)). predict*. |
| CINAHL | (MH “Lung Diseases, Obstructive”) OR (MH “Bronchitis+”) OR (MH “Emphysema”) OR (MH “Pulmonary Disease, Chronic Obstructive+”) (MH “Telenursing”) OR (MH “Telepathology”) OR (MH “Remote Consultation”) OR (MH “Telemedicine”) OR (MH “Telehealth”) (MH “Telemetry”) (MH “Ambulatory Care”) (MH “Software”) OR (MH “Communications Software+”) OR (MH “Mobile Applications”) OR (MH “User-Computer Interface+”) (MH “Pulse”) OR (MH “Heart Rate”) (MH “Wireless Communications”) OR (MH “Telephone+”) OR (MH “Instant Messaging”) (MH “Respiratory Rate”) OR (MH “Respiratory Sounds”) (MH “Respiration+”) (MH “Oximetry+”) (MH “Oximeters+”) (MH “Oxygen”) (MH “Oxygenation”) OR (MH “Oxygen Saturation”) (MH “Monitoring, Physiologic”) (MH “Early Diagnosis”) | TX emphysema* TX bronchiti* TX (copd or coad or cobd or aecb) TX (obstruct* n3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) TX (telemonitor* or tele-monitor* or tele-health* or telehealth* or telemedicine or tele-medicine or tele-rehabilitat* or telerehabilitat*) TX (e-health or ehealth or m-health or mhealth or “mobile health”) TX (telemetr* or tele-metr*) TX monitoring n4 (ambulatory or home*) TX Domiciliary TX (app# or iphone or ipad or android or smartphone* or smart-phone*) OR TI software* OR AB software* TX (exacerbat* or deteriorat*) TX ((heart* or pulse* or cardiac) n3 rate*) TX (respirat* or breath*) n3 rate* TX oximetr* TX SPO2 TX oxygen TX (physiological n4 (variable* or measure*)) TX (early n4 (detect* or diagnos*)) TX predict* |
| Cochran | [mh “lung diseases, obstructive”] [mh bronchitis] [mh “pulmonary disease, chronic obstructive”] [mh telemedicine] [mh telerehabilitation] [mh Telemetry] [mh “Monitoring, Ambulatory”] [mh software] [mh “mobile applications”] [mh “user-computer interface”] [mh “heart rate”] [mh pulse] [mh “respiratory rate”] [mh Respiration] [mh Oximetry] [mh Oxygen] [mh “early diagnosis”] | COPD emphysema* bronchiti* (obstruct* near/3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) (copd or coad or cobd or aecb) (telemonitor* or tele-monitor* or tele-health* or telehealth* or telemedicine or tele-medicine or tele-rehabilitat* or telerehabilitat*) (e-health or ehealth or m-health or mhealth or mobile health) (telemetr* or tele-metr*) (monitoring near/4 (ambulatory or home*)) Domiciliary (software* or app or apps or iphone or ipad or android or smartphone* or smart-phone*) (exacerbat* or deteriorat*) ((heart* or pulse* or cardiac) near/3 rate*) ((respirat* or breath*) near/3 rate*) oximetr* SPO2 Oxygen (physiological near/4 (variable* or measure*)) (early near/4 (detect* or diagnos*)) predict* |
| First Author | Study Title | Reason |
|---|---|---|
| Malliopoulos, C., 2008 | Continuous mobile services for healthcare: The health wear project | Article not available and no response from the author |
| Antoniades, N.C., 2012 | Pilot study of remote telemonitoring in COPD | No physiological data shown and it does not address the prediction of COPD exacerbation |
| Jensen, M.H., 2012 | Clinical impact of home telemonitoring on patients with chronic obstructive pulmonary disease | Not relevant (evaluated the impact of tele-health on patients, not in predicting exacerbation) |
| Jakobsen, A.S., 2013 | Hospital-admitted COPD patients treated at home using telemedicine technology in The Virtual Hospital Trial: methods of a randomized effectiveness trial | Recruited non-stable COPD patients for preventing readmission |
| Jehn, M., 2013 | Tele-monitoring reduces exacerbation of COPD in the context of climate change-a randomized controlled trial | Looked at the association between the weather and exacerbation. |
| Pinnock, H., 2013 | Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial | No physiological variation reported and not for predicting exacerbation |
| San Miguel, K., 2013 | Telehealth remote monitoring for community-dwelling older adults with chronic obstructive pulmonary disease | No physiological variation reported and not for predicting exacerbation |
| Schou, Lone, 2013 | A randomised trial of telemedicine-based treatment versus conventional hospitalisation in patients with severe COPD and exacerbation—Effect on self-reported outcome | Not for predicting exacerbation and recruited non-stable COPD patients |
| van der Heijden, M., 2013 | An autonomous mobile system for the management of COPD | Designing a mobile system |
| Zhang, J., 2013 | MIOTIC study: A prospective, multicenter, randomized study to evaluate the long-term efficacy of mobile phone-based internet of things in the management of patients with stable COPD | No physiological variation reported and not for predicting exacerbation |
| Ding, H., 2014 | A pilot study of a mobile-phone-based home monitoring system to assist in, remote interventions in cases of acute exacerbation of COPD | Did not report any monitored physiological data |
| Ko, F.W.S., 2014 | COPD care programme can reduce readmissions and in-patient bed days | Recruited non-stable COPD patients |
| Minami S., 2014 | Ambulatory pulse oximetry monitoring in Japanese COPD outpatients not receiving oxygen therapy | Monitored the patient’s SPO2% for a 24 h period only. |
| Jakobsen, A.S., 2015 | Home-Based Telehealth Hospitalization for Exacerbation of Chronic Obstructive Pulmonary Disease: Findings from “The Virtual Hospital” Trial | Recruited non-stable COPD patients |
| Ringbaek, T., 2015 | Effect of telehealthcare on exacerbations and hospital admissions in COPD: A randomised controlled trial | No physiological variation reported and not for predicting exacerbation |
References
- World Health Organization (WHO). Burden of COPD. Available online: http://www.who.int/respiratory/copd/burden/en/ (accessed on 24 August 2016).
- World Health Organization (WHO). Chronic Obstructive Pulmonary Disease (COPD). 2016. Available online: http://www.who.int/mediacentre/factsheets/fs315/en/ (accessed on 24 August 2016).
- Murray, C.J.L.; Lopez, A.D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997, 349, 1498–1504. [Google Scholar] [CrossRef]
- The Global Strategy for the Diagnosis. Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2016. Available online: http://www.goldcopd.org/ (accessed on 22 July 2016).
- Hurst, J.R.; Donaldson, G.C.; Quint, J.K.; Goldring, J.J.P.; Patel, A.R.C.; Wedzicha, J.A. Domiciliary pulse-oximetry at exacerbation of chronic obstructive pulmonary disease: Prospective pilot study. BMC Pulm. Med. 2010, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Brooks, D.; Marques, A. Home telemonitoring effectiveness in COPD: A systematic review. Int. J. Clin. Pract. 2014, 68, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Segrelles Calvo, G.; Gomez-Suarez, C.; Soriano, J.B.; Zamora, E.; Gonzalez-Gamarra, A.; Gonzalez-Bejar, M.; Jordán, A.; Tadeo, E.; Sebastián, A.; Fernández, G.; et al. A home telehealth program for patients with severe COPD: The PROMETE study. Respir. Med. 2014, 108, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ringbaek, T.; Green, A.; Laursen, L.C.; Frausing, E.; Brondum, E.; Ulrik, C.S. Effect of tele health care on exacerbations and hospital admissions in patients with chronic obstructive pulmonary disease: A randomized clinical trial. Int. J. COPD 2015, 10, 1801–1808. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Risk of Bias Tool (Modified) For Quality Assessment of Randomized Controlled Trial. Available online: http://www.tc.umn.edu/~msrg/caseCATdoc/rct.crit.pdf (accessed on 18 August 2016).
- Adapted Version of a Modified Newcastle-Ottawa Scale for Single Use in Specific Context. Available online: http://www.biomedcentral.com/content/supplementary/2046-4053-3-45-S2.pdf (accessed on 28 August 2016).
- Seemungal, T.A.D.G.; Bhowmik, A.; Jeffries, D.J.; Wedzicha, J.A. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000, 161, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.; Doughty, K. Preliminary results of a medical telecare pilot in Wrexham. J. Assist. Technol. 2009, 3, 36–42. [Google Scholar] [CrossRef]
- Sund, Z.M.; Powell, T.; Greenwood, R.; Jarad, N.A. Remote daily real-time monitoring in patients with COPD—A feasibility study using a novel device. Respir. Med. 2009, 103, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.H.; Cichosz, S.L.; Dinesen, B.; Hejlesen, O.K. Moving prediction of exacerbation in chronic obstructive pulmonary disease for patients in telecare. J. Telemed. Telecare 2012, 18, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Van den Berge, M.; Hop, W.C.J.; van der Molen, T.; van Noord, J.A.; Creemers, J.P.; Schreurs, A.J.; Wouters, E.F.; Postma, D.S.; COSMIC (COPD and Seretide: A Multi-Center Intervention and Characterization) Study Group. Prediction and course of symptoms and lung function around an exacerbation in chronic obstructive pulmonary disease. Respir. Res. 2012, 13, 1–9. [Google Scholar]
- Yanez, A.M.; Guerrero, D.; Perez de Alejo, R.; Garcia-Rio, F.; Alvarez-Sala, J.L.; Calle-Rubio, M.; de Malo Molina, R.; Valle Falcones, M.; Ussetti, P.; Sauleda, J.; et al. Monitoring breathing rate at home allows early identification of COPD exacerbations. Chest 2012, 142, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lesende, I.; Orruno, E.; Bilbao, A.; Vergara, I.; Cairo, M.C.; Bayon, J.C.; Reviriego, E.; Romo, M.I.; Larrañaga, J.; Asua, J.; et al. Impact of telemonitoring home care patients with heart failure or chronic lung disease from primary care on healthcare resource use (the TELBIL study randomised controlled trial). BMC Health Serv. Res. 2013, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Pedone, C.; Chiurco, D.; Scarlata, S.; Incalzi, R.A. Efficacy of multiparametric telemonitoring on respiratory outcomes in elderly people with COPD: A randomized controlled trial. BMC Health Serv. Res. 2013, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Hardinge, M.; Rutter, H.; Velardo, C.; Shah, S.A.; Williams, V.; Tarassenko, L.; Farmer, A. Using a mobile health application to support self-management in chronic obstructive pulmonary disease: A six-month cohort study. BMC Med. Inform. Decis. Mak. 2015, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Mohktar, M.S.; Redmond, S.J.; Antoniades, N.C.; Rochford, P.D.; Pretto, J.J.; Basilakis, J.; Lovell, N.H.; McDonald, C.F. Predicting the risk of exacerbation in patients with chronic obstructive pulmonary disease using home telehealth measurement data. Artif. Intell. Med. 2015, 63, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Granero, M.A.; Sanchez-Morillo, D.; Leon-Jimenez, A. Computerised Analysis of Telemonitored Respiratory Sounds for Predicting Acute Exacerbations of COPD. Sensors 2015, 15, 26978–26996. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.; Pinnock, H.; McKinstry, B. Changes in telemonitored physiological variables and symptoms prior to, exacerbations of chronic obstructive pulmonary disease. J. Telemed. Telecare 2015, 21, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Borel, J.-C.; Pelletier, J.; Taleux, N.; Briault, A.; Arnol, N.; Pison, C.; Tamisier, R.; Timsit, J.F.; Pepin, J.L. Parameters recorded by software of non-invasive ventilators predict COPD exacerbation: A proof-of-concept study. Thorax 2015, 70, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.A.; Nrook, M.; Morice, A.H. The value of telehealth in the early detection of chronic obstructive pulmonary disease exacerbations: A prospective observational study. Health Inform. J. 2016, 22, 406–413. [Google Scholar] [CrossRef] [PubMed]
| Author | Subjects and COPD Severity | Country | Measures | Quality | Detailed Description | Results |
|---|---|---|---|---|---|---|
| Seemungal et al., 2000 [12] | N = 101 severe COPD | United Kingdom | PEFR FEV1 Symptoms | Moderate quality | Period: 2.5 years. PEFR and symptoms measured daily, post morning medication. In a subgroup of 34, FEV1 was measured | Analysis of 504 exacerbations: Lung function changed significantly on the day of onset (p < 0.001). A decrease in the median of: PEFR by 8.6 L/m FEV1: 24.0 mL FVC: 76.0 mL |
| Cooper et al., 2009 [13] | N = 19 mild−severe COPD | United Kingdom | HR SpO2% PEFR FEV1 Symptoms | High risk of bias | Period: 4 months. Participants measured their vital signs and recorded their symptoms twice a week in the morning | Analysis of four exacerbations: Concluded that SpO2% was the variable most closely associated with exacerbation but no statistical significance reported |
| Sund et al., 2009 [14] | N = 18 severe COPD | United Kingdom | FEV1 Symptoms | Low quality | Period: 6 months. Daily electronic diary and performed three spirometry manoeuvres daily in the evening | Analysis of 75 exacerbations: 55 exacerbations were detected via tele-health (symptoms) and 6/55 exacerbations were detected via FEV1 alone (p = not significant). Exacerbation detected via FEV1 was defined as a 10% fall in FEV1 for ≥2 consecutive days. |
| Hurst et al., 2010 [5] | N = 31 severe COPD | United Kingdom | HR SpO2% PEFR Symptoms | Moderate quality | Period: 9 months. Daily paper diary cards | Analysis of 13 exacerbations: Variation was noted prior and during the onset of exacerbation in PEFR, HR, and SpO2%. Maximal change in SpO2% and HR occurred two days into exacerbation: SpO2% had fallen by −1.24 SD, HR increased by +3.09 SD. Maximal change in PEFR occurred four days into exacerbation: −2.97SD Composite Score to detect exacerbation: AUC = 0.832, p < 0.05. |
| Jensen et al. in 2012 [15] | N = 57 moderate−severe COPD | Denmark | HR SpO2% BP | Moderate quality | Period: 4 months. Daily diary cards | Analysis of 9 exacerbations: Their algorithm classified variables into 273 features and was able to detect seven exacerbations via vital signs with 70% sensitivity, 95% specificity, AUC = 0.73. |
| Berge et al., 2012 [16] | N = 137 severe COPD | Netherlands | Salbutamol use PEFR Symptoms | Moderate quality | Period: 15 months. Daily diary cards | Analysis of 101 exacerbations: Significant decrease in PEFR 15 L/min at exacerbation compared to baseline. |
| Yanez et al. in 2012 [17] | N = 89 severe COPD (On O2 therapy) | Spain | Respiratory Rate (RR) | Moderate quality | Period: 3 months. Daily monitoring of respiratory rate, using a sensor inserted into the domiciliary oxygen supply system | Analysis of 10 exacerbations: Increase in the mean respiratory rate in 21/30 exacerbations, 1–5 days prior to hospitalisation Mean of respiratory rate raised: Five days: 15.2 ± 4.3 min−1 to 19.1 ± 5.9 min−1 Two days: 2.3 min−1 (15% from baseline) One day: 4.4 min−1 (30% from baseline) All p-value < 0.05 |
| Martin Lesende et al. 2013 [18] | N = 58 Heart failure (27.6%) + O2 therapy (57.1%) + moderate−very severe COPD and asthma 25.9% | Spain | HR SpO2% BP RR Weight Temperature Symptoms | High risk of bias | Period: 12 months. Daily monitoring | In the five days preceding hospital admission: Mean SpO2% fell from 93.1% to 91.0% (4.6 SD), and mean HR increased from 77.8 to 84.2 min−1 (17.1 SD) p = 0.003 for both. No significant change for respiratory rate, body temperature and blood pressure. |
| Pedone et al. 2013 [19] | N = 99 moderate−severe COPD | Italy | HR SpO2% TemperaturePhysical activity | High risk of bias | Period: 9 months. Automatic recording of vital signs, a mean of four times per day. | Analysis of 13 exacerbations: SpO2% fell three days before an exacerbation, which permitted timely intervention, and was associated with a 33% reduction in hospitalisation rate (p = not shown, data displayed in a Figure only). |
| Segrelles et al., 2014 [7] | N = 60 severe COPD (On O2 therapy) | Spain | HR SpO2% BP PEFR | High risk of bias | Period: 7 months. Participants monitored their vital signs every morning, but PEFR was three times/week. | Analysis of 50 red flags: confirmed red flag defined as moderate, severe or very severe exacerbation. Tele-health was associated with significant reduction in acute NIV usage (p < 0.0001), ER visits (p = 0.001), admissions (p = 0.015) and bed days (p = 0.018). Reported that SpO2% and PEFR were the most predictive parameters (but data not reported). |
| Harding et al., 2015 [20] | N = 18 moderate−very severe COPD | United Kingdom | HR SpO2% Symptoms | Moderate quality | Period: 6 months. Each participant asked to fill a daily symptom diary card. | Analysis of 37 exacerbations: 15/37 exacerbations were identified in three days prior to medication self-initiation.Alerts related to events: 47 symptom alerts (16 patients)80 HR alerts (18 patients), and 62 SpO2% alerts (17 patients). p = not shown. |
| Mohktar et al., 2015 [21] | N = 21 moderate−very severe COPD | Australia | HR SpO2% BP RR Weight Temperature FEV1 Symptoms | Moderate quality | Period: 11 months. Participants daily monitored their vital signs and symptoms | Analysis of 90 exacerbations: The designed algorithm identified 55/90 true exacerbations (71.8% sensitivity 80.4% specificity). FEV1 value (k = 0.21), mean of distribution of SpO2% (k = 0.27) and the weight (k = 0.21) were the most predictive variables (p = not shown). |
| Fernandez-Granero et al., 2015 [22] | N = 16 severe−moderate COPD | Spain | Respiratory sound | Moderate quality | Period: 6 months. Daily recorded respiratory sounds using a microphone over the super-sternal notch | Analysis of 33 exacerbations: 25 out of 33 exacerbations were detected 5 ± 1.9 days prior to the onset of exacerbation by changes in sounds (p = not shown). |
| Burton et al., 2015 [23] | N = 33 mild−very severe COPD | United Kingdom | HR SpO2% FEV1 PEFR Symptoms | Moderate quality | Period: >200 days. Each participant asked to fill a symptom questionnaire, and measure heart rate, and SpO2% daily. FEV1 and PEFR monitored weekly. | Analysis of 172 exacerbations: Increase in HR (87 min−1–94 min−1) at the onset of exacerbation and mean SpO2% fell (93.6% to 92.4%) around the onset of exacerbation. Exacerbation associated with a reduction of 0.1 L in FEV1. |
| Borel et al., 2015 [24] | N = 44 severe COPD (On NIV and O2 therapy) | France | RR %Triggering NIV usage Questionnaire | Moderate quality | Period: 6 months. Daily monitoring via the ventilator and daily EXACT-Pro questionnaire. | Analysis of 21 exacerbations: 21 exacerbations detected, and the risk of exacerbation was high if high value noted on ≥ two days out of five for RR P = 0.01, and %Triggered Breaths p = 0.037, but not total NIV usage p = 0.097). |
| Hamad et al., 2016 [25] | N = 183 COPD * | United Kingdom | HR SpO2% Temperature Physical activity Symptoms | Moderate quality | Period: 4 months. Daily monitoring. | Analysis of 98 exacerbations: 80/98 showed changes on one or more tele-health parameters prior to hospitalisation/exacerbation onset. 30 exacerbations resulted in hospitalisation and 7/30 had significant SpO2% reduction (significant defined for each patient individually, p = 0.049) 12/98 exacerbations had a significant SpO2% fall (p < 0.05). |
| Author | Definition of Exacerbation |
|---|---|
| Seemungal et al., 2000 [12] | Anthonisen criteria. |
| Cooper et al., 2009 [13] | Not explained. |
| Sund et al., 2009 [14] | Increase of two symptoms and/or ≥10% reduction of FEV1 for ≥2 consecutive days; or the use of antibiotics and/or prednisolone. |
| Hurst et al., 2010 [5] | ≥2 of new or worsening symptoms (one should be increased breathlessness, sputum volume of sputum purulence) for ≥2 days. |
| Jensen et al. in 2012 [15] | Admission to hospital, or started antibiotics or steroids with specific symptoms. |
| Berge et al., 2012 [16] | Not explained. |
| Yanez et al., 2012 [17] | Clinical diagnosis by an emergency room clinician. |
| Martin Lesende et al., 2013 [18] | Not explained. |
| Pedone et al., 2013 [19] | Change in symptoms that lead to a change in medication. |
| Segrelles et al., 2014 [7] | GOLD definition. |
| Harding et al., 2015 [20] | Initiation of antibiotics or steroids or both. |
| Mohktar et al., 2015 [21] | GOLD definition. |
| Fernandez-Granero et al., 2015 [22] | Use of medication for exacerbation, and/or unplanned emergency room visits and/or hospital admissions. |
| Burton et al., 2015 [23] | Anthonisen criteria or started antibiotics. |
| Borel et al., 2015 [24] | If abnormal values of respiratory rate and % triggered breaths were reported for two days or more, or abnormal values of NIV daily usage were reported for three days or more out of five. Abnormal values were defined as “value of a parameter was >75th or <25th percentile, the day was recorded as abnormal value’ (‘high value’ > 75th, ‘low value’ < 25th). |
| Hamad et al., 2016 [25] | Admission to hospital, or started antibiotics or/and steroids. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Rajeh, A.M.; Hurst, J.R. Monitoring of Physiological Parameters to Predict Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review. J. Clin. Med. 2016, 5, 108. https://doi.org/10.3390/jcm5120108
Al Rajeh AM, Hurst JR. Monitoring of Physiological Parameters to Predict Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review. Journal of Clinical Medicine. 2016; 5(12):108. https://doi.org/10.3390/jcm5120108
Chicago/Turabian StyleAl Rajeh, Ahmed M., and John R. Hurst. 2016. "Monitoring of Physiological Parameters to Predict Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review" Journal of Clinical Medicine 5, no. 12: 108. https://doi.org/10.3390/jcm5120108
APA StyleAl Rajeh, A. M., & Hurst, J. R. (2016). Monitoring of Physiological Parameters to Predict Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review. Journal of Clinical Medicine, 5(12), 108. https://doi.org/10.3390/jcm5120108





